Abstract
Photo-active metal oxides (PMOs) have outstanding physical and chemical properties which are ideal to disintegrate wastewater pollutants. Titanium oxide (TiO2) initially found popularity in wastewater treatment. TiO2 utilization on wastewater degradation was attributed particularly to its wider bandgap. Nonetheless, TiO2 retains antibacterial activity during the application and that renders it to rapid recombination of photogenerated electron–hole pairs. In a subsequent search of alternative PMO, zinc oxide (ZnO) was obtained, and it was found to have a wider bandgap equivalent to that of TiO2. However, ZnO suffers from photo-corrosion and poor response to visible light. This rigorously proved that an application of a single PMO leads to both inefficiency and ineffectiveness in wastewater treatment. This phenomenon necessitates the hybridization of photocatalysts and improvement of their surface properties.
The present chapter details organic pollutants which are found in wastewater and the methods which are used to remove them from the wastewater. Further discussions are made intensively on photocatalysis and advance oxidation methods. Furthermore, photocatalysts and their advancements are clearly stated and elaborated. Finally, surface-modified photoanodes and their applications using the photoelectrochemical technique have been thoroughly explained. From overall analyses, several deductions have been documented:
-
POMs on their singular existence are packed with pros and cons, and that makes wastewater treatment dynamic.
-
AOMs, particularly the photoelectrochemical technique, are worthwhile for the degradation of wastewater pollutants.
-
Factors that affect degradation processes on wastewater pollutants include light captivation properties, reduction and oxidation rates on the surface by the photogenerated electrons and holes and a recombination rate of such charges.
-
Surface modification of photoanodes is carried through nanostructured materials, the addition of metals particularly noble ones such as gold (Au), silver (Ag), platinum (Pt), and through the use of novel titanium alloys and cubic double-perovskite.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The rapid increase of organic pollutants (OPs) in wastewater is linked to various health hazards and environmental pollution. Major OPs found in wastewater are from azo dyes, and 15% of their annual production goes into the wastewater [1,2,3,4,5]. Hazardous minerals from organic pollutants such as sodium, potassium, calcium, chloride, and bromide have been reported to be recalcitrant during wastewater treatment, and their direct contact with living species leads to various chronic illnesses [6,7,8,9,10,11,12,13,14,15]. Hence, it is a matter of urgency to remove organic pollutants from the wastewater.
Photo-active materials (PMs), particularly in a form of metal oxides, have various significances [16, 17] which make them able to degrade wastewater pollutants. Some of the predominantly utilized PMs are ZnO, CU2O, MnO2, Ag2O/Ag3VO4/AgVO3, TiO2, Fe2O3, NiO, SrZrO3, BiOI/Ag3VO4, MoS2, ZnS and WS2 [18,19,20,21,22,23,24,25,26,27,28]. In recent applications, PMs are synthesized from nanostructured materials through rigorous steps. Typically, PMs are fabricated to form a conductive photoanode that directly oxidizes and degrade wastewater pollutants from the wastewater using electrochemistry techniques.
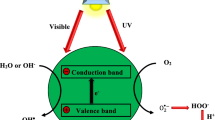
Presently, the photoelectrochemical technique (PT) is regarded as a superior electrochemistry method for the degradation of wastewater pollutants. The utilization of PT on degradation goes along with various photoanodes. Typically, PT is engaged in wastewater treatment through the aid of bias potential which is supplied from the potentiostat. Herein, photoanode is immobilized and hydroxyl species form from occurring reactions. Ultimately, electrons from the photoanode react with some oxygen, and that consequently develop oxygen reactive radicals (O2−) which react with protons to produce H2O−. Both O2− and H2O− are then able to degrade wastewater pollutants [29, 30].
In light of the wastewater pollutants’ nature and their dynamic processes, this chapter elucidates factors that affect degradation processes on wastewater pollutants. Three factors that generally affect degradation processes have been discussed. Most importantly, surface modifications of different photoanodes which make in-roads on wastewater treatment are detailed.
6.2 Wastewater and Organic Pollutants
6.2.1 Wastewater
Wastewater comprises organic pollutants from a wide range of applications: domestic, industrial, commercial, and agricultural activities. Most notably, wastewater consists of about 15% of annually produced organic dyes [31, 32] and some other pollutants. Figure 6.1 demonstrates the wastewater profile consists of different organic pollutants.
