Abstract
Purpose
In this study, we determined normal serum butyrylcholinesterase (BChE) and carboxylesterase (CbE) activities in Tupinambis merianae in order to obtain reference values for organophosphorus pesticide monitoring.
Methods
Forty-two T. merianae individuals were grouped by sex and size to identify potential differences in their enzyme levels to allow for proper representation of normal values for females, males, juveniles, and hatchlings. Mean CbE was determined using two model substrates: alpha-naphtylacetate (α-NA) and p-nitrophenyl valerate (4-NPV). BChE and CbE sensitivity to malaoxon (Mx) was also evaluated as well as the possibility of BChE reactivation with pyridine-2-aldoxime methochloride (2-PAM).
Results
Mean adult females’ BChE was significantly higher than adult males, juveniles, and hatchlings. No significant differences were found between groups regarding CbE. CbE (4-NPV) activity showed slightly negative correlation with lizard snout–vent length, while BChE and CbE (α-NA) showed no correlation with body size. Apparent IC50 values for BChE and CbE (α-NA) suggested different sensitivities among groups. CbE (4-NPV) could not be inhibited. All Mx-inhibited groups treated with 2-PAM in a final concentration of 2.8 mM showed clear signs of reactivation.
Conclusions
In conclusion, the results demonstrate that (1) plasma esterase activity did not vary with age and sex, except for BChE activity, and (2) because biological and environmental variables could be confounding factors in the response of plasma cholinesterases, complementary biomarkers like CbE inhibition and oxime-induced reactivation of esterases are strongly recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wildlife exposure to anticholinesterase (anti-ChE) agrochemicals usually implies the determination of acetylcholinesterase (AChE, EC 3.1.1.7) inhibition by nondestructive sampling methods, particularly when the species of interest are endangered, rare, or they are under a regulatory status of protection (Nunes 2011). Nevertheless, this exposure biomarker, considered a specific indicator of organophosphate (OP) and carbamate (CM) exposure, should not be used alone for assessing wildlife exposure to these classes of agrochemicals. Determination of blood AChE inhibition presents a set of limitations such as a high interindividual variation of its normal hydrolytic activity, a rapid recovery of its activity after inhibition by OP insecticides, and finally, the fact that this esterase is not directly involved in the acute toxicity by OPs or CMs, making the prediction of detrimental effects at whole-individual level risky (Sanchez-Hernandez 2001). The use of reactivating agents such as pyridine-2-aldoxime methochloride (2-PAM) to provide additional evidences of OP-inhibited AChE activity is a common strategy in the field monitoring of wild vertebrate exposure by these agrochemicals (Parsons et al. 2000; Maul and Farris 2005). Likewise, other blood esterases such as butyrylcholinesterases (BChEs, EC 3.1.1.8) and carboxylesterases (CbEs, EC 3.1.1.1) have gained a growing concern in the assessment of pesticide exposure in wildlife vertebrates (Sogorb et al. 2007; Wheelock et al. 2008). These esterases are able to modulate the toxicity of OPs and CMs through the stoichiometrical binding with these agrochemicals. This detoxification pathway has led to consider these esterases as efficient bioscavengers of anti-ChE pesticides reducing therefore their impact on the nervous AChE activity (Maxwell 1992; Masson and Lockridge 2010).
Measurement of multiple biomarkers rationally involved in the toxic mechanism and detoxification pathways of a pesticide in particular would be a recommended approach to assess the observed detrimental effects from the pesticide at whole individual level (Beliaeff and Burgeot 2002; Hagger et al. 2006); however, it is necessary to establish the naturally occurring variation of esterase responses and the environmental and biological factors contributing to their normal fluctuation to avoid misinterpretations (Forbes et al. 2006; Hagger et al. 2006). In the case of BChE and CbE activities, because of their direct implication in the modulation of OP and CM toxicity, a marked variation of their natural activity levels by factors such as light/dark cycles, sex, and age could be useful to identify the moment of a higher risk of pesticide toxicity or a group of individuals more sensitive to pesticide exposure (Thompson 1993; Maul and Farris 2004). As an example, the affinity of liver CbE activity to chlorpyrifos-oxon exposure in male and female rats was the same, whereas the higher number of CbE molecules in male liver accounted for a higher tolerance to chlorpyrifos-oxon exposure compared to females (Chanda et al. 1997); moreover, Kramer et al. (2002) determined that methyl parathion detoxification period depends substantially with route of exposure. Furthermore, serum BChE and CbE activities of some bird species such as buzzards (Buteo buteo), Japanese quail (Coturnix coturnix japonica), or European starlings (Sturnus vulgaris) display a large circadian variation (Thompson 1993). Furthermore, Fairbrother and Rattner (1991, in Thompson 1999) listed species, age, sex and diurnal, seasonal, and interindividual changes among the biological factors potentially affecting ChE activity. Also, size-dependent ChE activity was reported in both passerine (Mayack and Martin 2003) and nonpasserine (Roy et al. 2005; Strum et al. 2008) birds and crocodiles (Schmidt 2003). In addition, sex-dependent variations have also been observed in birds (Rattner and Franson 1984) and lizards (Bain et al. 2004). Taken all together, these examples illustrate that when blood is the unique biological material available to measure biomarkers (e.g., esterases) of pesticide exposure, naturally occurring fluctuations and the main biological and environmental variables contributing to their changes should be established.
