Abstract
Electrochemical oxidation is an effective wastewater treatment method. Metal oxide-coated substrates are commonly used as anodes in this process. This article compiles the developments in the fabrication, application, and performance of metal oxide anodes in wastewater treatment. It summarizes the preparative methods and mechanism of oxidation of organics on the metal oxide anodes. The discussion is focused on the application of SnO2, PbO2, IrO2, and RuO2 metal oxide anodes and their effectiveness in wastewater treatment process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes, toxic organic molecules, inorganic salts, adsorbable organohalogens (AOX), aromatic pesticide residues, drugs, and surfactants are the major components of wastewater released from textile, leather, pulp and paper, printing, photograph, cosmetic, pharmaceutical, and food industries (Fornazari et al. 2012; Zaviska et al. 2009). The environmental concern has led to stringent rules and it is mandatory to treat the wastewater before disposing into natural water streams. Chemical, physicochemical, biological/enzymatic, advanced oxidation processes, and electrochemical methods are commonly employed in wastewater treatment (Brungs et al. 1996; Cheng-chun and Jia-fa 2007; Guinea et al. 2009; Hou et al. 2009; Martínez-Huitle and Brillas 2009; Rosales et al. 2009). In the past few years, “electrochemical method” has drawn much attention. It is economical and easy to construct and operate. More importantly, electrodes serve as immobilized active surfaces for the oxidation of organics (Fornazari et al. 2012). In addition, unlike chemical methods, electrochemical method does not necessitate the addition of chemical reagents. However, the addition of electrolyte is inevitable if the wastewater sample is not sufficiently conducting. In many cases, the electrolyte is deliberately added to enhance the degradation process. The added electrolyte strongly influences the efficiency of the process. In exploiting this method as a powerful tool in wastewater treatment, the key part of the electrolytic system—“electrode”—acquires utmost importance.
On oxidation, the organic contaminant undergoes complete oxidation to CO2 and H2O (combustion) or results in the formation of simpler fragments (conversion) (Comninellis 1994). On the other hand, reduction of organics cannot completely lower the chemical oxygen demand (COD) of the wastewater sample, but only leads to poor decontamination (Martínez-Huitle and Brillas 2009). Electrooxidation favors the effective removal of organics over electro-reduction.
Anode is the oxidation site in any electrolytic system. Long service life, large surface area, wide operating potential window between hydrogen and oxygen evolution reaction (OER) overpotentials, high catalytic activity, physically stable, resistant to corrosion, cheap, and easy to fabricate are the prerequisite properties intended to be fulfilled by the anodes employed in electrochemical wastewater treatment.
The anode materials like stainless steel, glassy carbon, Ti/RuO2, Ti/Pt-Ir, activated carbon fibers, MnO2, Pt carbon black, porous carbon and reticulated vitreous carbon, and electrodes made of lead, iron, and graphite usually undergo deformation during electrolysis (Martínez-Huitle and Ferro 2006; Rodgers et al. 1999). Beer introduced the titanium substrate-based RuO2 and TiO2-coated electrode, referred as dimensionally stable anodes (DSA®s) which possessed high catalytic activity and stability (Beer 1980; Chandler et al. 1997). Figure 1 is the flow chart showing the development of DSA® and DSA®-type metal oxide electrodes (Chandler et al. 1997; Chen et al. 2001, 2002; Klamklang et al. 2012; Nikolić et al. 2012; Panić and Nikolić 2007; Trasatti 2000). The DSAs are good conductors, exhibit high catalytic activity, dimensionally stable, and contribute towards energy savings (see Table 1). They exhibit extended operating life of 8–9 years, without deformation, and their durability has been established in many fields (Chandler et al. 1997; Chen et al. 2002).
The metal oxide electrodes with stability comparable with DSA tempted several research teams to investigate their performance in the electrochemical wastewater treatment. The metal oxide electrodes prepared by Comninellis and Vercesi (RuO2, IrO2, TiO2, ZrO2, and Ta2O5) over Ta substrate exhibited high oxygen evolution overpotential. But, due to high cost of Ta, the other metal Ti was selected as the substrate, and ever since, Ti has been the favorite substrate for the fabrication of metal oxide-coated anodes. Titanium is a cheap “self-healing” substrate which anodically generates a layer of TiO2 and protects itself against corrosion (Beer 1980). Moreover, the water quality is not affected by the dissolution of Ti in low concentrations (Niu et al. 2012). Ti/tin dioxide (SnO2), Ti/lead dioxide (PbO2), and platinum group metal oxide coated on valve metals (Ti, Zr, Ta, Nb, Hf, W) (DSA®s) are the exhaustively studied metal oxide electrodes.
Numerous combinations of these modified metal oxides have been fabricated and tested for their stability, catalytic activity, and ability to incinerate the organics. The ultimate goal is to fabricate an electrode which satisfies the prerequisite properties of the anode. Though few research teams have been successful in fabricating the electrodes with excellent catalytic activity and stability, the search for the best recipe still continues! This review outlines the developments in the preparation, properties, and application of metal oxide-coated anodes in the electrochemical wastewater treatment.
Fabrication of metal oxide anodes
The metal oxide anodes are cheap and easy to fabricate (Gaber et al. 2012; Klamklang et al. 2012; Kusmierek et al. 2011; Yang et al. 2009). Designing and fabricating an electrode of desired composition and characteristics is a challenging task. Thin film metal oxide coatings are prepared by solution phase and gas phase chemical methods. The solution phase method involves dip coating, spin coating, painting, spray pyrolysis, and sol–gel techniques, which employ a precursor solution (Perednis and Gauckler 2005). The gas phase methods are chemical vapor deposition (CVD) and atomic layer deposition (ALD) (Perednis and Gauckler 2005). Electrochemical method (electrochemical anodization and electrochemical deposition) is also widely utilized in the preparation of metal oxide-coated electrode.
A precursor solution is generally prepared by dissolving stoichiometric quantities of metal salts in a solvent or mixture of solvents. The resultant solution is applied on the surface of a substrate using suitable coating techniques [dip coating (Watts et al. 2008; Yang et al. 2009), spin coating (Fierro and Comninellis 2010), or painting (del Río et al. 2009)]. The spin coating technique gives uniform coat on flat substrates. For uneven substrates, dip coating or painting is preferred. The process of coating and drying is repeated until a desired thickness of the coat is obtained. Finally, the coated substrate is annealed to obtain superior morphological characteristics and wear resistance (Comninellis and Vercesi 1991). Thermal decomposition method was adopted in the fabrication of Ti/SnO2-Sb2O3 (Yang et al. 2009), Ti/TiO2-RuO2 (Kusmierek et al. 2011; Fachinotti et al. 2007), and Ti/SnO2-Pt-antimony (Sb) (del Río et al. 2009) electrodes.
Spray pyrolysis is an aerosol process. It is relatively simple and cost effective (Lipp and Pletcher 1997; Perednis and Gauckler 2005; Vicent et al. 1998; Yusta et al. 1997). It involves the atomization of precursor and conversion of these droplets into solid particles by heating. The nature of the deposit obtained on the substrate is dependent on the transformations undergone by the droplets. The temperature gradient between the spraying nozzle and the substrate, the carrier gas and precursor solution flow velocity, and shape and nature of the substrate influence the transformations undergone by the droplets (Correa-Lozano et al. 1996a, 1997). Correa-Lozano et al. (1996a) prepared Ti/SnO2-Sb2O5 electrode using spray pyrolysis technique and have thoroughly studied its characteristics (Correa-Lozano et al. 1996b, 1997).
The sol–gel method is also widely used in the fabrication of metal oxide anodes (Attia et al. 2002; Brinker et al. 1991; Choi et al. 2007; Panić et al. 2003a, b, 2010; Lu et al. 2010; Ozer et al. 1995). In this method, the microstructure of the coating depends on pH and viscosity (Ozer et al. 1995). Generally, the precursor salts are hydrolyzed by dispersing them in an appropriate media and vigorously stirred for certain ageing time. The formation of solid phase particles of the sol from hydrolyzed precursor is dependent on ageing time. Electrodes prepared at different ageing time differ in their electrochemical behavior (Panić et al. 2003a).
A uniform metal oxide coating of desired thickness can be achieved by electrochemical method by adjusting its operating conditions. In electrochemical anodization, a pretreated metal substrate is anodized in a suitable electrolytic bath. A layer of metal oxide coat is generated on the surface of the anodized metal. In electrochemical deposition, precursor salts are dissolved in the electrolytic bath, which are electrodeposited on the substrate. The electrochemical method is advantageous in achieving effective doping and a uniform metal oxide coat on the substrate. Electrochemical method was adopted in the preparation of the following electrodes: Ti/SnO2-Sb2O3-Nb2O5/PbO2 (Yang et al. 2009), Er-chitosan-F-PbO2 (Wang et al. 2010b), C/PbO2, Pb + Sn/PbO2-SnO2, Pb/PbO2 (Gaber et al. 2012), Ti/PbO2 (Panizza and Cerisola 2008), Ti/SnO2-Sb2O5/PbO2 (Liu and Liu 2008), Pb/PbO2 (Martínez-Huitle et al. 2008), and Ti/SnO2-Sb/PbO2-Ce (Niu et al. 2012).