Different organic pollutants consisted in wastewater under treatment [32]
6.2.2 Organic Pollutants and Their Impact
Dyes are used mainly in the production of food, textiles, leather, fabrics, paper, cosmetic, electroplating, distillation, and pharmaceutical products [33,34,35,36,37,38,39,40]. With global industrialization, the uses of dyes increase at an alarming rate. The presence of pollutants in wastewater causes major problems on wastewater treatment and leads to environmental pollution and hazardous to living species [41,42,43,44]. In particular, hazardous minerals from organic pollutants such as sodium, potassium, calcium, chloride, and bromide are recalcitrant to wastewater treatment, and their direct contact with living species could lead to chronic diseases [45].
6.3 Photocatalysists and Their Applications
6.3.1 Photocatalysts
PMs are capable of captivating light under either direct sunlight or a solar application. Due to their nature, PMs are commonly known as photocatalysts. There are quite a several photocatalysts, and some of the heavily engaged photocatalysts include ZnO, CU2O, MnO2, Ag2O/Ag3VO4/AgVO3, TiO2, Fe2O3, NiO, SrZrO3, BiOI/Ag3VO4, MoS2, ZnS and WS2 [18,19,20,21,22,23,24,25,26,27,28]. Among other significant characteristics listed photocatalysts and the likes, photocatalysts generally possess bandgaps which enable them to possess a high level of photodegradation to remove pollutants from the wastewater.
6.3.2 Nanostructured Materials and Photoanodes
6.3.2.1 Nanostructured Materials
Nanotechnology has recently found vast application across the world. Among the most prominent applications, nanotechnology is used on medical equipment, car paintings, chronic illness treatment, etc. In wastewater treatment, nanotechnology is applied in a form of nanostructured materials. Nanostructured materials are commercially purchased as nanoparticles (powders less than 100 nm). From their purchases to laboratories, they are molded into conductive semiconductors which are popularly termed photoanodes.
6.3.2.2 Photoanodes
Photoanodes are fabricated from well-mixed nanostructured materials [37] in five major steps which are shown in Fig. 6.2. Firstly, nanostructured composite is taken to a hydraulic press, and it is subjected to higher temperatures. Secondly, the compressed pellet of about 1.3 cm diameters is obtained as a photoanode [38]. Thirdly, a copper wire is inserted and passed out through a glass tube with an opening at both ends and coiled onto the fabricated nanocomposite pellet. Fourthly, conduction between the Cu wire and the pellet is created with the use of conductive silver paste. Finally, epoxy resin is applied to the glass tube to cover up all the ends.
6.3.2.3 Coupling of Photoanode to an Electrochemical Cell for Wastewater Pollutants Degradation
Besides an electrolyte which simulates wastewater pollutant, an electrochemical cell is comprised of three conductive electrodes. The simulated electrolyte is mostly made up of potassium ferricyanide, ferrocyanide, and potassium chloride. The incompleteness of wastewater pollutant, sodium sulfate, sodium hydroxide, and hydrochloric acid are added as complementary solutions to make wastewater pollutant conductive [45]. The photoanodes is popularly known as the working electrode (WE) as it is the one degrading organic pollutants from the wastewater. Then, there is a reference electrode (RE), and it is specifically used to measure the potential difference produced from the electrochemical cell. Lastly, the counter electrode (CE) is used to count a current that is produced as the photoanode is applied to wastewater pollutant (electrolyte).
6.4 Photocatalysis and Advance Oxidation Measurements
Photocatalysis is a fundamental technique in both wastewater treatment and renewable energy systems. Photocatalysis is carried out both spontaneously and synthetically. Naturally, direct sunlight is exposed to the photoanode, and consequently, hydrogen and oxygen are produced, and they are used to degrade wastewater pollutants [46]. Synthetically, however, the light from solar light is directed to the PMs, and that produces electrical energy. Relative to sunlight utilization, this method is comprised of insufficient energy, difficult storage and transportation [46].
Although all photo-active materials ideal for wastewater degradation, their singular existence is packed with distinctive limitations and that makes their uses on wastewater treatment to be dynamic. For instance, TiO2 has a wider bandgap and that makes it provide inefficient degradation processes. On the other hand, ZnO suffers from photo-corrosion, has a poor response to visible light, and has higher recombination of electron–hole pairs. Photocatalysis has been a standard method for the degradation of wastewater. However, photocatalysis has been reported to inefficient due to an occurrence of higher recombination of electron–hole pairs and inferior solar light absorption. In light of limitations, singular photocatalysts have and photocatalysis as a technique, alternative methods which work on hybridization of photoanodes had to be sought out.