This laboratory study is a preliminary phase of a broader project aimed to assess the impact of agrochemicals applied in Argentinean soy crops (Province of Santa Fe) on reptiles that frequent this agroecosystem. The objectives of this initial phase were to determine natural levels of plasma esterase (BChE and CbE) activities in the lizard Tupinambis merianae as well as the impact of both age and sex on esterase activity, and to examine the sensitivity of plasma esterases to in vitro exposure with a model OP insecticide, i.e., malaoxon, which is the main active metabolite of malathion. Finally, chemical reactivation of malaoxon-inhibited BChE in the presence of 2-PAM was also examined in an attempt to propose this methodology as a complementary index of OP exposure in this lizard species. We selected T. merianae because of several ecological features, e.g., the Tupinambis genus is widely distributed in South America (Péres and Colli 2004), and T. merianae (formerly Tupinambis teguixin) is frequently found in both natural ecosystems and cultivated areas (Fitzgerald et al. 1991; Péres 2003). Furthermore, this lizard species is a diet generalist and feeds on a wide range of animals and fruits (de Castro and Galetti 2004), its conservation status is considered of “Least Concern” (Embert et al. 2009), and finally, the implementation of captive breeding programs (Noriega et al. 1996) facilitates some aspects of its study. Considering all the above mentioned, we believe that this lizard species is a good sensor of the impact of pesticide application in soy crops.
2 Materials and methods
2.1 Reagents
Sodium dodecyl sulfate (SDS) was purchased from Calbiochem® (Canada). Butyrylthiocholine iodide (BuSCh), 2-PAM, 5, 5-dithiobis-2-nitrobenzoic acid (DTNB), α-naphthyl acetate (α-NA), 4-nitrophenyl valerate (4-NPV), and Fast Red ITR salt were obtained from Sigma-Aldrich® (Germany). The pesticide malaoxon (CAS RN 1634-78-2, 99.2% purity) was acquired from Applied Science® (USA). All other chemicals used in this study were obtained from Biopack® (Argentina).
2.2 Experimental animals and condition
We obtained experimental lizards from the “El Gringo” captive breeding tegu farm (Sa Pereira, Santa Fe Province). In this farm, lizards are reproduced and reared under natural conditions (e.g., sunlight, temperature, rain) and fed with a diet mostly consisting of meat and eggs.
Blood samples (1–2 ml) were obtained in March, during the post-hatching period (Manes et al. 2007) between 1000 and 1300 hours by puncturing of the caudal vein with heparinized sterile syringes and transported on ice to the laboratory where plasma was separated by centrifugation at 4,500 rpm for 15 min at 4°C, and subsequently, plasma was frozen at −20°C.
Snout–vent length (SVL) was recorded in each individual with a retractable flexible rule (±0.1 mm precision). In accordance to these data, the randomly captured lizards were grouped by size and sex according to Noriega et al. (2002) and Manes et al. (2007) thus creating an “adult” group subdivided in males (>35 cm SVL) and females (>32 cm SVL), a “juveniles” group (22–31.9/34.9 cm SVL) and a “hatchlings” group (12–21.9 cm SVL) in order to determine age- and sex-related differences in esterase activity. Juveniles and hatchlings were not subdivided by sex since no differences were found in preliminary studies (Basso, personal observation).
2.3 Esterase assays
Plasma BChE activity was determined by the Ellman et al. (1961) colorimetric method. The reaction mix was composed by 1,870 μl 25 mM Tris–HCl containing 1 mM CaCl2 (pH = 7.6), 100 μl DTNB (3 × 10−4 M, final concentration [FC]), 20 μl BuSCh (2 × 10−3 M, FC), and 10 μl of plasma. The variation in the optical density was measured in triplicate at 410 nm for 1 min at 25°C using a Jenway 6405 UV–VIS spectrophotometer. The activity of plasma BChE was expressed in micromoles of hydrolyzed substrate per minute per milliliter of plasma using a molar coefficient extinction of 13.6 × 103 M−1 cm−1. Plasma AChE activity was not measured due to the fact that total ChE activity in the plasma of reptiles is primarily due to BChE activity (75–80% of total ChE activity; Sanchez-Hernandez and Moreno Sanchez 2002; Bain et al. 2004).
Plasma carboxylesterase was determined using two substrates: α-NA and 4-NPV. The hydrolysis of α-NA by CbE was measured as described by Gomori (1953) adapted by Bunyan and Jennings (1968). The enzymatic assay was made with 1,940 μl 25 mM Tris–HCl, 1 mM CaCl2 (pH = 7.6), and 10 μl of diluted (1:50) plasma. The reaction was initiated by addition of 50 μl α-NA (1.04 mg ml−1 in acetone) and stopped after 10 min of incubation at 25°C with the addition of 500 μl 2.5% SDS and, subsequently, 500 μl 0.1% of Fast Red ITR in 2.5% Triton X-100. Samples were left in the dark for 30 min to enable the development of the color, and the absorbance was read at 530 nm. Hydrolysis of α-NA was expressed in micromoles of hydrolyzed substrate per minute per milliliter of plasma using a molar extinction coefficient of 33.225 × 103 M−1 cm−1. Determination of CbE activity towards 4-NPV followed the methods of Carr and Chambers (1991). A 20-μl aliquot of diluted (1:50) plasma was added to 1,940 μl 50 Mm Tris–HCl (pH = 7.5) and incubated for 5 min at 25°C. The reaction was initiated by the addition of 20 μl 4-NPV (5 × 10−4 M, FC) and stopped 10 min later by the addition of a solution of 0.5 ml 2% (w/v) SDS and 0.5 ml of 2% (w/v) Tris base. The formation of 4-nitrophenolate was monitored at 405 nm and quantified using a standard curve made with 4-nitrophenolate.