The gas phase techniques are also well known, but they require sophisticated instruments and are relatively costly. In CVD, generally the reactant (precursors) gases are pumped into the reaction chamber maintained at optimum temperature and pressure, where these reactive gases adsorb and react with the substrate to form a thin film. Metal organic chemical vapor deposition utilizes organometallic compounds as precursors (Amjoud et al. 1998; Duverneuil et al. 2002; Devilliers et al. 2003). The ALD technique is closely related to CVD, but the factor which distinguishes it from CVD is that the precursors are led on to the substrate alternately, one at a time (Riihelä et al. 1996). Using CVD, thin films with high uniformity can be reproduced. However, it involves complex processes, high temperature, and toxic gases. Few teams have used gas phase methods for the preparation of thin film metal oxide-coated electrodes (Watts et al. 2008; Yusta et al. 1997; Amjoud et al. 1998; Duverneuil et al. 2002). However, the earlier studies revealed that in majority of the cases, solution method was preferred for the preparation of metal oxide electrodes. The conditions used in the fabrication of different metal oxide-coated electrodes by solution method are presented in Table 2.
Mechanism of electrochemical oxidation of organics on metal oxide anodes
Figure 2 displays various electrochemical methods used for wastewater treatment. The electroflocculation and electrocoagulation methods produce large amount of sludge, whereas such problem is not encountered in the case of electrooxidation (Mao et al. 2008). The organics in the wastewater are converted into simple molecules and/or completely incinerated to CO2 and H2O by electrochemical oxidation (Comninellis 1994). The morphology and composition of the metal oxide coating, nature of the contaminating organic molecule, and wastewater matrix influence the mechanism of oxidation of organics and hence the products (Martínez-Huitle and Ferro 2006).
Electrochemical methods. Adapted from Martínez-Huitle and Brillas (2009)
Mechanism of oxidation
Oxidation of organics proceeds as follows:
-
By direct electron exchange with the anode (direct oxidation)
-
By the active species during electrolysis (indirect oxidation)
The direct exchange of electrons between the anode material and the organic molecule at the electrode solution interface is referred as “direct oxidation.” This reaction is viable at potentials well below the oxygen evolution overpotential. But the anode surface gradually undergoes passivation due to adsorption of organic molecules (Panizza and Cerisola 2009).
A theoretical model for the mechanism of oxidation of organics on metal oxide anodes was proposed by Comninellis (1994), and it has been accepted by the scientific community. During electrolysis, hydroxyl (•OH) radicals are generated on the anode surface by water discharge at high anodic potentials (Panizza and Cerisola 2009; Watts et al. 2008). These •OH radicals adsorb physically (Eq. 1) or chemically (Eq. 2) on the metal oxide anode (MO x ).
As a result of strong interaction between hydroxyl radical (•OH) with the anode metal and availability of a higher oxidation state for the anode metal leads to the formation of MO x + 1 (Martínez-Huitle and Ferro 2006). Electrodes which can undergo such interaction are referred as “active” (carbon, graphite, IrO2, RuO2, platinum) (Panizza and Cerisola 2009). “Non-active” anodes (SnO2, PbO2, or boron-doped diamond (BDD)) only act as inert electron sink for the removal of electrons from organics, without providing any adsorptive site (Comninellis 1994; Ozer et al. 1995). Chemisorbed oxygen in MO x + 1/MO x couple acts as an active site for the selective oxidation of organics (Eq. 5), whereas physisorbed •OH radicals completely incinerate the organics into CO2 and H2O (Eqs. 3 and 4) (Comninellis 1994).
The oxygen evolution reaction (Eqs. 6 and 7) always competes with the oxidation reaction of organics.
Figure 3 is the schematic diagram of electrochemical oxidation of organics on metal oxide anodes.
Oxidation by electrochemically generated oxidants
On electrolysis, species generated by the discharge of water (hydroxyl radical, hydrogen peroxide, ozone) react with themselves or with the electrolytes to produce active oxidants. These oxidants are utilized in the oxidation of organics and this type of oxidation is referred as “indirect oxidation” (Jüttner et al. 2000; Li et al. 2009; Martínez-Huitle and Ferro 2006; Panizza et al. 2000; Scialdone 2009; Scialdone et al. 2009b; Simond et al. 1997b; Zhao et al. 2003). Mediated electrooxidation involves metal ions with high oxidation potential. In this method, oxidants are electrochemically generated in a closed cycle and can completely incinerate the organics (Jüttner et al. 2000).
Different electrolytes could be found in any industrial effluent. Chloride salts are most common. It is observed that the presence of chloride (Cl−) ions in the wastewater increases the organic removal efficiency (Comninellis 1994; Scialdone et al. 2009b). Electrochemically generated active chlorine species efficiently oxidize the organics as per Eqs. 8–11 (Jüttner et al. 2000; Martínez-Huitle and Ferro 2006; Panizza et al. 2000; Scialdone et al. 2009b).
The equilibrium in Eq. 10 is pH dependent. HOCl predominates in the pH range 3–8 and ClO− predominates above pH 8 (Fornazari et al. 2012; Malpass et al. 2012). Hence, oxidation of organics by HOCl is favorable at near neutral pH (Fornazari et al. 2012; Malpass et al. 2012). The Cl2/Cl− and HOCl/Cl2 couples also oxidize the organics completely (Fornazari et al. 2012; Song et al. 2010b). The electrochemical reactions at the anode surface involving chloride ions are given in Eqs. 12 and 13 (Scialdone et al. 2009b):
MO x (HOCl) mediates unselective oxidation and thereby provides substantial total organic carbon (TOC) removal (Fornazari et al. 2012). However, the presence of chloride ions is associated with the following disadvantages: the formation of AOX (Malpass et al. 2012; Sakalis et al. 2007) and the generation of ClO–. AOXs are toxic and recalcitrant to degradation, and ClO− reduces the service life of the metal oxide anode (Costa et al. 2010). Several other electrolytes like ClO4 −, NO3 −, PO4 2−, SO4 2−, CO3 2−, and Br− were tested for electrochemical degradation process (Fornazari et al. 2012; Gaber et al. 2012). But the degradation efficiency in presence of Cl− ions was found to be better than that achieved in the presence of these electrolytes. The peroxidisulfate is a powerful oxidizing agent and as effective as hydroxyl radical (Malpass et al. 2012). Excellent degradation of organics is possible in presence of sulfate ions, provided that the electrode and the wastewater environment matrix favors the formation of sulfate radical or peroxidisulfate ion (Martínez-Huitle et al. 2008; Martínez-Huitle et al. 2008; Brillas et al. 2009). However, the COD/TOC removal not only depends on the electrode and electrolytes, but also on the organic molecule itself (Marugán et al. 2007).
Factors influencing the anode performance
The performance of an electrode is rated in terms of its ability to incinerate the organics with minimum consumption of energy and time. The nature of the electrode, applied current density, duration of electrolysis, supporting electrolyte, pH, temperature, and cell geometry significantly contribute to the efficiency and energy consumption of the electrochemical oxidation process. The nature of the electrode is dependent on its fabrication technique, composition, and structure.
Fabrication technique
Both physical (surface morphology, metal oxide film thickness, composition, dimension, microstructure) (Wang et al. 2009) and chemical (availability of higher oxidation state, its affinity towards hydroxyl radicals, stability in different pH, active species generated in presence of different electrolytes, and wear-tear) properties of an anode influence its ability to oxidize the organics (electrochemical activity) or to generate active oxidants (electrocatalytic activity). The fabrication technique can modulate both the chemical and physical nature of the coating and hence its electrocatalytic and electrochemical activities.
Proper substrate pretreatment is the key to produce a strong adhesion of metal oxide coating on the surface of the substrate (Beer 1980). The solvents used in the precursor solution, dopant and its content, coating technique, dip withdrawal rate, and annealing temperature are crucial factors which contribute in altering the characteristics of an anode. The schematic representation of the steps involved in the fabrication of metal oxide anodes by thermal decomposition is shown Fig. 4 (Comninellis and Guohua 2010).
Schematic diagram of steps involved in the fabrication of metal oxide electrodes by thermal decomposition technique. Adapted from Comninellis and Guohua (2010)
The solvents used in the preparation of precursor solution play a role of great consequence in altering the morphology and hence the characteristics associated with the morphology (exposure of catalytic and adsorptive sites, porosity, and effective surface area) of the metal oxide coating. The difference in the thermal expansion coefficient of the base metal and the coating leads to the formation of cracks in the coating and results in typical cracked mud coating (Jiang-tao et al. 2007; Wang et al. 2009). Cracked mud coating gradually undergoes erosion. The adsorption capacity is favored by the irregular electrode surface. But it involves undesired strong adsorption of hydroxyl radicals as well. Weak interaction between the electrode surface and hydroxyl radicals increases electrochemical activity of the electrode towards the oxidation of organics, whereas strong interaction results in oxygen evolution (Costa et al. 2010; Kapałka et al. 2008). The efficiency of the process decreases with oxygen evolution.