Advance oxidation measurements (AOMs) have so far been worthwhile on wastewater treatment. There are about three distinct AOMs for wastewater treatment: electrochemical, chemical, and photoelectrochemical technique. Nonetheless, the photoelectrochemical technique (PT) has been proven to be superior in the degradation of organic pollutants [47]. PT is engaged in wastewater treatment with the aid of bias potential which is supplied by the potentiostat. As the potential on a full set of electrochemical cell components discussed in Sect. 3.2.3, a nanocomposite from the working electrode becomes immobilized and that results in a formation of hydroxyl species. Furthermore, electrons from the photoanode react with some oxygen and that consequently develop (oxygen reactive radicals) which react with protons to produce H2O−. Both O2− and H2O− are then able to degrade wastewater pollutants [31].
6.5 Factors that Affect Degradation Processes on Wastewater Pollutants Using a Photoelectrochemical Technique
There are about three factors that affect degradation processes on wastewater pollutants, and they are light captivation properties, reduction and oxidation rates on the surface by the photogenerated species, and a recombination rate of such charges.
6.5.1 Light Captivation Properties
PMs absorb and transmit light differently, and they are normally characterized using UV measurements to dictate light absorption of a certain photo-active material within a specific wavelength range. As can be seen in Fig. 6.3, three photoanodes exhibit different curves on the absorption of light. Cautiously, photo-active materials have different significances and limitations. In the hybridization of photoanodes, improved light captivation is obtained. Significantly, faster degradation processes are also reached under the hybridization of photoanodes [48].
UV–Vis spectra absorbance for three photoanodes: EG, EG-MoO3, and MoO3 [48]
6.5.2 The Reduction and Oxidation Rates on the Surface by the Photo-generated Electrons and Holes
In applications of PET, a composite from the photoanode is oxidized (electrons are emitted from it), and then, the wastewater pollutant, in a form of liquid, is degraded. The faster the electrons emission from the photoanode, the higher the wastewater pollutant is being degraded (reduced). This is the most important factor which is particularly examined from the photoanode material [49].
6.5.3 Recombination Rate of Electron Charges
As mentioned earlier that an extreme recombination rate of electrons occurs particularly from TiO2, most surface modification has been carried on TiO2 [15, 24, 29, 39, 41, 42]. With such modifications, better degradation efficiencies have been reached.
6.6 Surface-Modified Photoanodes
Surface modification of photoanodes is mainly carried out in three ways. The first step is on morphological adjustments. Herein, nanostructured materials are added particularly in the form of noble metals such as Au, Ag, and Pt. Furthermore, novel titanium alloys and cubic double-perovskite are sometimes used. In the second step, synthetic techniques are varied on different novel materials. Finally, nanostructured materials in the forms of nanotubes, nanorods, nanowire, and nanoclusters are applied in a variety of parameters such as growth time, temperature, initial reactant concentration, acidity, and additives [61].
6.7 Morphological Adjustments
With the hybridization of photoanodes, there is a particular form of structure in terms of morphologies and phases. This phenomenon leads to a new characteristic of the fabricated photoanode. The surface characteristics on newly fabricated photoanode are normally verified with surface characterization techniques such as scanning electron microscopy (SEM) and X-ray diffraction (XRD).
6.8 Synthetic Technique on Novel Materials
PT being reckoned as the most efficient technique on wastewater treatment, its outstanding application has been corroborated by a considerable number of studies on improvement of limitations possessed by major photoanodes (TiO2 and ZnO). Firstly, Chakrabarti et al. [62] combined both TiO2 and ZnO to improve degradation efficiency on methylene blue (MB). An enhanced degradation efficiency was recorded to be 99.41% within 3 h of measurements. With the addition of novel material, Mirzaei et al. [63] established TiO2 nanotube arrays enfolded with g-C3N4 to degrade Phenol. Enhanced degradation efficiency of about 90% was obtained from the TiO2/g-C3N4 photoanode. Similarly, Ahmed et al. [64] invented A/RTiO2251 photoanode to degrade orange II dye, and 96% degradation efficiency was obtained. This, therefore, proves that the application of PT with an appropriately hybridized photoanode, on specific wastewater pollutant, provides improved degradation processes.
