Abstract
Inhibition of cholinesterases has been frequently used as a biomarker for contamination of aquatic environments, because these enzymes are frequent targets for toxic effects of contaminants, such as insecticides derived from phosphoric and carbamic acids. However, this enzyme is also responsive to other contaminants, including metals. The use of cholinesterase inhibition as effect criterion in ecotoxicology studies requires the previous characterization of the specific enzymatic forms that can be present in the different tissues and/or organs of species. This work characterized the soluble ChEs present in the brain and dorsal muscle of three marine fish species, namely Scomber scombrus, Sardina pilchardus and Chelidonichthys lucerna. Pesticides (chlorpyrifos) and metals (copper sulphate) in vitro assays were conducted to quantify the effects of these contaminants on cholinesterases activity. The results of this study showed that acetylcholinesterase (AChE) was the predominant form present in the brain tissues of the three species and in the muscle tissue of one species (Sardina pilchardus). For Scomber scombrus and Chelidonichthys lucerna, the cholinesterase form present in the muscle tissue evidenced properties between the classic acetylcholinesterase and those of pseudocholinesterase forms. The results for the metal (copper) and pesticide (chlorpyrifos) showed that this species may be suitable for monitoring contaminations for these types of contaminants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cholinesterases (ChEs) are a family of enzymes designated as esterases. Among these, one may find specific forms, acetylcholinesterases (AChEs), which are crucial for nerve response and function (Thompson and Walker 1992). AChEs are capable of catalysing the hydrolysis of the neurotransmitter acetylcholine, a reaction necessary to prevent the accumulation of acetylcholine at the synaptic cleft and allow cholinergic neurons to return to their resting state after activation (Payne et al. 1996).
The activity of ChEs has been widely used as a marker of neural changes in organisms exposed via the environment to a large set of chemical contaminants. This use occurs since the inhibition of these enzymes may happen due to exposure of several species to varied environmental contaminants (Labrot et al. 1996; Payne et al. 1996; Guilhermino et al. 1998; Howcroft et al. 2010). However, the main focus of cholinesterase inhibition as effect criteria has been its use to assess the environmental presence and exposure of wild biota to anticholinesterasic pesticides, namely of the carbamate and organophosphate classes (Payne et al. 1996; Van der Oost et al. 2003). These chemicals act by specifically binding to the active site of AChE, blocking the access of the physiological substrate to this location, thereby impairing its activity (Koelle and Gilman 1949; Sanchez-Hernandez and Walker 2000).
AChE activity is thus the main target of organophosphate and carbamate pesticides, which block the function of this enzyme, causing the accumulation of acetylcholine in the synaptic cleft (Key and Fulton 2002; Caselli et al. 2006). Depression on the activity of ChEs (especially AChEs) often indicates sublethal toxicity and provides an early warning of detrimental effects caused by such chemicals in the wild (Day and Scott 1990).
It is possible that different cholinesterases may coexist in the same organism, but their relative abundance may change from tissue to tissue, being this factor related to their physiological role (Nunes et al. 2005; Xuereb et al. 2007; Nunes and Resende 2017). Different types of ChEs can be functionally distinct, according to their affinity for a specific substrate and to their susceptibility to selective inhibitors. In regard to differentiate the cholinesterasic forms present in each species and/or tissue, it is important to test them to analyse their sensitivity towards different substrates and inhibitors of cholinesterase activity. This same differential sensitivity is likely to happen towards environmental toxicants, and it is mandatory to know in advance the response of the ChEs of each species/tissue, before using them as test organisms or bioindicator species in environmental monitoring or testing.
The differentiation of cholinesterases, designated as their characterization, is a process aiming to identify their properties, according to already defined groups of cholinesterases, which share common properties. The major forms of cholinesterases are acetylcholinesterase (AChE, also known as true cholinesterases), butyrylcholinesterase (BChE) and propionylcholinesterase (PChE), being these forms commonly designated as pseudocholinesterases; their preferential substrates are acetylcholine, butyrylcholine and propionylcholine, respectively. The use of inhibitors also contributes for the identification of cholinesterasic forms; eserine is a general inhibitor of cholinesterases, BW284C51 (1,5-bis-(4allyldimethyl-ammoniumphenyl)-pentan-3-one-dibromide) is a specific inhibitor of acetylcholinesterase, and Iso-OMPA (tetraisopropylpyrophosphoramide) is a specific inhibitor of pseudocholinesterases.
The predominant types of ChEs vary across species. In most vertebrates, two types of ChEs have been identified, namely acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), whereas in invertebrates, this distinction is sometimes difficult to establish (Massoulié and Bon 1982; Karczmar 2010; Cacciatore et al. 2012). The occurrence of cholinesterases in living organisms is generally organ specific; while AChE is especially abundant in the central nervous system (where it plays a key role in the regulation of neuronal processes), BChE has a wider tissue distribution, and previous studies suggest that BChE could be involved in several processes such as detoxification, lipid metabolism, cell regeneration, neurogenesis, regulation of cell proliferation, and the beginning of differentiation during early neuronal development (Mack and Robitzki 2000). In addition, ChEs may also be extensively distributed across numerous tissues, such as serum, liver, heart, brain, vascular endothelia, and the nervous system (Leticia and Gerardo 2008; Valbonesi et al. 2011).
Accordingly, the variability and complexity of ChEs require their characterization before their use as biomarkers. Many studies have already shown the multiplicity of cholinesterasic forms in aquatic organisms, in distinct aquatic species. Cholinesterases from aquatic organisms have been already characterized in a diverse group of species such as fish, Lepomis gibbosus (Rodrigues et al. 2011), Haemulon plumieri (Leticia and Gerardo 2008), Gambusia holbrooki (Nunes et al. 2005), Gasterosteus aculeatus, Limanda limanda, Platichthys flesus and Serranus cabrilla (Sturm et al. 1999), and invertebrates, Solen marginatus, Diopatra neopolitana (Nunes and Resende 2017), Palaemonetes pugio (Key and Fulton 2002) and Gammarus pulex (Xuereb et al. 2007). However, the characterization of cholinesterases present in marine fish species are still scarce, and considering the future use of these animals in monitoring programs of the marine environment, their characterization is now of fundamental importance.
The main purpose of this work was to characterize the predominant cholinesterasic forms found in the muscle and brain tissue of three marine fish species, namely Scomber scombrus Linnaeus, 1758 (common name mackerel), Sardina pilchardus Walbaum, 1792 (common name sardine) and Chelidonichthys lucerna Linnaeus, 1758 (common name tub gurnard). These are marine fish species of commercial interest, being routinely captured by the commercial fishing fleet in Europe; this is extremely important since their availability is high, and no efforts of capture or collection are involved if these species are to be used in biomonitoring programs in the future. A secondary objective of this study was to analyse and compare the sensitivity of main cholinesterases of each species, determined in the previous step, towards specific contaminants, namely the metal copper (implicated in anticholinesterasic effects; Cunha et al. 2007; Nunes et al. 2011; De Lima et al. 2013) and the pesticide chlorpyrifos (an anticholinesterasic substance, highly toxic for marine fish, even at low concentrations; Humphrey et al. 2004).