Three different precursor solutions prepared in ethanol, n-butanol and a polymeric precursor were used in the fabrication of SnO2 + Sb2O3 interlayer by thermal decomposition (Wang et al. 2009). The scanning electron microscopy (SEM) images in Fig. 5 clearly reveal the morphological differences in the oxide interlayer obtained from these precursor solutions. The oxide interlayer from the polymeric precursor assisted the structural alignment and produced more agglomerated coating, whereas alcoholic precursors produced typical cracked mud coating.
SEM micrographics of SnO2 + Sb2O3 coatings prepared with different precursor solvents: a polymeric precursor, b ethanol, and c n-butanol. Reprinted from Wang et al. (2009). Copyright 2008 Elsevier
The nature of the precursor solution can significantly affect the host metal and dopant content in the coating. It was found that viscous precursor solutions aid in the formation of agglomerated uniform coating and accumulation of the dopant on the surface of the coating, whereas alcoholic precursor solutions lead to cracked mud coating. Precursor solution prepared in isopropanol and sol–gel was used in the fabrication of Sb-SnO2/Ti electrode by thermal decomposition. The Sn/Sb content in the coating was found to be higher than in the alcoholic precursor solution (Wang et al. 2010a; He and Mho 2004). On the contrary, the Sb content was found to be higher in the coating than in the solution when sol–gel method was adopted (Wang et al. 2006).
Antimony is the most commonly used dopant in the preparation of SnO2 electrodes and it is the hydroxyl radical generation site (Jiang-tao et al. 2007). Hence, its accumulation on the surface of the coating is recommended. Variation in the Sb content considerably influences the morphology of the coating.
The electrocatalytic activity can be enhanced by minimizing the interlayer thickness and maximizing the outer active surface area by choosing the right combination of techniques in the preparation of metal oxide electrodes (Yeo et al. 2010). Different preparative methods were used in combination by Yeo et al. (2010) to obtain a compact layer of metal oxide coat. They prepared Ti/Pb(E)/PbO2(T,E) (E-electrodeposition, T-thermal decomposition) electrode by electrodepositing a layer of Pb on Ti, followed by coating a layer of PbO by thermal decomposition. The PbO was then electrooxidized to PbO2. This electrode acquired large surface area and roughness. The relative roughness factor of this electrode was found to be 8.98, where the arbitrary roughness factor of Ti/Pb(E)/PbO2 (E) electrode was set as unity. Watts et al. (2008) found that the dopant content, coating technique, and the annealing temperature are the major factors which contribute significantly in controlling the characteristics of a metal oxide coat. The effect of number of layers of the coating and dip withdrawal rate on the performance of the electrode in eliminating the organics was found to be very feeble.
Interfacial layer or undercoat
The oxygen which evolved at the anode surface creeps through the metal oxide coating and passivates the substrate metal. This is eliminated by the inclusion of an interfacial layer connecting the active metal oxide coating and the substrate. The chemical stability, resistance against corrosion, and service life of the anode are enhanced by the insertion of an interlayer (Kong et al. 2012). An undercoat of stable metal oxide is applied on a substrate and then an outer active layer is overlaid. It is represented as substrate/interlayer/active surface layer as shown in Fig. 6. The interlayer supports the active metal oxide layer to adhere strongly onto the substrate and helps in guarding the stability and enhancing the electrocatalytic activity of the electrode. It is important for the interlayer to be highly conducting and itself does not undergo passivation. The constituent metals of the interlayer should satisfy the Hume-Rothery limit (15 %) between their ionic radii, so that a solid solution between the three layers is possible.
The direct deposition of PbO2 on Ti substrate by electrodeposition leads to the formation of a passive layer of TiO2 (Yang et al. 2009). This can be avoided by depositing an interlayer on Ti substrate. The interlayer acts as a barrier against such passivation of the substrate by avoiding the diffusion of oxygen. For the interlayer in Ti/solid solution interlayer/PbO2 electrode, the metals chosen by Kong et al. (2012 were such that the ratios of their corresponding ionic radii (Sn/Sb, Ru/Ti, Ir/Ta) were below the Hume-Rothery limit. Figure 7 is the SEM images of the interlayer surfaces SnO2-Sb2O5, RuO2-TiO2, and IrO2-Ta2O5 (Kong et al. 2012). The ionic radii ratio of the metals in the interlayer to Ti4+ obtained from the oxidation of Ti substrate also lays within the limit; hence, excellent binding was obtained between the interlayer and the substrate. Similarly, the interlayer and the electrodeposited PbO2-active layer formed a continuous solid solution, except in the case of RuO2-TiO2 interlayer, which provided only partial solid solution with the PbO2 due to large difference in the ionic radii of Ti4+ and Pb4+. The interlayer enhanced the orientation of the crystal structure of the electrodeposited PbO2. Ti/IrO2-Ta2O5/PbO2 electrode coating showed high catalytic activity for oxygen evolution. The inclusion of IrO2-Ta2O5 interlayer in Ti/PbO2 electrode increased its service life from 11 to 672 h as determined by the accelerated lifetime test in 1 M H2SO4 under galvanostatic condition (4 A cm−2). RuO2-TiO2 and SnO2-Sb2O5 interlayers showed intermediate enhancement in service life of 207 and 58 h, respectively. The interlayer effectively eliminated the passivation of Ti substrate and enhanced the performance of the anode towards oxidation of organics. Similar results were obtained from the electrodes with interlayers of different composition (see Tables 2, 3, and 4).
SEM images of the interlayer surface. a Ti/SnO2-Sb2O5. b Ti/RuO2-TiO2. c Ti/IrO2–Ta2O5. Reprinted from Kong et al. (2012). Copyright 2012 Springer Science + Business Media B.V.
Metal oxides in wastewater treatment
Several mixed metal oxide electrodes have been used in the electrochemical wastewater treatment. Nevertheless, it is found from the literature that majority of these are the derivatives of four metal oxides, SnO2, PbO2, RuO2, and IrO2, and hence we limit our discussion to these metal oxide electrodes.
SnO2
Tin oxide is an insulator in its stoichiometric form. The distortions created by the oxygen vacancies make it an n-type and conductive material. Doped SnO2 anodes (non-active) show excellent conductivity, high oxygen and chlorine evolution overpotential, electrocatalytic activity for the oxidation of organic compounds, ability to adhere strongly to the base metal, and stability over a large pH range (Correa-Lozano et al. 1997; del Río et al. 2009; Jiang-tao et al. 2007; Lipp and Pletcher 1997; Vicent et al. 1998; Xu et al. 2012; Yusta et al. 1997). The deviation in the native defects, nature, and effect of doping into the crystal lattice influences the activity of SnO2. The oxygen vacant sites are responsible for the conductivity of SnO2 and also the availability of these vacant sites determines the ratio of MO x + 1 and MO(•OH) on the anode surface. If the crystal defect increases with the formation of more of oxygen vacant sites, the formation of MO x + 1 is favored. The physically adsorbed hydroxyl radical reacts chemically to fill the vacant sites of the crystal lattice to form MO x + 1. MO x + 1 favors only the conversion of organics into oxidized intermediates, whereas MO(•OH) completely incinerates the organics into CO2 and H2O (Feng et al. 2008). The electrocatalytic activity of Ti/SnO2 electrode is higher than that of PbO2 and RuO2/IrO2 electrodes (Radjenovic et al. 2011; Mao et al. 2008). Moreover, it is comparatively cheaper than the noble metal oxide electrodes and easy to fabricate (Mao et al. 2008).
The empty electron orbitals available on the f-block elements can be used as channels for electron transaction from the substrates, if doped into metal oxide coating (Fenga and Li 2003). Electrocatalytic activity of the electrodes for the oxidation of organics markedly improves on doping (Fenga and Li 2003). The dopant and its molar ratio with the host metal oxide influence the physicochemical properties of the electrode. Among the various dopants (B, Bi, Ce, F, Fe, Gd, Ir, La, Ni, Pt, Ru, and Sb) used to enhance the characteristics of the SnO2, Sb has shown remarkable impact (Jiang-tao et al. 2007; Costa et al. 2010). Sn and Sb ionic radii satisfy the Hume-Rothery limit, and hence, a homogeneous solid solution between the two metals is possible. A list of modified SnO2 electrodes and conditions used in their fabrication is given in Table 2.
The content of the dopant has a critical role to play in directing the microcrystalline structure, morphology, electrocatalytic activity, and capability of generation of hydroxyl radicals and hence the oxidation of organics. Ti/SnO2-Sb electrode possesses high OER potential which varies with the composition of Sn/Sb ratio. For the electrodes prepared with varying concentrations of Sb, OER showed potential greater than 1.9 V (vs standard calomel electrode (SCE)) in all the cases and it increased with an increase in Sb content (Mao et al. 2008). The highest catalytic activity was found with 8 mol % [SnO2-Sb(0.08)] and generated high concentration of oxidants.