6.9 Variation of Synthetic Process Parameters
Synthetic parameters could positively or negatively affect degradation processes. Some of the most crucial synthetic parameters affecting degradation processes are listed below.
6.9.1 Temperature
Generally, nanostructured materials are affected by temperature treatment [65]. Synthetic processes which form photoanode composites have been reported to go with changes in phases and crystalline structures. This has been attributed to temperature, particularly higher temperatures.
6.9.2 pH Range
An impact of pH on degradation processes was initially profiled on TiO2 as the standard photoanode. From some analyses, a similar temperature range has been realized to negatively affect degradation processes. For instance, the pH range between 3 and 10 corresponds was found to produce higher degradation efficiency in one study. In attest from the other study, TiO2 has been reported to agglomerates and possesses reduced surface area under acidic conditions. Therefore, a particular effect on the surface area of the photoanode is due to the pH range. Pinpointing the effect of pH range on degradation processes, Baran et al. [66] lowered the pH from 8.0 to 4.5 during their degradation processes. Significantly, magnificent degradation efficiency was attained at the lower pH values. Moreover, from the other similar work [67], higher degradation efficiency was also attained from lower pH values. This, therefore, proves that acidic pH is more favorable on most photoanodes for effective degradation processes.
6.10 Summary
6.10.1 Wastewater Pollutants and Their Impacts
-
Wastewater pollutants come mainly from domestic, industrial, commercial, and agricultural activities.
-
Major wastewater pollutant is organic dyes.
-
Wastewater pollutants cause environmental pollution and chronic illnesses on people and animals.
6.10.2 Photocatalysis and Advanced Oxidation Methods
-
The photoelectrochemical technique is a state-of-the-art electrochemistry technique for the degradation of wastewater pollutants and the enhancement of renewable energy systems.
-
Photoelectrochemical technique engages an immobilization of photoanode electrode through an application of bias potential.
6.10.3 Factors that Affect Degradation Processes on Wastewater Pollutants Using a Photoelectrochemical Technique
There are mainly three factors that affect degradation processes on wastewater pollutants, and these are light captivation properties, reduction and oxidation rates on the surface by the photogenerated species, and a recombination rate of electron charges during electrochemical measurements.
6.10.4 Methods Modifying Surface Properties of Photoanodes and Their Applications
Surface modification of photoanodes is mainly carried out through morphological adjustments, synthetic technique on novel material, and variation of synthetic process’s parameters.
6.11 Conclusions
The present chapter has outlined organic pollutants which are found in wastewater and methods which are used to remove them from the wastewater. Further discussions have been made on photocatalysis and advance oxidation methods. Photocatalysts and their advancements have also been clearly stated and elaborated. Most importantly, surface-modified photoanodes and their applications using the photoelectrochemical technique have carefully been explained. Overall, the following conclusions are drawn up from the main findings:
-
Photo-active materials (POMs) in their singular existence are packed with advantages and limitations and that makes wastewater treatment to be dynamic.
-
Advance oxidation methods (AOMs), particularly photoelectrochemical technique, are worthwhile for the degradation of wastewater pollutants.
-
Factors that affect degradation processes on wastewater pollutants include light captivation properties, reduction and oxidation rates on the surface by the photogenerated electrons, and holes and a recombination rate of such charges.
-
Variation of synthetic process parameters such as temperature and ph can either be positive or be negative on degradation processes. Higher temperatures and higher pH ranges are toxic to degradation processes.
-
Surface modification of photoanodes is carried through nanostructured materials, the addition of metals particularly noble ones such as gold (Au), silver (Ag), platinum (Pt), and using novel titanium alloys and cubic double-perovskite.