2.4 In vitro inhibition of B-esterase activity
Sensitivity of plasma BChE and CbE (α-NA and 4-NPV) activities to OPs was tested using the oxon metabolite of malathion. Insecticide solutions were initially prepared in dimethylsulfoxide, and serial dilutions of the OP solutions kept the solvent concentration below 1% in the reaction medium. Pools including equal amounts of plasma from five random lizards belonging to the same group were preincubated for 15 min at 25°C with multiple malaoxon concentrations to generate a range of esterase inhibition between 10% and 90%. The percentage of enzyme inhibition was calculated by comparison with controls, which received an equal volume of deionized water. Samples of 10 μl (BChE), 10 μl 1:50 (α-NA) and 20 μl 1:50 (4-NPV) were used to measure the esterase activities. All the incubations were run in triplicate. The molar concentration of malaoxon causing 50% inhibition of the observed maximum enzyme activity (apparent IC50) was estimated by plotting the percentage of remaining esterase activity against the molar inhibitor concentration. The inhibition curves were fit to the four-parameter logistic model \( y = { \min } + \left( {{ \max } - { \min }} \right)/{1} + \left( {x/{\text{I}}{{\text{C}}_{{{5}0}}}} \right) - {\text{HillSlope}} \), where y is the percentage of residual CbE activity compared to controls after a 30-min incubation with malaoxon, min and max are the y responses to the highest and lowest concentrations of the pesticide, x is the logarithmic of inhibitor molar concentration, and Hillslope describes the steepness of the dose–response relationship (Motulsky and Christopoulos 2003). A level of probability less than 0.05 was taken as statistically significant.
2.5 Chemical reactivation of BChE
Pralidoxime-induced reactivation of plasma BChE activity previously inhibited with malaoxon was examined in order to suggest the inclusion of this methodology in the ecotoxicological assessment of OP exposure. Plasma samples were incubated with 4.7 × 10−5 M malaoxon for 30 min at 25°C (Laguerre et al. 2009). After incubation, 2-PAM (0.28 mM or 2.8 mM, FC) was added to the samples. BChE activity was measured every 15 min over a 75-min period. Two aliquots of each plasma samples were used as controls: the first received an equal volume of distilled water and the second 2-PAM in order to test an already existing inhibition. The results are expressed in percentage of remaining BChE activity which was calculated considering the control activities (without malaoxon) using the equation by Laguerre et al. (2009):
where “reactivated” is the BChE activity after 75 min of 2-PAM treatment; “inhibited” is the BChE activity after 30 min of incubation in the presence of malaoxon; and “control” is the BChE activity of the sample without the OP after 75 min.
The chemical reactivation rate, the observed reactivation rate (k r), and the time (minutes) to 50% of BChE activity with respect to the corresponding controls (t 1/2) were estimated from the equation
where the coefficient a is the maximal activity of BChE activity after 2-PAM treatment (expressed as percentage of BChE activity), and the b coefficient is the observed reactivation constant (k r), expressed in minutes. These parameters enabled the comparison of the ability of 2-PAM to reverse the phosphorylated BChE activity among the four groups of lizards.
2.6 Data analysis
The data were statistically analyzed using a nonparametric Kruskal–Wallis KS test; Dunn’s test was used for post hoc paired comparisons between the four groups of T. merianae (adult males, adult females, juveniles, and hatchlings). The Spearman correlation test was also made to determine the association between SVL and enzyme activities. Data were tested for variance homogeneity and normality (Kolmogorov–Smirnov test and Levene test). A level of probability below 0.05 was considered significant. The analyses were made with InfoStat® 1.1 software (Grupo InfoStat Professional, FCA, Universidad Nacional de Córdoba, Argentina).
3 Results
3.1 Esterase activity levels
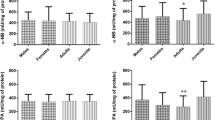
The mean plasma esterase activities measured in the four tegu groups are summarized in Table 1. Butyrylcholinesterase activity of adult female lizards was more than twofold higher than those of adult males, juveniles, or hatchlings. However, CbE (using either α-NA or 4-PNV as substrates) activity showed no statistical differences between groups. Moreover, no substrate-specific differences were found in plasma CbE activity. The mean length (SVL) for each group was: 39.44 ± 4.51 cm for the adult males group (n = 10), 39.72 ± 2.33 cm for the adult females group (n = 9), 28.66 ± 2.22 cm for the juveniles group (N = 10), and 15.42 ± 1.75 cm for the hatchlings group (n = 13). Lizard length had a significant effect on 4-NPV-CbE activity solely (r = − 0.33, p < 0.05), whereas no significant correlation was observed with α-NA-CbE (r = 0.14, p > 0.05) or BChE (r = 0.20, p > 0.05).
3.2 Malaoxon inhibition and chemical reactivation
We tested for age and sex-related differences in BChE and CbE sensitivity to malaoxon as model OP pesticides. Plasma BChE and α-NA-CbE activities followed a sigmoidal model when in vitro exposed to malaoxon (Fig. 1). Carboxylesterase activity was much more sensitive to the OP (apparent IC50s in the nanomolar level) than BChE activity was (Table 2). Interestingly, malaoxon had no effect on CbE activity towards 4-NPV at concentration as high as 6.02 × 10−5 M (Fig. 1b).
Stability of the enzyme-inhibitor complex was examined by incubation of the phosphorylated BChE activity in the presence of 0.28 mM and 2.8 mM 2-PAM following malaoxon inhibition (60–80% inhibition compared to controls). Plasma BChE activity of all lizard groups showed clear signs of 2.8 mM 2-PAM reactivation (47–52% of reactivated enzyme) even though full recovery was not achieved (Fig. 2); in this case, plasma BChE activity of hatchlings reactivated more slowly (k r = 0.07 min−1 and t 1/2 = 23 min) than the other groups (Table 2). The lowest concentration of 2-PAM (0.28 mM FC) resulted to be the least effective for reactivating plasma BChE in this lizard species (Fig. 2).