The competition between the oxidation reaction and the OER resulted in low efficiency of Ru0.7Ir0.3O2 anode to degrade β-blocker metoprolol and led to the persistence of halogenated derivatives, whereas on SnO2-Sb electrode, the production of hydroxyl radical was sufficient enough to degrade the metoprolol into simpler intermediates (Radjenovic et al. 2011).
Jiang-tao et al. (2007) doped SnO2 with 5, 10, and 15 % of Sb and used these electrodes in the electrooxidation of 4-chlorophenol. The oxygen evolution potential for Ti/Sb-SnO2 with 5 % Sb electrode skipped from 1.5 V (without Sb) to 1.65 V. The oxidizable species in this potential window are oxidized directly on the anode surface. COD removal (48.9 %) of 4-chlorophenol with 15 % current efficiency was obtained on 5 % Sb-doped electrode, whereas for 0, 10, and 15 %, Sb dopant the COD removal was 36.4, 44.8, and 26.6 %, respectively. However, it has also been reported in both cases (Mao et al. 2008; Jiang-tao et al. 2007) that effective and homogeneous distribution of Sb diminished with an increase in Sb content. This was attributed to the difference in the thermal expansion coefficients. This effect is not so strong at lower Sb contents, but at higher content of Sb, the variation in the surface morphology is clearly noticeable. On using high content of dopant, the electrode follows conversion mechanism, due to selective oxidation by the chemisorbed oxygen, as discussed earlier (Kong et al. 2012). At low content of dopant (Sb centers are sites for hydroxyl radical generation), physisorbed hydroxyl radicals are involved in the oxidation leading to complete combustion of the organics. The inhomogeneous distribution of Sb reduces the catalytic activity, and the base metal gets exposed to the electrolytic bath solution leading to its passivation. The dopant not only influences the morphology of the oxide coating, but also controls the mechanism of oxidation of organics on the anode surface. Hence, it is important to choose the right dopant and monitor its content very carefully during the fabrication of an electrode.
Gd (2 mol %)-doped SnO2-Sb possesses low oxygen vacancies in the crystal lattice of SnO2 than undoped SnO2-Sb (Feng et al. 2008). Lower oxygen vacancies enhance the production of hydroxyl radicals on the surface of the electrode and hence the oxidizing ability. Gd covered the electrode surface with an increase in its content and reduced the availability of Sb (Feng et al. 2008). It is worth noting that not only the dopant, but also its content substantially influences the performance of an electrode.
Other dopants like La and Ru are also advantageous in modifying the characteristics of an electrode. On doping SnO2-Sb with La and Ru, distortion effects led to the hindrance in the crystal growth and the crystallite size was reduced (Xu et al. 2012). The smaller crystallite size not only increases the surface area, but also increases the number of active sites on the surface of the anode. XPS measurements of this electrode revealed two different oxygen 1s peaks, one with lower binding energy (BE) component of 530.08–530.36 eV (assigned to lattice oxygen Olat) incorporated into SnO2 lattice and the other higher BE component of 531.86–531.89 eV (assigned to adsorbed oxygen Oads) is of the weakly bonded oxygen species. The adsorbed oxygen is very reactive towards the oxidation of organics. The Oads content on the surface of Ti/ SnO2-Sb-La was found to be the highest (41.78 %), followed by Ti/SnO2-Sb-Ru (39.42 %) and Ti/SnO2-Sb (29.13 %) electrodes. Evidently, the addition of La and Ru as dopants substantially enhanced the performance of the Ti/SnO2-Sb electrode in terms of COD removal and current efficiency (see Table 3).
Zhuo et al. (2011) reported that the service of Ti/SnO2-Sb electrode doubled on doping with Bi. The degradation of perfluorooctanoic acid (PFOA) was successfully achieved (~90 %) on both Ti/SnO2-Sb and Ti/SnO2-Sb-Bi. Liu et al. (2013) have recently shown that the OER potential for the carbon nanotube (CNTs) coated with SnO2 nanoparticles is higher than that of bulk SnO2. A comparison was made between SnO2 (TO-CNT), SnO2-Sb (ATO-CNT), and SnO2-Bi nanoparticle-coated CNT (BTO-CNT) for the electrochemical filtration of oxalate. SnO2-Bi/CNT showed 1.5 to 3.5 times greater current efficiency and 4 to 5 times lesser energy consumption as compared to bare CNT for the electrooxidative filtration of ethanol, methanol, formaldehyde, and formate. Also, BTO-CNT showed higher OER potential and TOC removal as compared to ATO-CNT, suggesting that Bi can be an alternative for Sb. However, Lin et al. (2013a) reported that the Ti/SnO2-Sb-Ce electrode yielded lower perfluorocarboxylic acid (PFCA) (perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA)) removal and observed secondary products of Sb due to the dissolution.
Modifications in the composition of SnO2 electrode fabrication and investigation of their performance in the wastewater treatment are summarized in Table 3. The conditions used in the electrochemical wastewater treatment process, maximum COD/TOC removal achieved, and percent current efficiency have been summarized in this table. As mentioned earlier, the extent of degradation of an organic molecule is dependent on its chemical nature, structure, and stability. The data in Table 3 is helpful in selecting a particular electrode for the degradation of different organic pollutant and vice versa.
PbO2
Generally, there are two kinds of PbO2. Orthorhombic α-PbO2 (brown color) and tetragonal β-PbO2 (black color), where β-PbO2 is a disordered close packed structure, possessing comparatively higher conductivity and higher overpotential for oxygen evolution are highly preferred (Abaci et al. 2005; Aquino et al. 2010; Gaber et al. 2012; Li et al. 2011; Yang et al. 2009; Zhoua and He 2008). β-PbO2 is a non-active anode, highly conducting, chemically inert, cheaper than noble metals, and possesses high oxygen evolution overpotential and high catalytic activity for the production of oxidants. Direct oxidation of organics on the surface of PbO2 anode is allowed (Fenga and Li 2003; Liu and Liu 2008; Yang et al. 2009). Thermal decomposition, electrodeposition (both in acidic and alkali media), and electrooxidation (anodization) are the techniques commonly employed in the fabrication of PbO2 electrodes (Fenga and Li 2003; Liu and Liu 2008; Martínez-Huitle et al. 2008; Niu et al. 2012; Panizza and Cerisola 2008; Panizza and Cerisola 2009; Sala and Gutiérrez-Bouzán 2012; Soloman et al. 2009; Yang et al. 2009). Liu et al. (2009) have been able to fabricate both kinds of PbO2 coat on Ti substrate. Pavlov (1992) carried out detailed work on the PbO2 electrodes and proposed a complex mechanism of formation of hydroxyl radicals on the surface of lead dioxide anodes. A gel-crystal system was suggested which follows the equilibrium shown below (Liu et al. 2009; Pavlov 1992):
Precisely, according to Pavlov, in the gel zone, the hydrated PbO2 forms linear polymer chains which allow the electron hopping from one Pb4+ ion to the other along the chain and from one polymer chain to the other, which accounts for its conductivity. The crystal zone is made up of PbO2 and allows electrical conductivity. There exists certain number of active centers on the surface of the PbO2 crystal layer. The hydroxide ions in the solution give out the electrons to these active centers which lead to the formation of hydroxyl radicals and create a weak interactive bond with the active centers. These hydroxyl radicals are utilized in the oxidation of organics (Liu et al. 2009).
PbO2 as itself has been used in the electrochemical treatment of wastewater and also in its doped form. On adding suitable dopant, PbO2 exhibits exceptional electrochemical properties. Ni, Bi, Co, Er, and Ce are the most commonly used dopants (Yang et al. 2009; Liu and Liu 2008; Liu et al. 2009; Niu et al. 2012; Wang et al. 2010b). Niobium-doped PbO2 has showed excellent hydrophilicity and conductivity due to the improvement in its microcrystal structure (Yang et al. 2009). It has also been reported that doping roughened the thin film surface and increased the effective surface area for the generation of hydroxyl radicals (Yang et al. 2009). The effect of bismuth and cobalt as dopants in the fabrication of PbO2 by electrodeposition was studied by Liu et al. (2009) and Liu and Liu (2008). This electrode was used in the degradation of nitrophenols. The addition of bismuth into the PbO2 matrix remarkably decreased the crystallite size of the deposit and improved its electrocatalytic activity. Out of Ti/β-PbO2, Ti/Bi-PbO2, Ti/Co-PbO2, Ti/Bi-Co-PbO2 electrodes, the morphology of Ti/Bi-PbO2 was found to be compact and porous with small crystallite size. This was attributed to the variations caused by the formation of Bi2O5 in the nucleation and crystal growth of the film (Liu and Liu 2008; Liu et al. 2009). In addition, this anode showed the highest oxygen evolution overpotential of 1.75 V (vs. SCE) and stability. Ti/Bi-PbO2 and Ti/Bi-Co-PbO2 favored the generation of hydrogen peroxide and hypochlorite ion. In contrast, no effect on the PbO2 crystallite size was found by doping cobalt. Ti/Bi-PbO2 showed the highest electrocatalytic activity for the generation of •OH. Obviously, the COD removal was maximum in the case of Ti/Bi-PbO2. Though Ti/Bi-Co-PbO2 exhibited smallest removal rates, its current efficiency was higher than Ti/β-PbO2 and Ti/Co-PbO2. Thus, Bi doping into the PbO2 matrix dramatically improves its characteristics, but Co doping does not make any such differences.