References
R. Saravanan, V.K. Gupta, E. Mosquera, F. Gracia, V. Narayanan, A. Stephen, Visible light-induced degradation of methyl orange using β-Ag0. 333V2O5 nanorod catalysts by facile thermal decomposition method. J. Saudi Chem. Soc. 19(5), 521–527 (2015)
P. Li, G. Zhao, K. Zhao, J. Gao, T. Wu, An efficient and energy-saving approach to photocatalytic degradation of opaque high-chroma methylene blue wastewater by electrocatalytic pre-oxidation. Dyes Pigm. 92(3), 923–928 (2012)
M.A. Mahadik, G.W. An, S. David, S.H. Choi, M. Cho, J.S. Jang, Fabrication of A/R-TiO2 composite for enhanced photoelectrochemical performance: solar hydrogen generation and dye degradation. Appl. Surf. Sci. 426, 833–843 (2017)
X. Yuan, J. Yi, H. Wang, H. Yu, S. Zhang, F. Peng, New route of fabricating BiOI and Bi2O3 supported TiO2 nanotube arrays via the electrodeposition of bismuth nanoparticles for photocatalytic degradation of acid orange II. Mater. Chem. Phys. 196, 237–244 (2017)
Y.M. Hunge, M.A. Mahadik, S.S. Kumbhar, V.S. Mohite, K.Y. Rajpure, N.G. Deshpande et al., Visible light catalysis of methyl orange using nanostructured WO3 thin films. Ceram.442 Int. 42(1), 789–798 (2016)
O.M. Ama, K. Khoele, D.J. Delport, S.S. Ray, P.O. Osifo, Synthesis and fabrication of photoactive nanocomposites electrodes for the degradation of wastewater pollutants. Nanostruct. Metal-oxide Electrode Mater. Water Purif. Eng. Mater.
L.M. Reid, T. Li, Y. Cao & C.P. Berlinguette, Organic chemistry at anodes and photoanodes, NOPAGES & V (2018)
D. Rawat, V. Mishra, R.S. Sharma, Detoxification of Azo dyes in the context of environmental processes. Chemosphere 155, 591–605 (2016)
X.L. He, C. Song, Y.Y. Li, N. Wang, L. Xu, X. Han, D.S. Wei, Efficient degradation of Azo dyes by a newly isolated fungus Trichoderma tomentosum under non-sterile conditions. Ecotoxicol. Environ. Saf. 150, 232–239 (2018)
V.M. Daskalaki, M. Antoniadou, G. Li Puma, D.I. Kondarides, P. Lianos, Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol. 44(19), 7200–7205 (2010)
C. Yu, Y. Shu, X. Zhou, Y. Ren, Z. Liu, Multi-branched Cu2O nanowires for photocatalytic degradation of methyl orange. Mater. Res. Exp. 5(3), 035046 (2018)
Q. Zheng, C. Lee, Visible light photoelectrocatalytic degradation of methyl orange using anodized nanoporous WO3. Electrochim. Acta 115, 140–145 (2014)
N. Chaukura, W. Gwenzi, N. Tavengwa, M.M. Manyuchi, Biosorbents for the removal of synthetic organics and emerging pollutants: opportunities and challenges for developing countries. Environ. Dev. 19, 84–89 (2016)
S. Garcia-Segura, S. Dosta, J.M. Guilemany, E. Brillas, Solar photoelectrocatalytic degradation of acid orange 7 Azo dye using a highly stable TiO2 photoanode synthesized by atmospheric plasma spray. Appl. Catal. B 132, 142–150 (2013)
N. Lezana, F. Fernández-Vidal, C. Berríos, E. Garrido-Ramírez, Electrochemical and photo-504 electrochemical processes of methylene blue oxidation by Ti/TiO2 electrodes modified with 505 Fe-allophane. J. Chil. Chem. Soc. 62(2), 3529–3534 (2017)
H. Wu, Z. Zang, Photoelectrochemical water splitting and simultaneous photoelectrocatalytic degradation of organic pollutant on highly smooth and ordered TiO2 nanotubearrays. J. Solid State Chem. 184(12), 3202–3207 (2011)
R.M. Fernández-Domene, R. Sánchez-Tovar, B. Lucas-granados, M.J. Munoz-Portero, J. García-Antón, Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets Chem. Eng. J. 350, 1114–1124 (2018)
X. Meng, Z. Zang, X. Li, Synergetic photoelectrocatalytic reactors for environmental remediation: a review. J. Photochem. Photobiol. C 24, 83–101 (2015)
S. Garcia-Segura, S. Dosta, J.M. Guilemany, E. Brillas, Solar photoelectrocatalytic degra-397 dation of acid orange 7 Azo dye using a highly stable TiO2 photoanode synthesized by atmospheric plasma spray. Appl. Catal. B 132, 142–150 (2013)