Reactivation of Mx-inhibited plasma BChE activity in the presence of 2-PAM. Start point represents the plasma BChE activity incubated with 4.7 × 10−5 M of Mx for 30 min at 25°C. Zero represents the activity at the moment right after the treatment with two different concentrations of 2-PAM a 2.8 mM FC and b 0.28 mM FC. Enzyme activity was determined periodically (15-min intervals) after addition of 2-PAM. Reactivation is expressed as percentage of remaining BChE activity with respect to the corresponding controls. Each point represents the mean of three determinations
4 Discussion
4.1 Impact of confounding variables on esterase activity
Blood is the suitable biological material for assessing pesticide exposure in terrestrial wild vertebrates because of obvious regulatory, ethical, and conservation reasons. However, when biomarkers are included within the set of biological variables to be integrated in a weight-of-evidence framework for the environmental assessment of pesticide exposure, it is necessary to know the impact of biological (i.e., life stage or sexual development) and environmental factors (i.e., temperature or light/dark cycles) on biomarker responses (Peakall 1992; Sanchez-Hernandez 2001) (Forbes et al. 2006; Hagger et al. 2006). In the case of blood esterases, the understanding of normal fluctuation of their activities would enable to know the moment of critical vulnerability of the organism to pesticide intoxication as well as to identify the stressors responsible for esterase responses.
Few lizard esterases have been previously characterized (Table 3). In the present study, we found that sex had a significant impact on the plasma BChE activity of T. merianae; adult females had higher BChE activity than males. This observation does not corroborate other related studies with reptiles. For example, the mean (±SD) plasma ChE activity of the Australian agamid Pogona vitticeps was 0.66 ± 0.06 mmol/min/ml for males and 0.45 ± 0.06 mmol/min/ml for females (Bain et al. 2004). Likewise, Sanchez-Hernandez et al. (2004) reported no significant differences of serum BChE activity between males (4.42 ± 0.94 mmol/min/ml, mean ± SD) and females (3.93 ± 0.75 mmol/min/ml) of the lizard Gallotia galloti. Bain et al. (2004) suggested that sex-related differences of ChE activity found in P. vitticeps were due likely to the fact that their lizards came from different natural populations. However, lizards (Gallotia galloti palmae) collected from two different localities of the Palma Island (Canary Islands, Spain) did not show significant differences in plasma BChE activity even when animals were sampled in summer (3.07 ± 1.22 mmol/min/ml for males and 3.00 ± 1.09 for females, mean ± SD) and in autumn (3.61 ± 2.57 for males and 4.06 ± 1.30 for females) (Sanchez-Hernandez et al. 2004).
Taken all together, these studies suggest a previous analysis of interindividual normal variations of blood esterases as well as the potential stressor other than pesticides contributing to their basal responses. However, most of the studies with vertebrate esterases have been addressed on the following issues: (1) enzymatic characterization of blood ChE activity for enzyme assay purposes (e.g., Küster 2005; Attademo et al. 2007; Lajmanovich et al. 2010), (2) chemical reactivation of the phosphorylated ChE activity using oximes (e.g., Lajmanovich et al. 2008), (3) relationship between ChE inhibition and physiological or behavioral changes (e.g., Fildes et al. 2009; Junges et al. 2010), and (4) field monitoring of OP/CM pesticide exposure by comparing blood ChE activity between pesticide-exposed and nonexposed populations (e.g., Attademo et al. 2011). But most of the studies examining the sources of natural variation of blood esterases in wild vertebrates are particularly limited to birds. For example, seasonal variations of serum BChE activity have been observed in northern bobwhites (Colinus virginianus), whereas a wide variation of CbE activity has been recorded in the plasma of European starlings (S. vulgaris) which increased up to 150% during the day (Thompson 1993). Circadian variations of blood esterase activities have been also documented in other bird species such as buzzards (B. buteo), Japanese quails (C. coturnix japonica) or clay-colored robins (Turdus grayi) (Thompson 1993; Cobos et al. 2009). Sex, age, and diet are also confounding variables when depressing of bird esterase activity is linked to ChE-inhibiting agrochemicals (Westlake et al. 1983; Sanchez-Hernandez 2001; Roy et al. 2005; Sogorb et al. 2007).
The explanation on why blood ChE and CbE activities vary with light/dark cycles, season, or sex is not totally understood. Nevertheless, some authors postulate that feeding activity, hormone-related mechanism, or the presence of lipid-rich materials in diet are among the potential factors contributing to blood esterases interindividual variations (Rattner and Fairbrother 1991; Thompson 1993). Past studies show that CbE activity plays a notable role in the metabolism of lipids in invertebrates (Geering and Freyvogel 1974; Mommsen 1978). Similar findings have been documented in rats (Wassmer et al. 1988) and mice (Van Lith et al. 1991, 1992) fed with lipid-rich diets. More recently, a CbE showing triacylglycerol hydrolase activity was involved in the metabolism of neutral lipids in several tissues of mammals (Dolinsky et al. 2004). Nevertheless, our lizards were obtained from a specialized farm where they were fed with a standardized diet, and apparently, this confounding variable did not have a significant effect on plasma CbE activity in the T. merianae used in this study. Interestingly, our data suggest that food intake by T. merianae could be a determinant factor in the marked variation of plasma BChE activity between adult males and females. It is known that gravid females of T. merianae lose a significant percentage of the total body mass during reproduction season (Andrade et al. 2004). However, this loss of body weight is recovered before to initiate the dormancy, accumulating fat in their abdominal cavity up to a 5% of the total body weight (Andrade et al. 2004). If it is the same scenario for our animals, then the increase in food intake necessary to survive during hibernation might partially explain the higher plasma BChE activity in the female individuals.