Similar influence of doping was observed when Er was used as dopant. The Er doping into F-PbO2 improved the electrocatalytic activity and produced compact coating with decreased crystallite size. Wang et al. (2010b) prepared erbium-doped chitosan-F-PbO2 electrode and compared it with the electrode without Er doping and without chitosan for the degradation of 2,4-dichlorophenol (2,4-DCP). Maximum 2,4-DCP removal was achieved on the electrode obtained from 10 mM Er electroplating solution. The order of 2,4-DCP removal on different electrodes was found to be Ti/ SnO2-Sb2O3/Er-chitosan-F-PbO2 > Ti/SnO2-Sb2O3/Er-F-PbO2 > Ti/SnO2-Sb2O3/F-PbO2.
PbO2 was electrodeposited on Ti/SnO2-Sb(0.08) (8 mol % of Sb) interlayer using different concentrations of NaF in the electrodeposition bath solution (Mao et al. 2008). The NaF concentration not only affected the stability of PbO2, but also the morphology of the PbO2 deposit. Without the addition of NaF, the PbO2 coat obtained consisted irregular crystallite size and shape with dents of varying sizes on the surface. As the concentration of NaF increased, a more compact coat of PbO2 was obtained. Only 55 % color removal of the substrate AO7 was achieved on PbO2-F (0.75) electrode, whereas PbO2-F (0) showed 94 % removal for the same time period. This was attributed to more compact and uniform deposition which resulted with the inclusion of NaF. The higher color removal in case of PbO2-F (0.75) electrode is due to the rough surface area which facilitated the adsorption of dye. With 0.1 g L−1 of NaF (PbO2-F (0.1)), the PbO2 coat obtained was most stable to anodic dissolution in 1 M HOCl4 (Mao et al. 2008). β-PbO2 coated on Ti with SnO2-Sb2O3-Nb2O5 interlayer showed higher electrochemical activity than Ti/RuO2 and Ti/Sb-Sn-RuO2 anodes for the degradation of phenol. The interlayer enhanced the surface area, electrocatalytic activity, conductivity, and hence the efficiency. Evidently, the removal efficiency increased from 78.6 % (Na2SO4) to 97.2 % on adding 21.3 g L−1 of NaCl.
Niu et al. (2012) reported that the toxic PFCAs like perfluorobutanoic acid (PFBA), PFPA, PFHxA, PFHpA, and PFOA, which are recently driving attention as serious contaminants in various environmental matrices, can be successfully mineralized by electrochemical method using Ce-doped porous nanocrystalline PbO2 film electrode (Ti/SnO2-Sb/PbO2-Ce). The same electrode provided excellent mineralization of PFNA, PFDA, and sulfamethoxazole as reported by Lin et al. (2013a, b) under mild conditions.
The dissolution of PbO2 in strong basic conditions and on specific anodic polarization limits its usage in wastewater treatment (Carvalho et al. 2011; Niu et al. 2012). However, by adopting suitable fabrication technique, the wear resistance of PbO2 can be conditioned (Carvalho et al. 2011) and possible release of Pb2+ in acidic conditions can be avoided by the application of suitable anodic current (Niu et al. 2012). Unlike SnO2, passivation of surface due to the formation of hydroxides is avoided by the continuous production of hydroxyl radicals on the surface of PbO2 and offers high charge transfer. Table 4 summarizes the comparison between the various modified PbO2 anodes and conditions used in the electrochemical wastewater treatment process.
Ruthenium oxide and iridium oxide
RuO2- and IrO2-based anodes are active anodes, known for their electrocatalytic activity for oxygen and chlorine evolution. IrO2 is one of the cheapest dimensionally stable anodes and shows low chlorine and oxygen evolution overpotential used in the generation of active chlorine species (Zaviska et al. 2009). Both IrO2- and RuO2-coated Ti anodes undergo dissolution in basic medium on anodization (Li et al. 2009). Ti/RuO2 undergoes corrosion during electrolysis on prolonged usage (Papastefanakis et al. 2010). However, the service life of RuO2 enhances on doping with Ir (Chandler et al. 1997; Chen et al. 2001, 2002; Duby 1993; Nikolić et al. 2012). The electrochemical properties of iridium (Ti0.6Ir0.4O2/Ti) and ruthenium (Ti0.6Ru0.4O2/Ti) oxide anodes, prepared by sol–gel method, were investigated by Panić et al. (2003a, b, 2007, 2010). They found that the iridium oxide shows enhanced catalytic activity for both oxygen and chlorine evolution and stability against corrosion as compared to ruthenium oxide (Panić et al. 2003a, b, 2007, 2010). These electrodes provide only small range of operating potential within hydrogen and oxygen evolution reactions. It is difficult to realize the direct oxidation of variety of organics on these anodes within this potential range. Hence, practical relevance of these anodes in the wastewater treatment on large scale is unacceptable. However, few research teams have successfully degraded the organic pollutants using these anodes.
The low chlorine evolution overpotential on these anodes is advantageous in the production of active chlorine species in wastewater containing chloride ions. The addition of NaCl as supporting electrolyte improves the removal efficiency, indicating that electrochemical treatment using Ti/IrO2 and Ti/RuO2 involves both direct and indirect oxidation (Chatzisymeon et al. 2009; Li et al. 2009). Enhancement in the COD removal efficiency was observed due to oxidation mediated by the electrochemically generated oxidants in the presence of chloride ions using Ti/RuO2 anode in olive mill wastewater treatment (Papastefanakis et al. 2010). The degradation process involved the formation of intermediates followed by further oxidation to CO2 and water.
Due to high electrocatalytic activity and stability, IrO2-Ta2O5 anode finds wide industrial applications (Scialdone et al. 2009a). IrO2-Ta2O5 anode is known for its selective oxidation. It oxidizes the organics into simpler carboxylic acids, particularly oxalic acid (Scialdone et al. 2009a). IrO2-Ta2O5 is the suitable electrode for the degradation of OA (Scialdone et al. 2009a; Ferro et al. 2010).
Three different DSA®s, Ti/(RuO2)0.70(Ta2O5)0.30, Ti/Ru0.30Ti0.70O2, and Ti/Ru0.30Sn0.70O2, were used in the degradation of RB-4 and RO-16 (da Silva et al. 2011). The Ti/Ru0.30Ti0.70O2 showed the highest current efficiency among these electrodes. Indirect electrochemical oxidation by the active chlorine species generated in presence of NaCl enhanced the COD removal on this electrode. Ti/Ru0.30Ti0.70O2 anode showed higher OER potential as compared to the other two. The same electrode was used in the degradation of Reactive Blue 81 and Reactive Red 2 by Kusmierek et al. (2011). Complete mineralization was achieved when the electrochemical degradation was coupled with the UV irradiation. These results indicate that TiO2-RuO2 mixed metal oxide solid solution is effective in the generation of active chlorine species. As these dyes can be successfully mineralized by indirect electrochemical oxidation, Ti/Ru0.30Ti0.70O2 anode is suitable for the treatment of wastewater containing these dyes.
Chu et al. (2010) prepared Ti/RuO2/IrO2/TiO2 with two compositions: one electrode (#1) with 0.20, 0.85, and 0.40 mg cm−2 of Ru, Ir and Ti, respectively, whereas the other electrode (#2) composed of 1.10, 1.20, and 0.55 mg cm−2 of Ru, Ir, and Ti, respectively. The higher oxide loading in case of #2 led to relatively rough surface of the coating. Direct oxidation of 2,4-DCP was observed on this electrode (Chu et al. 2010). Pt doping increases the service life of IrO2 and RuO2 anodes and the inability of Pt to produce hydroxyl ions is compensated by the ruthenium and/or iridium oxides (Li et al. 2009).
Table 5 compares different modified RuO2 and IrO2 anodes. The conditions used in the electrochemical wastewater treatment process, COD and TOC removal, and current efficiency displayed are useful in comparing the affinity of electrodes for different pollutant molecules.
Comparison between main metal oxide anodes
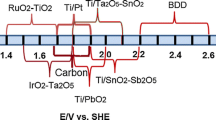
Table 6 reveals that the potential corresponding to the commencement of oxygen evolution reaction on the anode is related to OER overpotential and adsorption enthalpy of hydroxyl radicals (Kapałka et al. 2008). The ability of the anode to oxidize the organics increases with an increase in OER overpotential. Evidently, IrO2 and RuO2 are active anodes with low OER overpotential and are best suited for the production of chlorine and oxygen in chlor-alkali industries and electroorganic synthesis. They provide only partial decontamination with high energy consumption. The OER is highest for SnO2 anode, and hence, it possesses high electrocatalytic activity for the production of hydroxyl radicals. Therefore, complete incineration of organics is viable. Similarly, OER for PbO2 is also considerable and it is only slightly less than that of SnO2. The only problem encountered in the implementation of these anodes for the wasterwater treatment on large scale is their dissolution on electrolysis in severe wastewater matrix. Studies on appropriate modifications in the fabrication technique to enhance their stability are of much importance.