Material and methods
Chemicals
Acetylthiocholine (≥ 98%), Bradford reagent, butyrylthiocholine (≥98%), chlorpyrifos (97% purity) 5,5′-dithio-bis(ϒ-nitrobenzoic acid) (DTNB; ≥ 98%), ethanol (99% pure), physostigmine salicylate (98%), propionylthiocholine (≥ 98), 1,5-bis(4-allyldimethyl ammoniumphenyl)–pentan-3-one-dibromide (BW284C51; 97%), tetraisopropylpyrophosphoramide (Iso-OMPA; 100%).
Test organisms
Individuals of the three species of fish were purchased in a local market in Aveiro, Portugal, immediately after the landing of the fish. These fish species were captured in the wild by the traditional commercial coastal fishing fleet that is established in the commercial harbour of Aveiro. Their capture was undertaken in adjacent areas and the distances to their capture locations were limited by the autonomy of commercial fishing vessels. The animals, after being captured, were kept at 4 °C until arrival to the commercial harbour of Aveiro. Consequently, only dead animals (despite being refrigerated) were used. Upon arrival at the commercial harbour, the animals were immediately transported to the laboratory. A total of 5 individuals with similar sizes (Scomber scombrus measured 25 ± 1 cm; Sardina pilchardus measured 24 ± 1 cm; Chelidonichthys lucerna measured 28 ± 1 cm) of each species were selected to develop all procedures. After being purchased, fish were transported in refrigerated plastic boxes to laboratory facilities; between capture and sampling process, no more than 12 h elapsed. This sampling procedure was already adopted by Nunes et al. (2011) for the sampling of marine test organisms.
Sample processing and enzymatic analysis
Immediately upon arrival, fish were dissected on ice, and the brain (wet weight 4 ± 0.5 g) and dorsal muscle (wet weight 5.5 ± 0.5 g) of each individual were isolated. Samples were homogenized separate in phosphate buffer (0.1 M, pH = 7.2) and after that the samples were centrifuged at 3300 g for 3 min, and the supernatants were recovered and diluted 1:300 with the same buffer. This pool of homogenized tissues was used in all subsequent in vitro testing procedures. The enzymatic assay involved the monitorization of ChEs activity for a period of 15 min, by the formation of the conjugate thiocoline (resulting from the hydrolytic degradation of the substrates acetylthiocholine/butyrylthiocholine/propionylthiocholine by cholinesterases) + DTNB. The absorbance of this conjugate (yellow colour) was measured at a wavelength of 414 nm, as described by Ellman et al. (1961). The protein concentration in samples was determined by the method described by Bradford (1976) adapted to microplate and γ-globulin 1 mg/mL use as a standard. Enzymatic results were expressed as nanomole of hydrolysed substrate per minute, per milligram of protein.
Characterization of cholinesterases
Regarding the characterization of cholinesterases, a tiered strategy was followed. The first step was to analyse the hydrolytic preference of cholinesterases from the distinct tissues of the three species. To attain this objective, three different substrates were tested, namely acetylthiocholine, butyrylthiocholine and propionylthiocholine (substrates that are preferentially hydrolysed by AChE, BChE, and PChE, respectively). For each substrate, 13 solutions were prepared, with the following concentrations: 0.005, 0.01, 0.02, 0.04, 0.08, 0.16, 0.32, 0.64, 1.28, 2.56, 5.12, 10.24, and 20.48 mM (these are the concentrations in the final volume of incubation).
The second step was to study the hydrolytic inhibitory profiles caused by generic and specific inhibitors of cholinesterases, true cholinesterases, and also of pseudocholinesterases. Solutions of 3 different inhibitors were prepared: (1) eserine (physiostigmine hemisulfate salt) at the concentrations of 6.25, 12.5, 25, 50, 100, and 200 μM, as general cholinesterases inhibitor; (2) BW284C51 at the concentrations of 6.25, 12.5, 25, 50, 100, and 200 μM as acetylcholinesterases inhibitor; and (3) Iso-OMPA, at the concentrations of 0.25, 0.5, 1, 2, 4, and 8 mM, as pseudocholinesterases (BChE + PChE) inhibitor.
The stock solutions of both eserine and BW284C51 were prepared with distilled water, while Iso-OMPA stock solution was prepared in ethanol, since this inhibitor is not easily soluble in water and is required to this co-solvent to be dispersed in the incubation media. Following the procedure described by Nunes et al. (2005), a volume of 5 μL of each inhibitor solution were incubated with 495 μl of tissue homogenate (brain and muscle) at room temperature 25 ± 1 °C for 20 min. All the exposures were performed in triplicate, in which 3 Eppendorf microtubes (with the pooled homogenized samples, obtained from both tissues) were incubated with the selected concentrations for each of the three inhibitors. For eserine and BW284C51 inhibitors, a volume of 5 μL of ultra-pure water was used as control. For the incubation with Iso-OMPA, an additional control, incubated only with ethanol, was added to the experimental design to test for possible effects caused by the carrier solvent. For these tests, the substrates that were selected were those for which higher affinity was found in the previous step. For each species, the concentration of substrates that were used was of 0.075 M, as optimized by Ellman et al. (1961). With the exception of muscle tissues of S. scomber (whose activity was measured using PCh as substrate—which was the most hydrolysed substrate), the cholinesterase activities of all other tissues from the three species were measured using ACh as substrate.
In vitro exposure to pesticides and metals
We also studied the effect of chlorpyrifos and copper (copper sulphate) in order to determine the effects of pesticides and metals on in vitro ChE activity, respectively. Additional incubations were performed, identical to those previously described for the cholinesterases inhibitors. These tests were carried with the substrates that showed higher affinity to each species, and the concentration of substrate used was 0.075 M, as optimized by Ellman et al. (1961). The pesticide used was chlorpyrifos, in 6 concentrations (12.5, 25, 50, 100, 200, and 400 μM, dissolved in acetone). Besides these concentrations, a control group with acetone was also added to the experimental design. For the assay with copper, copper sulphate was used, and 5 concentrations (3.125, 6.25, 12.5, 25 and 50 mgL−1) were tested. The stock solution of copper was prepared with distilled water. All these exposures occurred in triplicate, and three technical replicates were tested with a pool of each tissue, in which 5 μl of each concentration of toxicant +495 μl of cellular suspension were distributed by 3 Eppendorf microtubes per condition. These microtubes were incubated at room temperature 25 ± 1 °C for 20 min with each concentration of the pesticide, and of the metal.
Data analysis
The obtained data were previously analysed to assure normality and uniformity of variance (Shapiro-Wilk and Levene tests, respectively). ANOVA was performed followed by Dunnett’s multicomparison test to compare the control group with the treatment groups. The adopted level of significance was of p < 0.05 to discriminate differences against the control treatments.
Results
Cholinesterase characterization: substrates
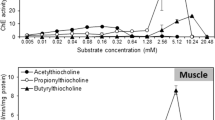
The cholinesterasic activity determined in the brain of the three different species is depicted in Fig. 1a, c and e. Cholinesterases activity of the brain tissue of all species showed higher affinity (with higher hydrolytic activity) towards acetylthiocholine as substrate. However, and despite this preference, the other two substrates were also extensively hydrolysed, but not in the same extent as acetylthiocholine. For the brain tissue of S. scombrus, the second most hydrolysed substrate was propionylthiocholine (Fig. 1a), followed by butyrylthiocholine; for the brain tissues of the two other species, S. pilchardus (Fig. 1c) and C. lucerna (Fig. 1e), data for butyrylthiocholine and propionylthiocholine were almost coincident.