4.2 Multiple plasma esterases of T. merianae for environmental biomonitoring
Inhibition of blood ChE activity is the traditional biomarker of pesticide exposure in wild vertebrates. However, convincing evidence accumulated over the last three decades has demonstrated that this biomarker shows a high interindividual variation that often makes the identification of pesticide-exposed individuals by comparing ChE activity levels difficult. A recommended strategy is the use of multiple biomarkers related to the mechanism of toxicity and detoxification pathways (Hagger et al. 2006) or the use of complementary methodologies of pesticide exposure such as the chemical reactivation of phosphorylated or carbamylated ChE activity employing oximes or water dilution, respectively (Sanchez-Hernandez et al. 2004). Measurement of BChE or CbE inhibition in the plasma of wild vertebrates is of growing concern because of the higher sensitivity to inhibition by OP or CM pesticides compared to AChE activity (Wheelock et al. 2008), and further, the role of these esterases in the detoxification of anticholinesterase and synthetic pyrethroid agrochemicals (Sogorb and Vilanova 2002).
In the present study, we have established the normal levels of plasma BChE and CbE activities in T. merianae in an attempt of using them as reference values in ongoing investigations on the impact of pesticides in these reptiles. Furthermore, we examined the sensitivity of these esterases to in vitro malaoxon exposure. This OP strongly inhibited both esterase activities, although apparent IC50s were lower for CbE activity using α-NA as substrate than those obtained for BChE activity. We found that inhibition of plasma CbE activity by malaoxon was substrate-specific, irrespective of the lizards’ age or sex. This is a clear evidence of the occurrence of multiple CbE isozymes in the plasma of T. merianae showing different sensitivities to OPs. It has been widely demonstrated that CbEs are expressed as multiple isozymes in many tissue and organs of many organisms (Satoh and Hosokawa 2006; Wheelock et al. 2008; Sanchez-Hernandez and Wheelock 2009). More interestingly, sensitivity of α-NA-CbE activity of females was higher to malaoxon in vitro inhibition compared to males, juvenile, or hatchings (Table 2). Merely speculating, it could be considered that if plasma CbE activity is a significant detoxification pathway because this esterase binds stoichiometrically OPs, then the comparatively higher levels of plasma CbE activity in the females as well as their high sensitivity to OPs would mean that T. merianae females are more resistant to the impact of OP exposure than males or subadult individuals at least during this time of the year.
Chemical reactivation of the phosphorylated ChE activity has shown to be a workable methodology of OP intoxication in wild vertebrates, providing further solid evidence of OP exposure (Table 4). The data reported in the present study show that malaoxon-inhibited BChE activity from all lizard groups exhibited approximately 50% of reactivation by 2-PAM treatment. This lack of full recovery of BChE activity could be due to many factors jointly interacting to reduce the potency of the oxime to reverse the phosphorylated esterase activity. For example, the concentration of the oxime in the reaction medium is critical to achieve a maximum reactivation of the phosphorylated ChE activity. As an example, while a 2-PAM concentration of 10−4 M caused inhibition in both fish and crab AChE activity (Monserrat and Bianchini 2000), the same range of 2-PAM concentration is optimum for reactivation of OP-inhibited blood BChE in birds and lizards (Table 4). Previous studies with the lizard G. galloti have recommended a 2-PAM concentration of ∼10−4 M to maximum recovery of phosphorylated ChE activity (Sanchez-Hernandez et al. 2004); however, we obtained a significant increase of the malaoxon-inhibited BChE activity in the presence of 2.8 mM compared to 0.28 mM. This unexpected observation could be explained by the chemical nature of the enzyme inhibitor which likely forms a stable complex. Some studies with human AChE activity and earthworm ChE activity have shown that the structure of the phosphoryl moiety at the active site of the esterase, which depends on the OP type, affects the reactivation potency of the oxime (Worek et al. 2004; Rodriguez and Sanchez-Hernandez 2007). Excess of free inhibitor in the reactivation procedure could be another significant factor to limit the reactivation potency of 2-PAM. To avoid this interference factor, many authors have developed multiple separating techniques to remove excess of the OP such as dialysis (Worek et al. 2004), gel permeation chromatography (Hovanec and Lieske 1972), solid-phase extraction (Hunt and Hooper 1993), or centrifuge filtration (Wheelock et al. 2006). These removing procedures are justified when an excess of the inhibitor is added to the incubation medium to guarantee the full inhibition of ChE activity, although these techniques have the risk for ChE aging or spontaneous reactivation during OP removal. We have used a concentration of malaoxon in the incubation medium that caused an inhibition of BChE activity between 20% and 40% compared to controls. We assumed, therefore, that free malaoxon would be minimal when 2-PAM was added to this medium to test for chemical reactivation of phosphorylated BChE activity. A similar strategy was used by Rodríguez and Sanchez-Hernandez (2007) to inhibit muscle ChE activity of earthworms.
Lastly, the marked variation in basal plasma BChE activity and its sensitivity to OPs between T. merianae males and females provide a unique animal model to examine the role that this plasma esterase plays as modulator of pesticide intoxication and, in turn, to link to whole individual adverse effects. Female behavior has a crucial influence on nest conditions during the whole incubation process (Chani et al. 1993; Noriega et al. 1996) keeping vital factors like temperature and moisture in optimum levels for embryo development (Manes et al. 2003). On the other hand, the exposure to some anticholinesterasic agents like OPs is known to temporarily, but significantly, reduce body temperature in mammals and birds (Rattner and Franson 1984; Gordon 1994). In mammals, this physiological response is usually followed by the search of cooler environments in order to reduce metabolic activity and adverse effects to the xenobiotics. In ectotherms, the response tends to be the opposite, inducing a searching for a warmer microenvironment so as to boost the metabolic response against intoxication (Grue et al. 2002). If these behavioral changes are verified in T. merianae females, they could also affect optimal nest conditions for the species, disfavoring their OP-exposed populations. Nest abandonment and extended time off nests have been already reported in adult free-living birds exposed to OPs (Bennet et al. 1991).