BDD anode recognized as the highly efficient anode for the oxidative degradation of organics in the past few years has acquired a lot of attention. Hydroxyl radical adsorption is very weak on the surface of BDD. The potential window between OER (2.2–2.6 V) and hydrogen evolution reaction is wide (>3 V) (Brillas et al. 2009; Koparal et al. 2007; Martínez-Huitle and Ferro 2006; Panizza and Cerisola 2008). However, BDD fabrication is very expensive as compared to metal oxide anodes. If appropriate modifications of metal oxide anodes to meet the characteristics of BDD and operating conditions are optimized to stretch the performance to the maximum, it only takes a mere part of the cost to achieve the same effectiveness as on BDD.
Conclusion
The electrochemical oxidation involving metal oxide electrodes for the abatement of organics in wastewater is an effective tool to keep up the aesthetic and environmental health. Nature of the electrode, pollutant, and operating conditions should coordinate suitably to successfully accomplish the goal of complete elimination of organics. The physical and chemical properties of the electrodes can be tuned by adopting appropriate fabrication technique to serve their purpose.
The structure, size, alignment, and morphology of the coated oxide thin film command the mechanism followed by the electrode. Electrodes with smooth surface interact very weakly with the hydroxyl radicals, and hence, these radicals are available for the oxidation of the organics, whereas on the electrodes with small crystallite size, large and rough surface areas provide sites for the hydroxyl radicals and organic molecules to adsorb strongly. The availability of hydroxyl radicals for the oxidation of organics diminishes on strong adsorption. Furthermore, with an increase in active centers on the surface of the oxide electrode and applied current density, the rate of formation of hydroxyl radicals also increases. Hence, metal oxide electrodes with smooth surface, small crystallite size, and high surface area are more effective. These prerequisite properties can be furnished with the help of suitable alterations made during the fabrication of the electrode. Selection of proper fabrication method, doping agent, interlayer insertion, and composition can bring out the best results.
Indirect oxidation of organics by the electrochemically generated oxidants is also a best method. Chloride is the most favorite electrolyte and ClO4 −, NO3 −, PO4 2−, SO4 2−, CO3 2−, and Br− are also effective under appropriate optimized operating conditions. Inclusion of chloride ions substantially enhanced the efficiency in color, COD, and TOC removal in many cases due to the formation of HOCl, ClO−, and Cl2. However, the risk of formation of AOX should be considered. Sulfate radicals, peroxydisulfate, peroxydicarbonate, and peroxydiphospates are also strong oxidants and can be effective under suitable conditions.
Among the three main metal oxide anodes discussed in this review, IrO2 and RuO2 show lowest OER potential. The oxygen evolution reaction on these electrodes competes with the oxidation reaction of organics. The complete removal of organics using these electrodes is possible only by indirect electrochemical oxidation. However, these electrodes are best suited for Cl2 generation and electroorganic synthesis. Both SnO2 and PbO2 exhibit very good electrochemical activity for the oxidation of organics and electrocatalytic activity for the production of hydroxyl radicals. The service life of PbO2 is greater than that of SnO2, and the OER for SnO2 is higher than that of PbO2. Metal dissolution is a serious concern in electrochemical treatment process. SnO2-Sb, PbO2, IrO2, and RuO2 electrodes undergo dissolution in basic medium and high anodic potentials. However, modified SnO2 and PbO2 electrodes show high stability, electrochemical, and electrocatalytic properties than modified IrO2 or RuO2 electrodes. The performance of SnO2 and PbO2 is comparable with the BDD electrode which is recognized as a highly efficient anode for the oxidative degradation of organics. This makes modified SnO2 and PbO2 electrodes as suitable candidates for the electrochemical wastewater treatment on large scale.
Abbreviations
- AO7:
-
Azo dye acid orange 7
- AOX:
-
Adsorbable organohalogens
- ALD:
-
Atomic layer deposition
- BDD:
-
Boron-doped diamond
- COD:
-
Chemical oxygen demand
- DCP:
-
Dichlorophenol
- DSA:
-
Dimensionally stable anode
- OA:
-
Oxalic acid
- OER:
-
Oxygen evolution reaction
- PFBA:
-
Perfluorobutanoic acid
- PFCA:
-
Perfluorocarboxylic acid
- PFDA:
-
Perfluorodecanoic acid
- PFHpA:
-
Perfluoroheptanoic acid
- PFHxA:
-
Perfluorohexanoic acid
- PFPA:
-
Perfluoropentanoic acid
- PFNA:
-
Perfluorononanoic acid
- PFOA:
-
Perfluorooctanoic acid
- RB-4:
-
Reactive Blue 4
- RO-16:
-
Reactive Orange 16
- SCE:
-
Standard calomel electrode
- SEM:
-
Scanning electron microscopy
- TOC:
-
Total organic content
- XPS:
-
X-ray photoelectron spectroscopy
References
Abaci S, Tamer U, Pekmez K, Yildiz A (2005) Performance of different crystal structures of PbO2 on electrochemical degradation of phenol in aqueous solution. Appl Surf Sci 240:112–119
Amjoud M’B, Maury F, Soukane S, Duverneuil P (1998) Making of specific electrodes by CVD. Surf Coat Technol 100–101:169–172
Andrade LS, Tasso TT, da Silva DL, Rocha-Filho RC, Bocchi N, Biaggio SR (2009) On the performances of lead dioxide and boron-doped diamond electrodes in the anodic oxidation of simulated wastewater containing the Reactive Orange 16 dye. Electrochim Acta 54:2024–2030
Aquino JM, Rocha-Filho RC, Bocchi N, Biaggio SR (2010) Electrochemical degradation of the Acid Blue 62 dye on a b-PbO2 anode assessed by the response surface methodology. J Appl Electrochem 40:1751–1757
Attia SM, Wang J, Wu G, Shen J, Ma J (2002) Review on sol–gel derived coatings: process, techniques and optical applications. J Mater Sci Technol 18(3):211–214
Beer HB (1980) The invention and industrial development of metal. Reviews and news. J Electrochem Soc 127(8):303C–307C
Brillas E, Sires I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton's reaction chemistry. Chem Rev 109:6570–6631
Brinker CJ, Frye GC, Hurd AJ, Ashley CS (1991) Fundamentals of sol–gel dip coating. Thin Solid Films 201:97–108
Brungs A, Haddadi-Asl V, Skyllas-Kazacos M (1996) Preparation and evaluation of electrocatalytic oxide coatings on conductive carbon-polymer composite substrates for use as dimensionally stable anode. J Appl Electrochem 26:1117–1123
Carvalho DA, Rocha JHB, Fernandes NS, da Silva DR, Martínez-Huitle CA (2011) Application of electrochemical oxidation as alternative for removing methyl green dye from aqueous solutions. Latin Am Appl Res 41:127–133
Chandler GK, Genders JD, Pletcher D (1997) Electrodes based on noble metals—essential components for electrochemical technology. Platinum Met Rev 41(2):54–63
Chatzisymeon E, Dimou A, Mantzavinos D, Katsaounis A (2009) Electrochemical oxidation of model compounds and olive mill wastewater over DSA® electrodes: 1. The case of Ti/IrO2 anode. J Hazard Mater 167:268–274
Chen X, Chen G, Yue PL (2001) Stable Ti/IrOx-Sb2O5-SnO2 anode for O2 evolution with low Ir content. J Phys Chem B 105:4623–4628
Chen G, Chen X, Yue PL (2002) Electrochemical behavior of novel Ti/IrOx-Sb2O5-SnO2 anodes. J Phys Che B 106:4364–4369
Cheng-chun J, Jia-fa Z (2007) Progress and prospect in electro-Fenton process for wastewater treatment. J Zhejiang Univ Sci A 8(7):1118–1125
Choi H, Stathatos E, Dionysiou DD (2007) Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 202:199–206
Chu YY, Wang WJ, Wang M (2010) Anodic oxidation process for the degradation of 2,4-dichlorophenol in aqueous solution and the enhancement of biodegradability. J Hazard Mater 180:247–252
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for wastewater treatment. Electrochem Acta 39:1857–1862
Comninellis Ch, Chen G (2010) Electrochemistry for the environment. Springer, Berlin. doi:10.1007/978-0-387-68318-8