Cholinesterase activities determined with different substrates: a brain of Scomber scombrus; b muscle of Scomber scombrus; c brain of Sardina pilchardus; d muscle of Sardina pilchardus; e brain of Chelidonichthys lucerna; f muscle of Chelidonichthys lucerna. ASCh acetylthiocholine, BSCh butyrylthiocholine, PSCh propionylthiocholine. Values are the mean of three replicates and corresponding standard deviation
Regarding the cholinesterases activity determined in the muscle tissues, results are depicted in Fig. 1 (b, d and f). ChEs muscle tissues from the two species S. pilchardus and C. lucerna showed higher affinity for acetylthiocholine as substrate, while S. scombrus showed higher affinity for propionylthiocholine as substrate. The calculated kinetic parameters (Vmax, Km and Vmax/Km) support the hypothesis for the preference of each enzyme of the three species (Table 1).
Cholinesterase characterization: inhibitors
The inhibitors eserine and BW284C51 showed higher inhibitory profiles than Iso-OMPA for both tissues (brain and muscle) of all species. For S. scombrus, brain tissues (Fig. 2a; F (6,14) = 6.3, p < 0.001) and muscle tissues (Fig. 2b; F (6,14) = 6.6, p < 0.001) were inhibited at the lowest concentration of eserine (6.25 μM). BW284C51 also caused inhibitory activities, at its lowest concentration (12.5 μM) for the brain (Fig. 2c; F (6,14) = 60.8; p < 0.001) and for all the concentrations in the muscle (Fig. 2d; F (6,14) = 10.1; p < 0.001) tissues. Yet for Iso-OMPA, the results were different; for the brain tissue (Fig. 2e; F (6,14) = 16.1, p = 0.708), Iso-OMPA caused no inhibitory effects, and for the muscle tissue (Fig. 2f; F (6,14) = 27.8, p < 0.001), it caused effects but only for the highest concentrations (2, 4 and 8 mM).
In vitro assay with cholinesterase inhibitors eserine, BW284C51 and Iso-OMPA. a brain of fish species with inhibitor eserine; b muscle of fish species with inhibitor eserine; c brain of fish species with inhibitor BW284C51; d muscle of fish species with inhibitor BW284C51; e brain of fish species with inhibitor Iso-OMPA; f muscle fish species with inhibitor Iso-OMPA. Ctrl, control group, incubation with water. Ctrl, EtOH ethanol control group, incubation with ethanol. Values correspond to the mean of three replicates and corresponding standard deviation bars. *Statistically significant differences, p < 0.05
The pattern of inhibition resulting from exposure to eserine, in the brain (Fig. 2a; F (6,14) = 3.8, p < 0.001) and muscle (Fig. 2b; F (6,14) = 0.15, p < 0.001) tissues of S. pilchardus, was similar to the one observed for S. scombrus; eserine caused an almost complete inhibition in both tissues. BW284C51 also showed a pattern like this; for the brain (Fig. 2c; F (6,14) = 13.8, p < 0.001) and muscle (Fig. 2d; F (6,14) = 0.078, p < 0.001) tissues, the inhibitory effect was notorious. Once again for Iso-OMPA, the results are different; for the brain (Fig. 2e; F (6,14) = 2.9, p < 0.001), all concentrations, except the lowest (0.25 mM), cause inhibitory effects, while for the muscle (Fig. 2f; F (6,14) = 0.49, p = 0.004), effects were only seen for the highest concentrations (8 mM).
Chelidonichthys lucerna presented a similar pattern for eserine as the other two species; for the brain (Fig. 2a; F (6,14) = 3.2; p < 0.001) and muscle (Fig. 2b; F (6,14) = 7.2; p < 0.001), the inhibitory effect was visible for all tested concentrations, showing a total inhibition. BW284C51 presented a total inhibition for the brain (Fig. 2c; F (6,14) = 0.74; p < 0.001); for the muscle (Fig. 2d; F (6,14) = 15.4, p < 0.001), all concentrations showed statistically significant differences, but the inhibitory profile was not so evident as the others. Concerning the inhibitory profile of the Iso-OMPA for the brain (Fig. 2e; F (6,14) = 1.9, p < 0.001), all the concentrations showed statistically significant differences and for the muscle (Fig. 2f; F (6,14) = 1.9, p < 0.001) as well, but the inhibition was much higher.
Chlorpyrifos
In general, all the three species showed to be sensitive to the pesticide chlorpyrifos. The brain (Fig. 3a; F (6,14) = 7.1, p < 0.001) and muscle (Fig. 3b; F (6,14) = 1.03, p < 0.001) tissues of S. scombrus presented statistically significant differences in all tested concentrations. However, for the muscle tissue, the inhibition was more evident, and results show an almost total inhibition of the cholinesterase activity.
In vitro effects of chlorpyrifos in the cholinesterases of fish species. a brain of Scomber scombrus; b muscle of Scomber scombrus; c brain of Sardina pilchardus; d muscle of Sardina pilchardus; e brain of Chelidonichthys lucerna; f muscle of Chelidonichthys lucerna. Ctrl AC, acetone control group, incubation with acetone. Values correspond to the mean of three replicates and corresponding standard deviation bars. *Statistically significant differences, p < 0.05
For S. pilchardus, not all tested concentrations showed effects. In the brain (Fig. 3c; F (6,14) = 7.8, p < 0.001), statistically significant differences were visible for all the concentrations, except one (50 μM), and the muscle (Fig. 3d; F (6,14) = 0.42, p < 0.001) only presented statistically significant differences for the highest concentrations (50, 100, 200 and 400 μM); in general, this was the less affected species.
Lastly, the species C. lucerna showed to be also sensitive to this pesticide. For the brain (Fig. 3e; F (6,14) = 2.09, p < 0.001), even the lowest concentration (12.50 μM) caused an inhibitory effect; a similar trend was also observed for the muscle tissue (Fig. 3f; F (6,14) = 5.9, p < 0.001), whose cholinesterase activity was highly inhibited.
Copper
Copper had effects that were dose dependent even in the lowest concentration (3.12 mg/L); for the brain (Fig. 4a; F (6,14) = 7.8, p < 0.001) tissue of S. scombrus and for the muscle (Fig. 4b; F (6,14) = 3.2, p < 0.001) tissue, this metal had no effects in the cholinesterase activity.
In vitro effects of copper in the cholinesterases of fish species. a brain of Scomber scombrus; b muscle of Scomber scombrus; c brain of Sardina pilchardus; d muscle of Sardina pilchardus; e brain of Chelidonichthys lucerna; f muscle of Chelidonichthys lucerna. Ctrl, control group, incubation with water. Values correspond to the mean of three replicates and corresponding standard deviation bars. *Statistically significant differences, p < 0.05
For S. pilchardus, the effects were made evident for both tissues. For the brain (Fig. 4c; F (6,14) = 6.3, p < 0.001), all concentrations tested had statistically significant effects in the cholinesterase activity; in the muscle (Fig. 4d; F (6,14) = 0.35, p < 0.001), the effects were only expressed for the 3 highest concentrations (12.5, 25, and 50 mg/L).
Copper changed the cholinesterase activity of the brain (Fig. 4e; F (6,14) = 1.6, p < 0.001) tissue of the C. lucerna for all concentrations, except for the lowest (3.12 mg/L); however, in the muscle (Fig. 4f; F (6,14) = 9.06, p < 0.001), the effects on the ChE activity were only present in the 3 highest concentrations (12.5, 25 and 50 mg/L).