5 Conclusion
Two main conclusions could be drawn from the current results, which should be taken into account when this lizard species is used in the field monitoring of sublethal effects from pesticides. First, plasma esterase activity did not vary with age and sex, except for BChE activity. Because of the significant contribution of both BChE and CbE activities in the natural tolerance of organisms to anticholinesterase pesticides—they are considered efficient endogenous scavengers of pesticides—female lizards could display a higher tolerance to pesticide exposure compared to males due to the higher level of normal BChE activity in females. Second, because environmental and biological variables could be confounding factors in the response of plasma ChE activity to pesticides, complementary biomarkers such as inhibition of CbE activity or exposure index such as oxime-induced reactivation of esterases are strongly recommended.
References
Andrade DV, Brito SP, Toledo LF, Abe AS (2004) Seasonal changes in blood oxygen transport and acid–base status in the tegu lizard, Tupinambis merianae. Resp Physiol Neurob 140:197–208
Attademo AM, Peltzer PM, Lajmanovich RC, Cabagna M, Fiorenza G (2007) Plasma B-esterases and glutathione S-transferase activities in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicol 16:533–539
Attademo AM, Cabagna Zenklusen M, Lajmanovich RC, Peltzer PM, Junges C, Basso A (2011) B-esterase activities and blood cell morphology in the frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicol 20:274–282
Bain D, Buttemer WA, Astheimer L, Fildes K, Hooper MJ (2004) Effects of sublethal fenitrothion ingestion on cholinesterase inhibition, standard metabolism, thermal preference, and prey-capture ability in the Australian central bearded dragon (Pogona vitticeps, Agamidae). Environ Toxicol Chem 23:109–116
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21(6):1316–1322
Bennett RS, Williams BA, Schmedding DW, Bennett JK (1991) Effects of dietary exposure to methyl parathion on egg laying and incubation in mallards. Environ Toxicol Chem 10:501–507
Bunyan PJ, Jennings DM (1968) Organophosphorus poisoning; some properties of avian esterase. J Agric Food Chem 16:326–331
Carr RL, Chambers JE (1991) Acute effects of the organophosphate paraoxon on schedule-controlled behaviour and esterase activity in rats: dose–response relationships. Pharmacol Biochem Behav 40:929–936
Chanda SM, Mortensen SR, Moser VC, Padilla S (1997) Tissue-specific effects of chlorpyrifos on carboxylesterase and cholinesterase activity in adult rats: an in vitro and in vivo comparison. Fundam Appl Toxicol 38:148–157
Chani JM, Cruz F, Perotti G, Aguirre M, Rufino S (1993) Rol de la hembra de Tupinambis teguixin (Teiidae), durante la nidificación. Acta Zool Lill 42:295–299 (In Spanish)
Cobos VM, Mora MA, Escalona G, Calme S, Jiménez J (2009) Variation in plasma cholinesterase activity in the clay-colored robin (Turdus grayi) in relation to time of day, season, and diazinon exposure. Ecotoxicol 19(2):267–272
de Castro ER, Galetti M (2004) Frugivoria e dispersão de sementes pelo lagarto teiú Tupinambis merianae (Reptilia: Teiidae). Pap Avulsos Zool (São Paulo) 44(6):91–97, (In Portuguese)
Dolinsky VW, Gilham D, Alam M, Vance DE, Lehner R (2004) Triacylglycerol hydrolase: role in intracellular lipid metabolism. Cell Mol Life Sci 61:1633–1651
Ellman L, Courtey KD, Andreas V Jr, Featherstone RM Jr (1961) A new rapid colorimetric determination of cholinesterase activity. Biochem Pharmacol 7:88–95
Embert D, Fitzgerald L, Waldez F (2009) Tupinambis merianae. In: IUCN 2010. IUCN Red List of Threatened Species. Version 2010.4. www.iucnredlist.org. Downloaded on 26 May 2011.