Y. Huang, H. Cai, D. Feng, D. Gu, Y. Deng, B. Tu et al., One-step hydrothermal synthesis of ordered mesostructured carbonaceous monoliths with hierarchical porosities. Chem.478 Commun. 23, 2641–2643 (2008)
A. Ray. Electrodeposition of thin films for low-cost solar cells, in Electroplating of Nanostructures (IntechOpen, 2015)
O.J. Ilegbusi, S.N. Khatami, L.I. Trakhtenberg, Spray pyrolysis deposition of single and mixed oxide thin films. Mater. Sci. Appl. 8(02), 153 (2017)
N. Liu, S.P. Albu, K. Lee, S. So, P. Schmuki, Water annealing and other low-temperature treatments of anodic TiO2 nanotubes: a comparison of properties and efficiencies in dye-sensitized solar cells and for water splitting. Electrochim. Acta 82, 98–102 (2012)
K. Nakata, A. Fujishima, TiO2 photocatalysis: design and applications. J. Photochem. Photobiol. C 13(3), 169–189 (2012)
S. Li, J. Qiu, M. Ling, F. Peng, B. Wood, S. Zhang, Photoelectrochemical characterization of hydrogenated TiO2 nanotubes as photoanodes for sensing applications. ACS Appl. Mater.490 Interfaces. 5(21), 11129–11135 (2013)
J.H. Pan, H. Dou, Z. Xiong, C. Xu, J. Ma, X.S. Zhao, Porous photocatalysts for advanced water purifications. J. Mater. Chem. 20(22), 4512–4528 (2010)
C. Fu, M. Li, H. Li, C. Li, X. Guo Wu, B. Yang, Fabrication of Au nanoparticle/TiO2 hybrid494 filmsfor photo electrocatalytic degradation of methyl orange. J. Alloy. Compd. 692, 727–733 (2017)
C. Fu, M. Li, H. Li, C. Li, X. Guo Wu, B. Yang, Fabrication of Au nanoparticle/TiO2 hybrid filmsfor photo electrocatalytic degradation of methyl orange. J. Alloy. Compd. 692, 727–733468 (2017)
G.P. Awasthi, S.P. Adhikari, S. Ko, H.J. Kim, C.H. Park, C.S. Kim, Facile synthesis of ZnO flowers modified graphene-like MoS2 sheets for enhanced visible-light-driven photocatalytic activity and antibacterial properties. J. Alloy. Compd. 682, 208–215 (2016)
Y. Li, C. Ji, Y.C. Chi, Z.H. Dan, H.F. Zhang, F.X. Qin, Fabrication and photocatalytic activity of Cu2O nanobelts on nanoporous Cu substrate. Acta Metallurgica Sinica (English Letters) 341 32(1), 63–73 (2019)
H.H. Cheng, S.S. Chen, S.Y. Yang, H.M. Liu, K.S. Lin, Sol-gel hydrothermal synthesis and visible light photocatalytic degradation performance of Fe/N co-doped TiO2 catalysts. Materials 11(6), 939 (2018)
T. Wright, State reviewing controversial wastewater treatment technique. Environment (2019)
M. Vaara, New approaches in peptide antibiotics. Curr. Opin. Pharmacol. 9(5), 571–576 (2009)
L.T. Nguyen, E.F. Haney, H.J. Vogel, The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 29(9), 464–472 (2011)
V. Vega-Sánchez, F. Latif-Eugenín, E. Soriano-Vargas, R. Beaz-Hidalgo, M.J. Figueras, M.G. Aguilera-Arreola, G. Castro-Escarpulli, Re-identification of Aeromonas isolates from rainbow trout and incidence of class1 integronand β-lactamase genes. Vet. Microbiol. 172(3–4), 528–533 (2014)
M. Mahboubi, G. Haghi, Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 119(2), 325–327 (2008)
R. Gothwal, T. Shashidhar, Antibiotic pollution in the environment: a review. Clean–Soil, Air, 386 Water 43(4), 479–489 (2015)
H, Wu, Z. Zang, Photoelectrochemical water splitting and simultaneous photoelectrocatalytic degradation of organic pollutant on highly smooth and ordered TiO2 nanotube arrays. J. Solid State Chem. 184(12), 3202–3207 (2011)
G.P. Awasthi, S.P. Adhikari, S. Ko, H.J. Kim, C.H. Park, C.S. Kim, Facile synthesis of ZnO flowers modified graphene like MoS2 sheets for enhanced visible-light-driven photocatalytic activity and antibacterial properties. J. Alloy. Compd. 682, 208–215 (2016)