Discussion
Cholinesterase characterization
The brain tissues of all tested species showed a preference for ASCh as substrate; for S. scombrus (Fig. 1a) and S. pilchardus (Fig. 1c), this preference was followed by PSCh and BSCH, while for C. lucerna (Fig. 1e), the second most hydrolysed substrate was BSCh, followed by PSCh; however, these differences in the hydrolytic activity of cholinesterases, obtained when using BSCh and PSCh as substrates and for S. pilchardus and C. lucerna, are almost imperceptible, and are likely to result from natural fluctuations caused by the experimental conditions. For S. pilchardus and C. lucerna, the hydrolytic degradation of acetylthiocholine was significantly higher than of the other two substrates. These data are consistent with previous studies. The majority of scientific works about this issue showed that acetylthiocholine is usually the most hydrolysed substrate by cholinesterases found in the nervous tissues of most vertebrates, namely fish. Rodrigues et al. (2011) observed that, for the head of the freshwater fish L. gibbosus, ASCh was the substrate of preference. Monteiro et al. (2005) concluded that for the head tissues of the fish species Pomatoschistus microps, cholinesterases showed more affinity for the ASCh when compared to other substrates. A study by Leticia and Gerardo (2008) also showed that for brain tissue of the marine fish species Haemulon plumeri, ASCh was the substrate of preference. Other studies done with fish species also support this result. Garcia et al. (2000) with Poecilia reticulata, Nunes et al. (2003) with Gambusia holbrooki, Osten et al. (2005) with Gambusia yucatana and also Sturm et al. (1999) with three species, Limanda limanda, Platichthys flesus and Serranus cabrilla, showed that ASCh was the most hydrolysed substrate by the mentioned species. This preference is not however limited to aquatic vertebrates. Scaps et al. (1996) obtained similar results for tissues of the polychaete species Nereis diversicolor.
The muscle tissues had a distinct behaviour in terms of substrate preferences, and no uniformity was reported, similar to the one described for brain tissues. For S. pilchardus (Fig. 1d) and C. lucerna (Fig. 1f), similarly to what occurred for the brain tissue, the substrate of preference was ASCh. For S. pilchardus, this result was more evident, since the hydrolytic degradation of acetylthiocholine was significantly higher compared to the other two substrates. Many studies from the literature showed similar results were ASCh was the preferential substrate in the muscle tissue. Rodrigues et al. (2011) identified ASCh as the preferential substrate of the muscle tissue of the fish species L. gibbosus. Similarly, this tendency was reported in the muscle tissue of the fish Haemulon plumeri (Leticia and Gerardo 2008). Some studies showed an identical pattern in larvae of the marine fish Sparus aurata (Arufe et al. 2007) in which cholinesterases had higher affinity towards ASCh as substrate. In addition to fish, this trend seems also to occur in invertebrates. Other studies also identified ASCh as the substrate of preference but in whole body homogenates of the freshwater snails Potamopyrgus antipodarum and Valvata piscinalis (Gagnaire et al. 2008).
However, for S. scombrus (Fig. 1b), the preferential substrate was PSCh; this result, despite not being a common feature of vertebrate species, is not totally surprising. Results from the literature have shown that there are other species showing preference for PSCh as substrate. This trend was observed in Eisenia fetida, as demonstrated by Scaps et al. (1996). This study showed that this preference for PSCh occurred for three different tissues, head, body, and whole animal. Nunes and Resende (2017) obtained comparable results in whole body homogenates of the mollusc S. marginatus. The mollusc Cerastoderma glaucum was also demonstrated to possess cholinesterasic forms whose activity was higher when PSCh was used, as shown by Jebali et al. (2011). Similarly, this same trend was observed in tissues of the freshwater clam species Corbicula fluminea (Mora et al. 1999). A similar finding was obtained by Talesa et al. (1990), in tissues of the marine mollusc Bolinus brandaris. A study with Daphnia magna Straus showed that cholinesterases of this species demonstrated higher affinity for PSCh as substrate (Diamantino et al. 2003).
In terms of the anticholinesterasic effects caused by inhibitors, the incubation of brain tissues from all the three species showed a consistent pattern, in which a significant inhibition of the cholinesterasic activity was attained even at the lowest concentration of eserine (6.25 μM) (Fig. 2a). Knowing that eserine is a generic inhibitor of cholinesterases, it is possible to suggest that the predominant form present in the brain tissues of the three species is a cholinesterase, rather than a nonspecific esterase. Exposure of brain tissue homogenates of all three species to BW284C51 (the specific inhibitor of acetylcholinesterases) also yielded an almost complete inhibition of all enzymatic hydrolytic activity, although for S. scombrus this inhibition was more evident for the highest concentration of this inhibitor (Fig. 2c). On the contrary, no uniform trend was envisaged after incubating brain tissues homogenates of the three species with Iso-OMPA. No inhibitory effect was observed after the incubation of the brain tissue homogenates of S. scombrus with this compound (Fig. 2e). For S. pilchardus, inhibitory effects were observed for all concentrations of Iso-OMPA, with the exception of the lowest. Concerning C. lucerna, inhibitory effects were observed for all concentrations. Although Iso-OMPA was capable of showing significant inhibitory effects, this only happened for high concentrations, and not following the same inhibitory trend that was observed for the other two inhibitors. In addition, the inhibition caused by exposure to Iso-OMPA was not complete, and incubations with this chemical only attained partial inhibition of the cholinesterases present in homogenates of tissues of the three tested species.
This may have several fundaments. Iso-OMPA did indeed inhibit cholinesterasic forms of all species. At first, this could be somewhat surprising, since the previous assumptions indicate that the predominant cholinesterasic form in tissues of the three fish was AChE. However, this effect only happened at concentrations of Iso-OMPA that were much higher than those of eserine and of BW28C51 (Nunes and Resende 2017). There are no totally specific inhibitors of each of the three distinct cholinesterasic forms. In fact, overlapping activities in terms of the inhibition capacity of each inhibitor were already described (Bhakta et al. 2016). This result is an additional indication that S. scombrus, S. pilchardus, and C. lucerna brain tissues possess a predominance of acetylcholinesterase, supported by the significant inhibition by eserine and BW284C51, and for the absence of strong, dose-dependent inhibitory effects after the incubation with Iso-OMPA. The result here obtained is in agreement with many others from the literature. Rodrigues et al. (2011) obtained similar results for two tissues (brain and muscle) of L. gibbosus and concluded that acetylcholinesterase was the main cholinesterasic form in this tissue. A similar conclusion was obtained by Leticia and Gerardo (2008), who showed that AChE was the main ChE found in the brain of H. plumieri. Another result in agreement with the here obtained data is the one from Frasco et al. (2008), showing that in the eyes of the shrimp Palaemon serratus, the main cholinesterasic form was AChE. Other studies supporting our assumptions were also performed with other species, such as Poecilia reticulata (Garcia et al. 2000), G. holbrooki (Nunes et al. 2003), G. yucatana (Osten et al. 2005) and Limanda limanda, Platichthys flesus and Serranus cabrilla (Sturm et al. 1999).