Fildes K, Astheimer L, Story P, Buttemer WA, Hooper MJ (2006) Cholinesterase response in native birds exposed to fenitrothion during locust control operations in eastern Australia. Environ Toxicol Chem 25:2964–2970
Fildes K, Astheimer LB, Buttemer WA (2009) The effect of acute fenitrothion exposure on a variety of physiological indices, including avian aerobic metabolism during exercise and cold exposure. Environ Toxicol Chem 28(2):388–394
Fitzgerald LA, Chani JM, Donadio OE (1991) Tupinambis lizards in Argentina: implementing management of a traditionally exploited resource. In: Robinson J, Redford K (eds) Neotropical wildlife: use and conservation. University of Chicago Press, Chicago, pp 303–316
Forbes VE, Palmqvist A, Bach L (2006) The use and misuse of biomarkers in ecotoxicology. Environ Toxicol Chem 25(1):272–280
Geering K, Freyvogel TA (1974) The distribution of acetylcholine and unspecific esterases in the midgut of female Aedes aegypti L. Comp Biochem Physiol B 49:775–784
Gomori G (1953) Human esterases. J Lab Clin Med 42:445–453
Gordon CJ (1994) Thermoregulation in laboratory mammals and humans exposed to anticholinesterase agents. Neurotoxicol Teratol 16:427–453
Grue CE, Gardner SC, Gibert PL (2002) On the significance of pollutant-induced alterations in the behaviour of fish and wildlife. In: Dell’Omo G (ed) Behavioural ecotoxicology. Wiley, Chichester, pp 1–90
Hagger JA, Jones MB, Leonard DRP, Owen R, Galloway TS (2006) Biomarkers and integrated environmental risk assessment: are there more questions than answers? Integr Environ Assess Manag 2(4):312–329
Hovanec JW, Lieske CN (1972) Spontaneous reactivation of acetylcholinesterase inhibited with parasubstituted phenyl inhibited by three organophosphates. Pestic Biochem Physiol 13:205–212
Hunt KA, Hooper MJ (1993) Development and optimization of reactivation techniques for carbamate-inhibited brain and plasma cholinesterases in birds and mammals. Anal Biochem 212:335–343
Junges CM, Lajmanovich RC, Peltzer PM, Attademo AM, Basso A (2010) Predator–prey interactions between Synbranchus marmoratus (Teleostei: Synbranchidae) and Hypsiboas pulchellus tadpoles (Amphibia: Hylidae): Importance of lateral line in nocturnal predation and effects of fenitrothion exposure. Chemosph 81:1233–1238
Kramer RE, Wellman SE, Zhu H, Rockhold RW, Baker RC (2002) A comparison of cholinesterase activity after intravenous, oral or dermal administration of methyl parathion. J Biomed Sci 9:140–148
Küster E (2005) Cholin- and carboxylesterase activities in developing zebrafish embryos (Danio rerio) and their potential use for insecticide hazard assessment. Aquat Toxicol 75(1):76–85
Laguerre C, Sanchez-Hernandez JC, Köhler HR, Triebskorn R, Capowiez Y, Rault M, Mazzia C (2009) B-type esterases in the snail Xeropicta derbentina: an enzymological analysis to evaluate their use as biomarkers of pesticide exposure. Environ Poll 157:199–207
Lajmanovich RC, Sanchez-Hernandez JC, Peltzer PM, Attademo AM, Fiorenza GS, Cabagna MC, Basso A (2008) Levels of plasma B-esterases and glutathione-S-transferase activities in three South American toad species. Toxicol Environ Chem 90:1145–1161
Lajmanovich RC, Peltzer PM, Junges CM, Attademo AM, Sanchez LC, Basso A (2010) Activity levels of B-esterases in the tadpoles of 11 species of frogs in the middle Paraná River floodplain: implication for ecological risk assessment of soybean crops. Ecotoxicol Environ Saf 73(7):1517–1524
Manes ME, Ibáñez MA, Manlla A (2003) Factores físicos y conductas de nidificación de lagartos Tupinambis merianae en cautiverio. Rev Arg Prod Anim 23:119–126 (In Spanish)
Manes ME, Noriega T, Campos Casal F, Apichela S (2007) Ovarian changes during the reproductive cycle of the Tupinambis merianae lizard raised in a temperate environment. Cuad Herpetol 21:21–29
Masson P, Lockridge O (2010) Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch Biochem Biophys 494(2):107–120
Maul JD, Farris JL (2004) The effect of sex on avian plasma cholinesterase enzyme activity: a potential source of variation in an avian biomarker endpoint. Arch Environ Contam Toxicol 47:253–258
Maul JD, Farris JL (2005) Monitoring exposure of northern cardinals, Cardinalis cardinalis to cholinesterase-inhibiting pesticides: enzyme activity, reactivations, and indicators of environmental stress. Environ Toxicol Chem 24:1721–1730
Maxwell DM (1992) The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol Appl Pharmacol 114:306–312
Mayack DT, Martin T (2003) Age-dependent changes in plasma and brain cholinesterase activities of house wrens and European starlings. J Wildl Dis 39:627–637
McInnes PF, Andersen DE, Hoff DJ, Hooper MJ, Kinkel LL (1996) Monitoring exposure of nestling songbirds to agricultural application of an organophosphorus insecticide using cholinesterase activity. Environ Toxicol Chem 15:544–552
Mommsen TP (1978) Digestive enzymes of a spider (Tegenaria atrica Koch)—III. Esterases, phosphatases, nucleases. Comp Biochem Physiol A 60:377–382
Monserrat M, Bianchini A (2000) Methodological and biological aspects to be considered in acetylcholinesterase reactivation assays using 2-PAM. Environ Toxicol Pharmacol 9:39–47
Motulsky HJ, Christopoulos A (2003) Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. GraphPad Software Inc, San Diego
Noriega T, Fogliatto O, Mignola L, Manes ME (1996) Ciclo biológico y patrones de comportamiento de una población de iguanas overas Tupinambis teguixin (L.) (Sauria, Teiidae) adaptada al cautiverio. Rev Agron NO Argent 28:109–127, (In Spanish)
Noriega T, Ibañez MA, Bru E, Manes ME (2002) The testicular cycle of captive Tupinambis merianae lizards in a temperate environment. Cuad Herpetol 16:119–127
Nunes B (2011) The use of cholinesterases in ecotoxicology. Rev Environ Contam Toxicol 212:29–59