Comninellis C, Vercesi GP (1991) Characterization of DSA®-type oxygen evolving electrodes: choice of a coating. J Appl Electrochem 21:335–345
Correa-Lozano B, Comninellis C, de Battisti A (1996a) Preparation of SnO2–Sb2O5 films by the spray pyrolysis technique. J Appl Electrochem 26:83–89
Correa-Lozano B, Comninellis C, de Battisti A (1996b) Electrochemical properties of Ti/SnO2-Sb2O5 electrodes prepared by the spray pyrolysis technique. J Appl Electrochem 26(7):683–688
Correa-Lozano B, Comninellis C, De Battisti A (1997) Service life of Ti/SnO2-Sb2O5 anodes. J Appl Electrochem 27:970–974
Costa CR, Montilla F, Morallón E, Olivi P (2010) Electrochemical oxidation of synthetic tannery wastewater in chloride-free aqueous media. J Hazard Mater 180:429–435
da Silva RG, Neto SA, De Andrade AR (2011) Electrochemical degradation of reactive dyes at different DSA® compositions. J Braz Chem Soc 22(1):126–133
del Río AI, Molina J, Bonastre J, Cases F (2009) Study of the electrochemical oxidation and reduction of C.I. Reactive Orange 4 in sodium sulfate alkaline solutions. J Hazard Mater 172:187–195
Devilliers D, Dinh Thi MT, Mahé E, Le Xuan Q (2003) Cr(III) oxidation with lead dioxide-based anodes. Electrochim Acta 48:4301–4309
Duby P (1993) The history of progress in dimensionally stable anodes. JOM 45(3):41–43
Duverneuil P, Maury F, Pebere N, Senocq F, Vergnes H (2002) Chemical vapor deposition of SnO2 coatings on Ti plates for the preparation of electrocatalytic anodes. Surf Coat Technol 151 –152:9–13
Fachinotti E, Guerrini E, Tavares AC, Trasatti S (2007) Electrocatalysis of H2 evolution by thermally prepared ruthenium oxide effect of precursors: nitrate vs. chloride. J Electroanal Chem 600:103–112
Feng Y, Cui Y, Logan B, Liu Z (2008) Performance of Gd-doped Ti-based Sb-SnO2 anodes for electrochemical destruction of phenol. Chemosphere 70:1629–1636
Fenga YJ, Li XY (2003) Electro-catalytic oxidation of phenol on several metal-oxide electrodes in aqueous solution. Water Res 37:2399–2407
Ferro S, Martínez-Huitle CA, De Battist A (2010) Electroxidation of oxalic acid at different electrode materials. J Appl Electrochem 40:1779–1787
Fierro S, Comninellis C (2010) Kinetic study of formic acid oxidation on Ti/IrO2 electrodes prepared using the spin coating deposition technique. Electrochim Acta 55:7067–7073
Fornazari ALT, Malpass GRP, Miwa DW, Motheo AJ (2012) Application of electrochemical degradation of wastewater composed of mixtures of phenol–formaldehyde. Water Air Soil Pollut 223:4895–4904
Gaber M, Ghalwa NA, Khedr AM, Salem MF (2012) Electrochemical degradation of reactive yellow160 dye in real wastewater using C/PbO2-, Pb + Sn/PbO2 + SnO2-, and Pb/PbO2 modified electrodes. J Chem 2013:1–9
Guinea E, Brillas E, Centellas F, Cañizares P, Rodrigo MA, Sáez C (2009) Oxidation of enrofloxacin with conductive-diamond electrochemical oxidation, ozonation and Fenton oxidation: a comparison. Water Res 43:2131–2138
He D, Mho S (2004) Electrocatalytic reactions of phenolic compounds at ferric ion co-doped SnO2:Sb5+ electrodes [J]. J Electroana Chem 568:19–27
Hou Y, Qu J, Zhao X, Lei P, Wan D, Huang CP (2009) Electro-photocatalytic degradation of acid orange II using a novel TiO2/ACF photoanode. Sci Total Environ 407:2431–2439
Jiancheng L, Jie Y, Weishan L, Qiming H, Hongkang X (2012) Electrochemical degradation of Reactive Brilliant Red K-2BP on Ti/RuTiIrSnMn oxide anode in a batch cell. J Electrochem Sci Eng 2:171–183. doi:10.5599/jese.2012.0022
Jiang-tao K, Shao-yuan S, Xiu-ping Z, Jin-ren N (2007) Effect of Sb dopant amount on the structure and electrocatalytic capability of Ti/Sb-SnO2 electrodes in the oxidation of 4-chlorophenol. J Environ Sci 19:1380–1386
Jüttner K, Galla U, Schmieder H (2000) Electrochemical approaches to environmental problems in the process industry. Electrochim Acta 45:2575–2594
Kapałka A, Fóti G, Comninellis C (2008) Kinetic modelling of the electrochemical mineralization of organic pollutants for wastewater treatment. J Appl Electrochem 38:7–16
Klamklang S, Vergnes H, Pruksathorn K, Damronglerd S (2012) Electrochemical incineration of organic pollutants for wastewater treatment: Past, present and prospect. In: Puzyn T, Mostrag-Szlichtyng A (eds) Organic pollutants ten years after the Stockholm convention – Environmental and Analytical update. doi:10.5772/1381. Available from: http://www.intechopen.com/books/organic-pollutants-ten-years-after-the-stockholm-convention-environmental-and-analytical-update/electrochemical-incineration-of-organic-pollutants-for-wastewater-treatment-past-present-and-prospec. Accessed Aug 2013
Kong H, Lu H, Zhang W, Lin H, Huang W (2012) Performance characterization of Ti substrate lead dioxide electrode with different solid solution interlayers. J Mater Sci 47:6709–6715
Koparal AS, Yavuz Y, Gürel C, Öğütveren UB (2007) Electrochemical degradation and toxicity reduction of C.I. Basic Red 29 solution and textile wastewater by using diamond anode. J Hazard Mater 145:100–108
Kusmierek E, Chrzescijanska E, Szadkowska-Nicze M, Kaluzna-Czaplinska J (2011) Electrochemical discolouration and degradation of reactive dichlorotriazine dyes: reaction pathways. J Appl Electrochem 41:51–62
Li M, Feng C, Hu W, Zhang Z, Sugiura N (2009) Electrochemical degradation of phenol using electrodes of Ti/RuO2–Pt and Ti/IrO2–Pt. J Hazard Mater 162:455–462
Li X, Pletcher D, Walsh FC (2011) Critical review: electrodeposited lead dioxide coatings. Chem Soc Rev 40:3879–3894
Lin H, Niu J, Xu J, Huang H, Li D, Yue Z, Feng C (2013a) Highly efficient and mild electrochemical mineralization of long-chain perfluorocarboxylic acids (C9 C10) by Ti/SnO2-Sb-Ce, Ti/SnO2-Sb/Ce-PbO2 and Ti/BDD electrodes. Environ Sci Technol. doi:10.1021/es4034414
Lin H, Niu J, Xu J, Li Y, Pan Y (2013b) Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: kinetics, reaction pathways, and energy cost evolution. Electrochim Acta 97:167–174
Lipp L, Pletcher D (1997) The preparation and characterization of tin dioxide coated titanium electrodes. Electrochim Acta 42(7):1091–1099
Liu Y, Liu H (2008) Comparative studies on the electrocatalytic properties of modified PbO2 anodes. Electrochim Acta 53:5077–5083
Liu Y, Liu H, Ma J, Wang X (2009) Comparison of degradation mechanism of electrochemical oxidation of di- and tri-nitrophenols on Bi-doped lead dioxide electrode: effect of the molecular structure. Appl Catal B 91:284–299
Liu H, Vajpayee A, Vecitis CD (2013) Bismuth-doped tin oxide-coated carbon nanotube network: improved anode stability and efficiency for flow-through organic electrooxidation. Appl Mater Interfaces. doi:10.1021/am402621v
Lu PJ, Chien CW, Chen TS, Chern JM (2010) Azo dye degradation kinetics in TiO2 film-coated photoreactor. Chem Eng J 163:28–34
Malpass GRP, Miwa DW, Santos RL, Vieira EM, Motheo AJ (2012) Unexpected toxicity decrease during photoelectrochemical degradation of atrazine with NaCl. Environ Chem Lett 10:177–182
Mao X, Tian F, Gan F, Lin A, Zhang X (2008) Comparison of the performances of Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Nb/BDD anodes on electrochemical degradation of azo dye. Russ J Electrochem 44:802–811
Martínez-Huitle CA, Andrade LS (2011) Electrocatalysis in wastewater treatment: recent mechanism advances. Quim Nova 34(5):850–858
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87:105–145
Martínez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35:1324–1340
Martínez-Huitle CA, de Battisti A, Ferro S, Reyna S, Cerro-López M, Quiro MA (2008) Removal of the pesticide methamidophos from aqueous solutions by electrooxidation using Pb/PbO2, Ti/SnO2, and Si/BDD electrodes. Environ Sci Technol 42:6929–6935
Marugán J, López-Muñoz MJ, van Grieken R, Aguado J (2007) Photocatalytic decolorization and mineralization of dyes with nanocrystalline TiO2/SiO2 materials. Ind Eng Chem Res 46:7605–7610
Nava JL, Quiroz MA, Martínez-Huitle CA (2008) Electrochemical treatment of synthetic wastewaters containing alphazurine A dye: role of electrode material in the colour and COD removal. J Mex Chem Soc 52(4):249–255