Incubation of muscle homogenates of S. pilchardus and C. lucerna with eserine yielded a significant inhibition of its cholinesterasic activity. This effect, similarly to what occurred for brain tissue, occurred even at the lowest concentration of eserine (6.25 μM) (Fig. 2b). The hydrolytic behaviour of muscle cholinesterases of the three species after the incubation with BW284C51 was made clear (Fig. 2d). There was a progressive, dose-response inhibition of the cholinesterasic activity, which was almost complete for low levels of exposure in the case of S. pilchardus. However, this effect was not so prominent for the muscle tissue of S. scombrus and C. lucerna. The incubation of the muscle tissue with Iso-OMPA resulted in inhibitory effects for the three highest concentrations (2, 4, and 8 mM) for S. scombrus (Fig. 2f), while in muscle homogenates of S. pilchardus, it was possible to observe an inhibition, but only for the highest concentration of this same inhibitor (8 mM). For muscle homogenates of C. lucerna, the incubation with Iso-OMPA resulted in inhibitory effects for all concentrations tested.
For S. pilchardus, the hydrolytic behaviour of the muscle ChE allows to classify its cholinesterases as AChE because it preferentially hydrolyses ASCh, being extremely sensitive to eserine and BW284C51; in addition, the activity of this enzymatic form was not significantly compromised by Iso-OMPA. Valbonesi et al. (2011) performed a study in Anguilla anguilla and similarly concluded that AChE was the predominant ChE form in the analysed tissues. In addition, the study performed by Varo et al. (2007) with Aphanius iberus indicated that AChE was the predominant form present in both sexes and tissues of this fish species. Ramos et al. (2013) obtained comparable results for the marine crustacean Pollicipes pollicipes.
The incubation of the muscle tissue of S. scombrus was made with the substrate PSCh, because of the higher affinity demonstrated by its cholinesterases towards this substrate. It is important however to note that the hydrolytic rate for the two other substrates was not much lower in this tissue and species, a trend that was not followed by the other tissues of the three species, for which strong and clear preferences for one of the substrates (especially for acetylthiocholine) were always noticed. Following this modification, muscle ChEs of this species were inhibited both by eserine and by BW284C51. Based on these results, it is possible to assume that muscle S. scombrus ChEs consisted mainly of AChE, despite being able to effectively degrade propionylthiocholine. This same tendency was observed by Caselli et al. (2006), when analysing the properties of cholinesterases from the earthworm Eisenia andrei. Additionally, the incubation of the muscle tissues with Iso-OMPA showed inhibitory effects, but only for the highest concentrations (2, 4, and 8 mM).
In summary, the results obtained for the muscle tissue of S. scombrus indicate the presence of a generalist cholinesterase, and not a specific form. This cholinesterase form shows intermediate properties, since its hydrolytic profile shows preference for propionylthiocholine, but the pattern of inhibition after using inhibitory compounds revealed an acetylcholinesterase-like behaviour. This type of results is not totally sporadic, and some data similar to these here mentioned were already documented in the literature. In muscle of fish, atypical ChEs displays like this have been previously reported, namely by Rodríguez-Fuentes et al. (2008), in the fish Pleuronectes vertulus and Pleuronichthys verticalis, and a similar effect was shown to occur in the fish Gasterosteus aculeatus (Sturm et al. 1999). Similar data are quite common for invertebrate species such as the mollusc Corbicula fluminea (Ramos et al. 2012) and the crustacean Daphnia magna (Diamantino et al. 2003).
The muscle tissue of C. lucerna presented quite different results. The substrate preference was for ASCh, but this preference was closely followed by the hydrolytic activity demonstrated when using the substrate PSCh. In addition, these muscle ChEs forms were not as sensitive to the inhibitor BW284C51 as those from the other two species, and their activity was compromised by Iso-OMPA in all its concentrations. It is thus not possible to conclude that this is a classic acetylcholinesterase. The cholinesterasic form present in the muscle tissue of C. lucerna has a hydrolytic activity between that of classic acetylcholinesterases and pseudocholinesterases, a pattern already described to occur by Gagnaire et al. (2008) in the freshwater snail P. antipodarum.
Inhibitory effects caused by chlorpyrifos
The cholinesterase inhibition of both brain and muscle tissues after in vitro exposures to chlorpyrifos showed that this organophosphate pesticide has the capacity of exerting significant effects in the three species. For S. scombrus, this pesticide caused effects in all the concentrations tested; however, for the muscle tissue, its sensitivity was more evident (Fig. 3a and b), since the inhibitory effects were more pronounced at lower concentrations of this pesticide. Tissues of S. pilchardus showed different results; in the brain, all concentrations of the pesticide caused inhibitory effects, except for the concentration of 50 μM (Fig. 3c); while in the muscle tissue, the inhibitory effects occurred only for the four highest concentrations (50, 100, 200 and 400 μM) (Fig. 3d). C. lucerna showed a pattern similar to that of S. scombrus, since the pesticide caused effects in all tested concentrations (Fig. 3e and f); however, in the muscle tissue, the inhibitory effect was more evident and occurred at lower levels of pesticide.
Acetylcholinesterase is considered a specific biomarker for organophosphate and carbamate pesticides (Leticia and Gerardo 2008; Costa 2013; Whitehead et al. 2005). Numerous studies reported a reduction in enzyme activity caused by this type of compounds in aquatic organisms. Alves et al. (2015) observed results similar to these in the brain and muscle of the shark Prionace glauca exposed to chlorpyrifos-oxon. A study conducted by Assis et al. (2010) concluded that individuals of the fish species Colossoma macropomum after being exposed to three organophosphates (dichlorvos, chlorpyrifos and tetraethyl pyrophosphate) showed inhibition of their ChEs activity. Other studies conducted by Karen et al. (1998, 2001) showed that even low concentrations of chlorpyrifos were enough to produce a strong inhibition of ChEs activity. Wheelock et al. (2005) found that young Chinook salmon, Oncorhynchus tshawytscha, exposed to chlorpyrifos at concentrations of 7.3 μg/l exhibited a ChE inhibition as high as 85% in the brain, and of 92% in the muscle. Varó et al. (2002) obtained a significant inhibition after exposing for 24 h two species of crustaceans, Artemia salina and Artemia parthenogenica, to all the tested concentrations of chlorpyrifos. Xuereb et al. (2007) and Barata et al. (2004) found similar results but with the crustacean Gammarus pulex and Daphnia magna, respectively. Samnuek et al. (2007) tested the responsiveness of the brain and muscle tissues of two species of Hydra catfish (Clarias macrocephalus and Clarias gariepinus) and showed that chlorpyrifos could indeed compromise their hydrolytic activity, being the brain tissue more sensitive than the muscle.
Such inhibitory effects may have consequences at higher levels of physiological integration, namely behaviour. By compromising behavioural traits, these anticholinesterasic effects may compromise the ecosystem balance, forcing ecological consequences of unpredictable consequences. To address this thematic, Sandahl et al. (2005) performed a study involving the exposure of the fish Oncorhynchus kisutch to chlorpyrifos. The endpoints of this study were changes in feeding behaviour and swimming, and also quantifying the effects of chlorpyrifos on AChE activity, to establish causal relationships between exposure to this neurotoxicant, impairment of neuronal regulation and behavioural alterations. The authors concluded that chlorpyrifos inhibited tissue AChE activity, and compromised all behavioural traits in a dose-dependent manner.