D. Liu, J. Zhou, J. Wang, R. Tian, X. Li, E. Nie et al., Enhanced visible light photoelectrocatalytic degradation of organic contaminants by F and Sn co-doped TiO2 photoelectrode. 498 Chem. Eng. J. 344, 332–341 (2018)
D. Liu, R. Tian, J. Wang, E. Nie, X. Piao, X. Li, Z. Sun, Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation. Chemosphere 185, 574–581 (2017)
D. Cao, Y. Wang, X. Zhao, Combination of photocatalytic and electrochemical degradation of organic pollutants from water. Current Opin. Green Sustain. Chem. 6, 78–84 (2017)
J. Tao, Z. Gong, G. Yao, Y. Cheng, M. Zhang, J. Lv et al., Enhanced photocatalytic and photoelectrochemical properties of TiO2 nanorod arrays sensitized with CdS nanoplates. Ceram. Int. 42(10), 11716–11723 (2016)
X.D. Li, T.P. Chen, P. Liu, Y. Liu, K.C. Leong, Effects of free electrons and quantum confinement in ultrathin ZnO films: a comparison between undoped and Al-doped ZnO. Opt. Express330 21(12), 14131–14138 (2013)
G. Li, N.M. Dimitrijevic, L. Chen, T. Rajh, K.A. Gray, J. Phys. Chem. C 112, 19040–19044 (2003)
D. Cao, Y. Wang, X. Zhao, Combination of photocatalytic and electrochemical degradation536 of organic pollutants from water. Curr. Opin. Green Sustain. Chem. 6, 78–84 (2017)
M.E. Osugi, G.A. Umbuzeiro, M.A. Anderson, M.V.B. Zanoni, Degradation of metallophtalocyanine dye by combined processes of electrochemistry and photoelectrochemistry. Electrochim. Acta 50(25–26), 5261–5269 (2005)
O.M. Ama, N. Kumar, F.V. Adams, S.S. Ray, Efficient and cost-effective photoelectrochemical degradation of dyes in wastewater over an exfoliated graphite-MoO3 nanocomposite electrode. Electrocatalysis (2018)
O.M Ama, O.A Arotiba , Exfoliated graphite/titanium dioxide for enhanced photoelectrochemical degradation of methylene blue dye under simulated visible light irradiationJ. Electroanalytical Chemistry, 157-164 (2017)
D. Pletcher, R.A. Green, R.C. Brown, Flow electrolysis cells for the synthetic organic chemistry laboratory. Chem. Rev. 118(9), 4573–4591 (2017)
A. Goshadrou, A. Moheb, Continuous fixed bed adsorption of C.I. Acid blue by exfoliated graphite: an experimental and modeling study. Desalination 269, 170–176 (2011)
G.W. An, M.A. Mahadik, W.S. Chae, H.G. Kim, M. Cho, J.S. Jang, Enhanced solar photoelectrochemical conversion efficiency of the hydrothermally-deposited TiO2 nanorod arrays: Effects of the light trapping and optimum charge transfer. Appl. Surf. Sci. 440, 688–699 (2018)
S. Chakrabarti, B.K. Dutta, Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 112(3), 269–278 (2004)
A. Mirzaei, Z. Chen, F. Haghighat, L. Yerushalmi, Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes–a review. Chemosphere 174, 665–688 (2017)
M.J. Ahmed, B.H. Hameed, Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: a review. Ecotoxicol. Environ. Saf. 149, 257–266 (2018)
T. Krishnakumar, N. Pinna, K.P. Kumara, K. Perumal, R. Jayaprakash, Microwave-assisted synthesis and characterization of tin oxide nanoparticles. Mater. Lett. 62, 3437–3440 (2008)
W. Baran, A. Makowski, W. Wardas, The effect of UV radiation absorption of cationic and anionic dye solutions on their photocatalytic degradation in the presence of TiO2. Dyes Pigm. 76, 226–230 (2008)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ama, O.M., Khoele, K., Govender, P.P., Ray, S.S. (2022). Application of Surface-Modified Electrode Materials in Wastewater Treatment. In: Ama, O.M., Sinha Ray, S., Ogbemudia Osifo, P. (eds) Modified Nanomaterials for Environmental Applications. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-85555-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-85555-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85554-3
Online ISBN: 978-3-030-85555-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)