Nikolić BŽ, Panić VV, Dekanski AB (2012) Intrinsic potential-dependent performances of a sol–gel prepared electrocatalytic IrO2–TiO2 coating of dimensionally stable anodes. Ectrocatalysis 3:360–368
Niu J, Lin H, Xu J, Wu H, Li Y (2012) Electrochemical mineralization of perfluorocarboxylic acids (PFCAs) by Ce-doped modified porous nanocrystalline PbO2 film electrode. Environ Sci Technol 46:10191–10198
Ozer N, DeSouza S, Lampert CM (1995) Optical and electrochemical properties of sol–gel spin coated CeO2-TiO2 films. SPIE 2531:143
Panakoulias T, Kalatzis P, Kalderis D, Katsaounis A (2010) Electrochemical degradation of Reactive Red 120 using DSA and BDD anodes. J Appl Electrochem 40:1759–1765
Panić VV, Nikolić BŽ (2007) Sol–gel prepared active ternary oxide coating on titanium in cathodic protection. J Serb Chem Soc 72(12):1393–1402
Panić V, Vidaković T, Gojkovic S, Dekanski A, Milonjić S, Nikolić B (2003a) The properties of carbon-supported hydrous ruthenium oxide obtained from RuOxHy sol. Electrochim Acta 48:3805–3813
Panić V, Dekanski A, Wang G, Fedoroff M, Milonjić S, Nikolić B (2003b) Morphology of RuO2–TiO2 coatings and TEM characterization of oxide sols used for their preparation. J Colloid Interface Sci 263:68–73
Panić VV, Dekanski AB, Mišković-Stanković VB, Milonjić SK, Nikolić BŽ (2010) Differences in the electrochemical behavior of ruthenium and iridium oxide in electrocatalytic coatings of activated titanium anodes prepared by the sol–gel procedure. J Serb Chem Soc 75(10):1413–1420
Panizza M, Cerisola G (2008) Electrochemical degradation of methyl red using BDD and PbO2 anodes. Ind Eng Chem Res 47:6816–6820
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Panizza M, Cerisola G (2010) Applicability of electrochemical methods to carwash wastewaters for reuse. Part 1: anodic oxidation with diamond and lead dioxide anodes. J Electroanal Chem 638:28–32
Panizza M, Bocca C, Cerisola G (2000) Electrochemical treatment of wastewater containing polyaromatic organic pollutants. Water Res 34(9):2601–2605
Papastefanakis N, Mantzavinos D, Katsaounis A (2010) DSA electrochemical treatment of olive mill wastewater on Ti/RuO2 anode. J Appl Electrochem 40:729–737
Pavlov D (1992) The lead-acid battery lead dioxide active mass: a gel-crystal system with proton and electron conductivity. J Electrochem Soc 139:3075–3080
Perednis D, Gauckler LJ (2005) Thin film deposition using spray pyrolysis. J Electroceram 14:103–111
Radjenovic J, Escher BI, Rabaey K (2011) Electrochemical degradation of the β-blocker metoprolol by Ti/Ru0.7Ir0.3O2 and Ti/SnO2-Sb electrodes. Water Res 45:3205–3214
Riihelä D, Ritala M, Matero R, Leskelä M (1996) Electronics, optics and opto-electronics, introducing atomic layer epitaxy for the deposition of optical thin films. Thin Solid Films 289:250–255
Rodgers JD, Jedral W, Bunce NJ (1999) Electrochemical oxidation of chlorinated phenols. Environ Sci Technol 33:1453–1457
Rosales E, Pazos M, Longo MA, Sanromán MA (2009) Influence of operational parameters on electro-Fenton degradation of organic pollutants from soil. J Enviro Sci Health Part A: Toxic/Hazard Subst Environ Eng 44:1104–1110
Sakalis A, Vanĕrková D, Holčapek M, Jandera P, Voulgaropoulos A (2007) Electrochemical treatment of a simple azodye and analysis of the degradation products using high performance liquid chromatography-diode array detection-tandem mass spectrometry. Chemosphere 67:1940–1948
Sala M, Gutiérrez-Bouzán MC (2012) Electrochemical techniques in textile processes and wastewater treatment. Int J Photoenergy 2012:1–12
Scialdone O (2009) Electrochemical oxidation of organic pollutants in water at metal oxide electrodes: a simple theoretical model including direct and indirect oxidation processes at the anodic surface. Electrochim Acta 54:6140–6147
Scialdone O, Randazzo S, Galia A, Filardo G (2009a) Electrochemical oxidation of organics at metal oxide electrodes: the incineration of oxalic acid at IrO2–Ta2O5 (DSA-O2) anode. Electrochim Acta 54:1210–1217
Scialdone O, Randazzo S, Galia A, Silvestri G (2009b) Electrochemical oxidation of organics in water: role of operative parameters in the absence and in the presence of NaCl. Water Res 43:2260–2272
Simond O, Schaller V, Comninellis C (1997a) Theoretical model for the anodic oxidation of organics on metal oxide electrodes. Electrochim Acta 42(13–14):2009–2012
Simond O, Schaller V, Comninellis C (1997b) Anodic oxidation of organics on Ti/IrO2 anodes using Nafion® as electrolyte. Electrochim Acta 42(13–14):2013–2018
Soloman PA, Basha CA, Velan M, Ramamurthi V, Koteeswaran K, Balasubramanian N (2009) Electrochemical degradation of remazol Black B dye effluent. Clean 37(11):8–900
Song S, Zhan L, He Z, Lina L, Tu J, Zhang Z, Chen J, Xu L (2010a) Mechanism of the anodic oxidation of 4-chloro-3-methyl phenol in aqueous solution using Ti/SnO2–Sb/PbO2 electrodes. J Hazard Mater 175:614–621
Song S, Fan J, He Z, Zhan L, Liu Z, Chen J, Xu X (2010b) Electrochemical degradation of azo dye C.I. Reactive Red 195 by anodic oxidation on Ti/SnO2–Sb/PbO2 electrodes. Electrochim Acta 55:3606–3613
Soni BD, Ruparelia JP (2012) Studies on effects of electrodes for decontamination of dyes from wastewater. J Environ Res Dev 6:973–979
Trasatti S (2000) Electrocatalysis: understanding the success of DSR®. Electrochim Acta 45:2377–2385
Vicent F, Morallón E, Quijada C, Vâzquez JL, Aldaz A, Cases F (1998) Characterization and stability of doped SnO2 anodes. J Appl Electrochem 28:607–612
Wang YH, Chan KY, Li XY (2006) Electrochemical degradation of 4-chlorophenol at nickel-antimony doped tin oxide electrode. Chemosphere 65:1087–1093
Wang YQ, Gu B, Xu WL (2009) Electro-catalytic degradation of phenol on several metal-oxide anodes. J Hazard Mater 162:1159–1164
Wang KS, Wei MC, Peng TH, Li HC, Chao SJ, Hsu TF, Lee HS, Chang SH (2010a) Treatment and toxicity evaluation of methylene blue using electrochemical oxidation, fly ash adsorption and combined electrochemical oxidation-fly ash adsorption. J Environ Manage 91:1778–1784
Wang Y, Shen Z, Li Y, Niu J (2010b) Electrochemical properties of the erbium–chitosan–fluorine-modified PbO2 electrode for the degradation of 2,4-dichlorophenol in aqueous solution. Chemosphere 79:987–996
Watts RJ, Wyeth MS, Finn DD, Teel AL (2008) Optimization of Ti/SnO2–Sb2O5 anode preparation for electrochemical oxidation of organic contaminants in water and wastewater. J Appl Electrochem 38:31–37
Xu H, Li AP, Qi Q, Jiang W, Sun YM (2012) Electrochemical degradation of phenol on the La and Ru doped Ti/SnO2-Sb electrodes. Korean J Chem Eng 29:1178–1186
Yang X, Zou R, Huo F, Cai D, Xiao D (2009) Preparation and characterization of Ti/SnO2–Sb2O3–Nb2O5/PbO2 thin film as electrode material for the degradation of phenol. J Hazard Mater 164:367–373
Yeo IH, Wen S, Mho SI (2010) Effect of interfacial oxides on the electrochemical activity of lead dioxide film electrodes on a Ti substrate. Ananl Sci 26:39–44
Yusta FJ, Hitchman ML, Shamlian SH (1997) CVD preparation and characterization of tin dioxide films for electrochemical applications. J Mater Chem 7(8):1421–1427
Zaviska F, Drogui P, Blais JF, Mercier G (2009) In situ active chlorine generation for the treatment of dye-containing effluents. J Appl Electrochem 39:2397–2408
Zhao WR, Xu XH, Shi HX, Wang DH (2003) Degradation mechanism of cationic red X-GRL by ozonation. Chin Chem Lett 14:1309–1312
Zhoua M, He J (2008) Degradation of cationic red X-GRL by electrochemical oxidation on modified PbO2 electrode. J Hazard Mater 153:357–363
Zhuo Q, Deng S, Yang B, Huang J, Yu G (2011) Efficient electrochemical oxidation of perfluorooctanoate using a Ti/SnO2-Sb-Bi anode. Environ Sci Technol 45:2973–2979
Acknowledgments
We thank the Department of Chemistry, Kuvempu University, Shankaraghatta for their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Subba Rao, A.N., Venkatarangaiah, V.T. Metal oxide-coated anodes in wastewater treatment. Environ Sci Pollut Res 21, 3197–3217 (2014). https://doi.org/10.1007/s11356-013-2313-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2313-6