Some studies have showed that AChE activity can be linked to alterations on the behaviour. Many authors showed modifications on the swimming performance of fish. Van Dolah et al. (1997) showed modifications on the swimming stamina of juvenile red drum (Sciaenops ocellatus) and of the mummichog (Fundulus heteroclitus) exposed to azinphosmethyl, Kumar and Chapman (1998) in eastern rainbow fish (Melanotaenia duboulayi) exposed to profenofos, and Beauvais et al. (2000) in larval rainbow trout (Oncorhynchus mykiss) exposed to diazinon and malathion. These effects are not however restricted to fish, and crustaceans also seem to be sensitive to these types of anticholinesterasic pesticides, evidencing strong behavioural alterations. Xuereb et al. (2007) performed a study in Gammarus fossarum exposed to chlorpyrifos and methomyl and established a relationship between the AChE activity and behaviour alterations. The authors concluded that, for both compounds, a concentration-dependent decrease was observed for the AChE activity and behavioural parameters. The author also reported that the feeding rate and locomotion impairment were directly correlated to levels of AChE inhibition for both pesticides. Rao et al. (2005) demonstrated that a period of 20 days of exposure to sublethal concentrations of chlorpyrifos were causative of changes in the locomotor behaviour of Gambusia affinis. The authors thus concluded that the triggering factor for this behavioural change was the inhibition of acetylcholinesterase activity. This type of effects can translate into alterations in a variety of other factors such as food seeking, predator avoidance, migration and also reproduction, which might be impaired, and have important deleterious alterations in higher levels of biological organization. So, we may assume that this is an important parameter, with extreme relevance in ecological terms, since individual alterations may translate into population changes of unpredictable nature and consequences.
Inhibitory effects caused by copper sulphate
Metals are natural compounds which are widely used nowadays; however, their interaction with living organisms can lead to harmful effects, namely with regard to toxicity on the central nervous system of exposed organisms. This neurotoxic effect is commonly a consequence of impairment of cholinesterase activity (Guilhermino et al. 1998; Mora et al. 1999; Castro et al. 2004; Rodríguez-Fuentes et al. 2008; Li 2008; Vieira et al. 2009). The here-obtained data showed that the metal copper, in general, was capable of causing a significant impairment of cholinesterase activity of all species and tissues. In vitro exposure to copper sulphate resulted in the inhibition of cholinesterase activity in all species, but not equally for all tissues. Results obtained for the species S. scombrus were ambiguous, because the metal caused effects in the brain tissue for all the concentrations tested (Fig. 4a), but muscle tissue did not evidence any significant alteration (Fig. 4b). In individuals of S. pilchardus, exposure to copper caused effects in both tissues. In the brain, effects were observed for all concentrations (Fig. 4c), while in the muscle, the effects were only reported for the three higher concentrations (12.5, 25 and 50 mg/L) (Fig. 4d). For C. lucerna, differences were reported between the two tissues. For the brain tissue, copper caused inhibitory effects for all concentrations, except for the lowest (Fig. 4e). In the muscle tissue, this metal only caused effect for the three highest concentrations (12.5, 25 and 50 mg/L) (Fig. 4f).
This set of results is in agreement with an already published data on cholinesterasic impairment by copper in aquatic organisms. Studies corroborating our data were published by Garcia et al. (2000), evidencing the inhibitory effects caused by copper in Poecilia reticulata. Pereira et al. (2019) also showed that copper could cause an in vitro inhibition of muscle acetylcholinesterase of the freshwater fish Astyanax altiparanae, Phalloceros harpagos and Pterygoplichthys pardalis. In addition, Cunha et al. (2007) showed that in vitro exposure to Cu2+ caused an inhibition of ChE activity in the molluscs Monodonta lineata and Nucella lapillus. Frasco et al. (2005) demonstrated that several metals could inhibit AChE activity, such as mercury, cadmium, copper, and zinc in purified AChE of the fly Drosophila melanogaster. In many aquatic organisms, ChEs have been found to be inhibited by copper in both in vivo and in vitro conditions (Garcia et al. 2000; Castro et al. 2004; Roméo et al. 2006; Rodríguez-Fuentes et al. 2008; Vieira et al. 2009). However, this effect by copper may not be assumed as a general rule for all metals, since some metallic compounds did not cause any effects (Varo et al. 2007; Frasco et al. 2008). In some cases, exposure to metals resulted in even increased levels of ChE activity (Dethloff et al. 1999; Romani et al. 2003). These results are somehow contradictory, and this can be related with the interference that the metals may have in the methodology used for the determination of ChE activity. Frasco et al. (2005) suggested that this pattern may be an artifact of the Ellman’s method. Some authors proposed that the apparent inhibition of ChEs caused by metals may not derive from interference with the catalytic activity of the enzyme; it may instead derive from an interference with a particle present in the reactive medium use for the determination of the enzymatic assay, namely 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB). Ellman et al. (1961) method is the most common methodology used for determination of ChE activity. In this method, the enzymatic catalytic activity is determined based on the degradation of acetylthiocholine (substrate) resulting in acetate and thiocholine, and the latter product complexes with DTNB (dithiobisnitrobenzoate), giving rise to a coloured compound (yellow colour). Metals can react with all metallic ions present in the reaction medium, reducing the formation of the coloured compound and therefore leading to the false assumption that ChEs are being inhibited by metals (Frasco et al. 2005; Nunes 2011).
Cholinesterase inhibition and behavioural traits
Cholinesterase inhibitory effects may have consequences at higher levels of physiological integration, namely behaviour, especially considering the role of these enzymatic forms in the central integration of nervous impulses, and the effector responses of muscular innervation at neuromuscular junctions (Nunes 2011). By compromising behavioural traits, these anticholinesterasic effects may compromise the ecosystem balance, forcing ecological consequences of unpredictable consequences. Consequently, the importance of the inhibition of cholinesterases by specific xenobiotics is not restricted to in vitro studies. However, this approach is mandatory to establish mechanistic linkages between environmental pollutants (such as those studied in this work) and putative adverse effects that may occur in the wild, namely involving behavioural changes. To address this thematic, Sandahl et al. (2005) performed a study involving the exposure of the fish Oncorhynchus kisutch to chlorpyrifos and observed a strong AChE inhibition that was associated to deleterious changes in feeding behaviour and swimming, in a dose-dependent manner. These data are not surprising, since many others authors showed modifications on the swimming performance of fish. Van Dolah et al. (1997) showed modifications on the swimming stamina of juvenile red drum (Sciaenops ocellatus) and of the mummichog (Fundulus heteroclitus) exposed to azinphosmethyl. Kumar and Chapman (1998) reported swimming modifications in eastern rainbow fish (Melanotaenia duboulayi) exposed to profenofos. Similar findings were reported by Beauvais et al. (2000) in larval rainbow trout (Oncorhynchus mykiss) exposed to diazinon and malathion. Rao et al. (2005) demonstrated that a period of 20 days of exposure to sublethal concentrations of chlorpyrifos were causative of changes in the locomotor behaviour of Gambusia affinis. The authors thus concluded that the triggering factor for this behavioural change was the inhibition of acetylcholinesterase activity. These effects are not however restricted to fish, and crustaceans also seem to be sensitive to these types of anticholinesterasic pesticides, evidencing strong behavioural alterations. Xuereb et al. (2007) exposed Gammarus fossarum to chlorpyrifos and methomyl, and established a relationship between the AChE activity and behaviour alterations, namely feeding rate and locomotion impairment, parameters that were directly correlated to levels of AChE inhibition for both pesticides. Despite not being possible, solely based on our data, to extrapolate to putative ecological consequences, it is important to fully describe mechanisms of toxic action that may underlie behavioural alterations. Based on our results, it is possible to unequivocally assume that chlorpyrifos and copper may exert adverse, inhibitory effects on cholinesterases of the tested species. It is also possible to hypothesize that these effects, as demonstrated by previous studies described in the literature, are likely to end up in behavioural alterations. This type of behavioural effects can translate into alterations in a variety of other interactions, such as food seeking, predator avoidance, migration and also reproduction. The impairment of such traits may have important deleterious alterations in higher levels of biological organization. So, we may assume that this is an important parameter, with extreme relevance in ecological terms, since individual alterations may translate into population changes of unpredictable nature and consequences. The putative relationship among environmental pollutants, effects of specific enzymatic forms, and adverse alterations in hydrolytic activities is thus mandatory to predict the type and extent of the most likely consequences if these chemicals indeed occur in the wild. Our study established a potential mechanistic link between adverse effects of two widespread environmental pollutants on the most significant forms of cholinesterases that occur in two tissues of three marine fish species and potential behavioural changes that these organisms may show.