Parsons KC, Matz AC, Hooper MJ, Pokras MA (2000) Monitoring wading bird exposure to agricultural chemicals using serum cholinesterase activity. Environ Toxicol Chem 19:1317–1323
Peakall D (1992) Animal biomarkers as pollutants indicators. Chapman & Hall, London
Péres AK Jr (2003) Sistemática e Conservação de Lagartos do Gênero Tupinambis (Squamata, Teiidae). Departamento de Zoologia, Universidade de Brasília, Tese de Doutorado
Péres Jr AK, Colli GR (2004) The taxanomic status of Tupinambis rufescens and T. duseni (Squamata: Teiidae), with a redescription of the two species. Occasional Papers of the Sam Noble Oklahoma Museum of Natural History 15:1–12
Rattner BA, Fairbrother A (1991) Biological variability and the influence of stress on cholinesterase activity. In: Mineau P (ed) Cholinesterase-inhibiting insecticides. Elsevier, Amsterdam, pp 89–107
Rattner BA, Franson JC (1984) Methyl parathion and fenvalerate toxicity in American kestrels: acute physiological responses and effects of cold. Can J Physiol Pharmacol 62:787–792
Rodriguez LC, Sanchez-Hernandez JC (2007) Earthworm biomarkers of pesticide contamination: current status and perspectives. J Pestic Sci 32:360–371
Roy C, Grolleau G, Chamoulaud S, Rivière JL (2005) Plasma B-esterase activities in European raptors. J Wild Dis 41:184–208
Sanchez JC, Fossi MC, Focardi S (1997) Serum B esterases as a non-destructive biomarker in the lizard Gallotia galloti experimentally treated with parathion. Environ Toxicol Chem 16:1954–1961
Sanchez-Hernandez JC (2001) Wildlife exposure to organophosphorus insecticides. Rev Environ Contam Toxicol 172:21–63
Sanchez-Hernandez JC (2003) Evaluating reptile exposure to cholinesterase-inhibiting agrochemicals by serum butyrylcholinesterase activity. Environ Toxicol Chem 22:296–301
Sanchez-Hernandez JC (2006) Ecotoxicological perspectives of B-esterases in the assessment of pesticide contamination. In: Plattenberg RH (ed) Environmental pollution: new research. Nova, New York
Sanchez-Hernandez JC, Moreno Sanchez B (2002) Lizard cholinesterases as biomarkers of pesticide exposure: enzymological characterization. Environ Toxicol Chem 21:2319–2325
Sanchez-hernandez JC, Wheelock CE (2009) Tissue distribution, isozyme abundance and sensitivity to chlorpyrifos-oxon of carboxylesterases in the earthworm Lumbricus terrestris. Environ Poll 157(1):264–272
Sanchez-Hernandez JC, Carbonell R, Henríquez Pérez A, Montealegre M, Gómez L (2004) Inhibition of plasma butyrylcholinesterase activity in the lizard Gallotia galloti palmae by pesticides: a field study. Environ Pollut 132:479–488
Satoh T, Hosokawa M (2006) Structure, function and regulation of carboxylesterases. Chem Biol Interact 162:195–211
Schmidt SR (2003) Reptile cholinesterase characterization and use in monitoring anti-cholinesterases. A thesis in Environmental Toxicology. Graduate Faculty of Texas Tech University.
Sogorb MA, Vilanova E (2002) Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett 128(1–3):215–228
Sogorb MA, Ganga R, Vilanova E, Soler F (2007) Plasma phenylacetate and 1-napththylacetate hydrolyzing activities of wild birds as possible non-invasive biomarkers of exposure to organophosphorus and carbamates insecticides. Toxicol Lett 168:278–285
Strum KM, Alfaro M, Haase B, Hooper MJ, Johnson KA, Lanctot RB (2008) Plasma cholinesterases for monitoring pesticide exposure in nearctic-neotropical migratory shorebirds. Ornitologia Neotropical 19:641–651
Strum KM, Hooper MJ, Johnson KA, Lanctot RB, Zaccagnini ME, Sandercock BK (2010) Exposure of migratory shorebirds to cholinesterase-inhibiting contaminants in the Western Hemisphere. Condor 112:15–28
Thompson HM (1993) Avian serum esterases: species and temporal variations and their possible consequences. Chem Biol Interact 87:329–338
Thompson HM (1999) Esterases as markers of exposure to organophosphates and carbamates. Ecotoxicol 8(5):369–384
Van Lith HA, Van Zutphen LF, Beynen AC (1991) Butyrylcholinesterase activity in plasma of rats and rabbits fed high-fat diets. Comp Biochem Physiol 98:339–342
Van Lith HA, Meijer GW, van der Wouw MJA, Bieman M, van Tintelen G, van Zutphen FLM, Beynen AC (1992) Influence of amount of dietary fat and protein on esterase-1 (ES-1) activities of plasma and small intestine in rats. Br J Nutr 67:379–390
Wassmer B, Augenstein U, Ronai A, De Looze S, Von Deimling O (1988) Lymph esterases of the house mouse (Mus musculus)—II. The role of esterase-2 in fat resorption. Comp Biochem Physiol 91B:179–185
Westlake GE, Martin AD, Stanley PI, Walker CH (1983) Control enzyme levels in the plasma, brain and liver from wild birds and mammals in Britain. Comp Biochem Physiol 76:14–24
Wheelock CE, Miller JL, Miller MJ, Phillip BM, Huntley SA, Gee SJ, Tjeerdema RS, Hammock BD (2006) Use of carboxylesterase activity to remove pyrethroid-associated toxicity to Ceriodaphnia dubia and Hyalella azteca in toxicity identification evaluations. Environ Toxicol Chem 25:973–984
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178
Worek F, Thiermann H, Szinicz L, Eyer P (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 68:2237–2248
Acknowledgments
We thank Hayde and Lider Mignola from “El Gringo” tegu farm for sharing their time and knowledge with us. We also are grateful to Eberhard Küster and Robert E. Kramer for a critical reading of the manuscript. This work was supported partially by PICT-SECYT no. 1148.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Basso, A., Attademo, A.M., Lajmanovich, R.C. et al. Plasma esterases in the tegu lizard Tupinambis merianae (Reptilia, Teiidae): impact of developmental stage, sex, and organophosphorus in vitro exposure. Environ Sci Pollut Res 19, 214–225 (2012). https://doi.org/10.1007/s11356-011-0549-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0549-6