Conclusions
The results from this study strongly suggest that the three species under study have not the same type of ChE form. The brain tissues of all the three species presented AChE as the main ChE form, and these forms were highly sensitive to eserine and BW284C51, and showed almost no inhibitory effects when exposed to Iso-OMPA. For the muscle tissue, the pattern is not so evident. Scomber scombrus, besides showing high preference for PSCh has substrate, also showed inhibitory profiles that suggest the existence of an atypical form of ChE. Sardina pilchardus showed a consistent pattern for the muscle tissue, similarly to what was shown for the brain tissue, since the results suggest that the main ChE form of both tissues of S. pilchardus is AChE. The brain tissue of C. lucerna has mainly an acetylcholinesterase as its predominant form; on the contrary, data on the muscle tissue of this species suggest otherwise, since the inhibitory profiles (partial inhibition caused by BW284C51 and also by Iso-OMPA) are not consistent with the predominance of AChE, being instead somewhere between the classic acetylcholinesterase and pseudocholinesterase forms. The data concerning pesticides and metals allows to conclude that the species may be suitable for monitoring pesticides and metals contaminations and can be used as test organisms in marine ecotoxicology, since the in vitro ChE sensitivity towards metals and pesticides showed inhibitory profiles. Although the different sensibilities of species and also they different habitat has to be considered since they can have different responses to que same contaminant.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alves LM, Lemos MFL, Correia JPS, da Costa NAR, Novais SC (2015) The potential of cholinesterases as tools for biomonitoring studies with sharks: biochemical characterization in brain and muscle tissues of Prionace glauca. J Exp Mar Biol Ecol 465:49–55
Arufe MI, Arellano JM, García L, Albendín G, Sarasquete C (2007) Cholinesterase activity in gilthead seabream (Sparus aurata) larvae: characterization and sensitivity to the organophosphate azinphosmethyl. Aquat Toxicol 84(3):328–336
Assis CRD, Castro PF, Amaral IPG, Carvalho EVMM, Carvalho LB, Bezerra RS (2010) Characterization of acetylcholinesterase from the brain of the Amazonian tambaqui (Colossoma macropomum) and in vitro effect of organophosphorus and carbamate pesticides. Environ Toxicol Chem 29(10):2243–2248
Barata C, Solayan A, Porte C (2004) Role of B-esterases in assessing toxicity of organophosphorus (chlorpyrifos, malathion) and carbamate (carbofuran) pesticides to Daphnia magna. Aquat Toxicol 66(2):125–139
Beauvais SL, Jones SB, Brewer SK, Little EE (2000) Physiological measures of neurotoxicity of diazinon and malathion to larval rainbow trout (Oncorhynchus mykiss) and their correlation with behavioral measures. Environ Toxicol Chem 19(7):1875–1880
Bhakta HK, Park CH, Yokozawa T, Min B-S, Jung HA, Choi JS (2016) Kinetics and molecular docking studies of loganin, morroniside and 7-O-galloyl-d-sedoheptulose derived from Corni fructus as cholinesterase and β-secretase 1 inhibitors. Arch Pharm Res 39(6):794–805
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cacciatore LC, Kristoff G, Verrengia Guerrero NR, Cochón AC (2012) Binary mixtures of azinphos-methyl oxon and chlorpyrifos oxon produce in vitro synergistic cholinesterase inhibition in Planorbarius corneus. Chemosphere 88(4):450–458
Caselli F, Gastaldi L, Gambi N, Fabbri E (2006) In vitro characterization of cholinesterases in the earthworm Eisenia andrei. Comp Biochem Physiol C Toxicol Pharmacol 143(4):416–421
Castro BB, Sobral O, Guilhermino L, Ribeiro R (2004) An in situ bioassay integrating individual and biochemical responses using small fish species. Ecotoxicology 13(7):667–681
Costa LG (2013) Chapter 22: toxic effects of pesticides in Casarett and Doull’s toxicology: the basic science of poisons, 8th edn. McGrawHill Education, New York
Cunha I, Mangas-Ramirez E, Guilhermino L (2007) Effects of copper and cadmium on cholinesterase and glutathione S-transferase activities of two marine gastropods (Monodonta lineata and Nucella lapillus). Comp Biochem Physiol C Toxicol Pharmacol 145(4):648–657
Day KE, Scott IM (1990) Use of acetylcholinesterase activity to detect sublethal toxicity in stream invertebrates exposed to low concentrations of organophosphate insecticides. Aquat Toxicol 18(2):101–113
De Lima D, Roque GM, de Almeida EA (2013) In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Mar Environ Res 91:45–51. https://doi.org/10.1016/j.marenvres.2012.11.005
Dethloff GM, Schlenk D, Hamm JT, Bailey HC (1999) Alterations in physiological parameters of rainbow trout (Oncorhynchus mykiss) with exposure to copper and copper/zinc mixtures. Ecotoxicol Environ Saf 42(3):253–264
Diamantino TC, Almeida E, Soares AMVM, Guilhermino L (2003) Characterization of cholinesterases from Daphnia magna Straus and their inhibition by zinc. Bull Environ Contam Toxicol 71(2):219–225
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2005) Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 10(5):360–375
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2008) Does mercury interact with the inhibitory effect of dichlorvos on Palaemon serratus (Crustacea: Decapoda) cholinesterase? Sci Total Environ 404(1):88–93
Gagnaire B, Geffard O, Xuereb B, Margoum C, Garric J (2008) Cholinesterase activities as potential biomarkers: characterization in two freshwater snails, Potamopyrgus antipodarum (Mollusca, Hydrobiidae, smith 1889) and Valvata piscinalis (Mollusca, Valvatidae, Müller 1774). Chemosphere 71(3):553–560
Garcia LM, Castro B, Ribeiro R, Guilhermino L (2000) Characterization of cholinesterase from guppy (Poecilia reticulata) muscle and it’s in vitro inhibition by environmental contaminants. Biomarkers 5(4):274–284
Guilhermino L, Barros P, Silva MC, Soares AMVM (1998) Should the use of inhibition of Cholinesterases as a specific biomarker for organophosphate and carbamate pesticides be questioned. Biomarkers 3(2):157–163
Howcroft CF, Gravato C, Amorim MJB, Novais SC, Soares AMVM, Guilhermino L (2010) Biochemical characterization of cholinesterases in Enchytraeus albidus and assessment of in vivo and in vitro effects of different soil properties, copper and phenmedipham. Ecotoxicology 20(1):119–130
Humphrey CA, Klumpp DW, Raethke N (2004) Ambon damsel (Pomacentrus amboinensis) as a bioindicator organism for the great barrier reef: responses to Chlorpyrifos. Bull Environ Contam Toxicol 72(5):888–895
Jebali J, Ben-Khedher S, Kamel N, Ghedira J, Bouraoui Z, Boussetta H (2011) Characterization and evaluation of cholinesterase activity in the cockle Cerastoderma glaucum. Aquat Biol 13:243–250. https://doi.org/10.3354/ab00365
Karczmar AG (2010) Cholinesterases (ChEs) and the cholinergic system in ontogenesis and phylogenesis, and non-classical roles of cholinesterases – a review. Chem Biol Interact 187(1–3):34–33
Karen DJ, Draughn R, Fulton M, Ross PE (1998) Bone strength and acetylcholinesterase inhibition as endpoints in chlorpyrifos toxicity to Fundulus heteroclitus. Pestic Biochem Physiol 60:167–175
Karen DJ, Klaine SJ, Ross PE (2001) Further considerations of the skeletal system as a biomarker of episodic chlorpyrifos exposure. Aquat Toxicol 52(3–4):285–296
Key PB, Fulton MH (2002) Characterization of cholinesterase activity in tissues of the grass shrimp (Palaemonetes pugio). Pestic Biochem Physiol 72(3):186–192
Koelle GB, Gilman A (1949) Anticholinesterase drugs. J Pharmacol Exp Ther 95:166–216
Kumar A, Chapman JC (1998) Profenofos toxicity to the eastern rainbow fish (Melanotaenia duboulayi). Environ Toxicol Chem 17(9):1799–1806
Labrot F, Ribera D, Denis MS, Narbonne JF (1996) In vitro and in vivo studies of potential biomarkers of lead and uranium contamination: lipid peroxidation, acetylcholinesterase, catalase and glutathione peroxidase activities in three non-mammalian species. Biomarkers 1(1):21–28
Leticia A-G, Gerardo G-B (2008) Determination of esterase activity and characterization of cholinesterases in the reef fish Haemulon plumieri. Ecotoxicol Environ Saf 71(3):787–797
Li M-H (2008) Effects of nonionic and ionic surfactants on survival, oxidative stress, and cholinesterase activity of planarian. Chemosphere 70(10):1796–1803
Mack A, Robitzki A (2000) The key role of butyrylcholinesterase during neurogenesis and neural disorders: an antisense-5’butyrylcholinesterase-DNA study. Prog Neurobiol 60(6):607–628
Massoulié J, Bon S (1982) The molecular forms of cholinesterase and Acetylcholinesterase in vertebrates. Annu Rev Neurosci 5(1):57–106
Monteiro M, Quintaneiro C, Morgado F, Soares AMVM, Guilhermino L (2005) Characterization of the cholinesterases present in head tissues of the estuarine fish Pomatoschistus microps: application to biomonitoring. Ecotoxicol Environ Saf 62(3):341–347
Mora P, Fournier D, Narbonne J-F (1999) Cholinesterases from the marine mussels Mytilus galloprovincialis Lmk. And M. edulis L. and from the freshwater bivalve Corbicula fluminea Müller. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 122(3):353–361
Nunes B (2011) The use of Cholinesterases in ecotoxicology. Rev Environ Contam Toxicol:29–59. https://doi.org/10.1007/978-1-4419-8453-1_2
Nunes B, Resende ST (2017) Cholinesterase characterization of two autochthonous species of Ria de Aveiro (Diopatra neapolitana and Solen marginatus) and comparison of sensitivities towards a series of common contaminants. Environ Sci Pollut Res 24(13):12155–12167
Nunes B, Carvalho F, Guilhermino L (2003) Characterization of total head cholinesterases of Gambusia holbrooki (mosquitofish), and the assessment of effects induced by two environmental contaminants. J Vet Pharmacol Ther 26:260–261
Nunes B, Carvalho F, Guilhermino L (2005) Characterization and use of the total head soluble cholinesterases from mosquitofish (Gambusia holbrooki) for screening of anticholinesterase activity. J Enzyme Inhib Med Chem 20(4):369–376
Nunes BS, Rodrigues S, Antunes SC, Castro BB, Gonçalves F (2011) The use of biomarkers to assess aquatic pollution by widespread agents (detergents – SDS; pesticides – chlorfenvinphos): feasibility, responsiveness and biological consequences in fish. Toxicol Lett 205:S67
Osten JR, Ortíz-Arana A, Guilhermino L, Soares AMVM (2005) In vivo evaluation of three biomarkers in the mosquitofish (Gambusia yucatana) exposed to pesticides. Chemosphere 58(5):627–636
Payne JF, Mathieu A, Melvin W, Fancey LL (1996) Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar Pollut Bull 32(2):225–231
Pereira BVR, Silva-Zacarin ECM, Costa MJ, Dos Santos ACA, Carmo JB, Nunes B (2019) Cholinesterases characterization of three tropical fish species, and their sensitivity towards specific contaminants. Ecotoxicol Environ Saf 173:482–493
Ramos AS, Gonçalves F, Antunes SC, Nunes B (2012) Cholinesterase characterization in Corbicula fluminea and effects of relevant environmental contaminants: a pesticide (chlorfenvinphos) and a detergent (SDS). J Environ Sci Health B 47(6):512–519
Ramos AS, Antunes SC, Gonçalves F, Nunes B (2013) The gooseneck barnacle (Pollicipes pollicipes) as a candidate sentinel species for coastal contamination. Arch Environ Contam Toxicol 66(3):317–326
Rao J, Begum G, Pallela R, Usman P, Rao R (2005) Changes in behavior and brain Acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal exposure to chlorpyrifos. Int J Environ Res Public Health 2(3):478–483. https://doi.org/10.3390/ijerph2005030013
Rodrigues SR, Caldeira C, Castro BB, Gonçalves F, Nunes B, Antunes SC (2011) Cholinesterase (ChE) inhibition in pumpkinseed (Lepomis gibbosus) as environmental biomarker: ChE characterization and potencial neurotoxic effects of xenobiotics. Pestic Biochem Physiol 99(2):181–188
Rodríguez-Fuentes G, Armstrong J, Schlenk D (2008) Characterization of muscle cholinesterases from two demersal flatfish collected near a municipal wastewater outfall in Southern California. Ecotoxicol Environ Saf 69(3):466–471
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145(3):321–329
Roméo M, Gharbi-Bouraoui S, Gnassia-Barelli M, Dellali M, Aïssa P (2006) Responses of Hexaplex (Murex) trunculus to selected pollutants. Sci Total Environ 359(1–3):135–144
Samnuek C, Cheevaporn V, Saengkul C, Beamish FWH (2007) Variability in Acetylcholinesterase upon exposure to Chlorpyrifos and Carbaryl in hybrid catfish. Science Asia 33(3):301–305
Sanchez-Hernandez JC, Walker CH (2000) In vitro and in vivo cholinesterase inhibition in lacertides by phosphonate- and phosphorothioate-type organophosphates. Pestic Biochem Physiol 67(1):1–12
Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL (2005) Comparative thresholds for ACETYLCHOLINESTERASE inhibition and behavioral impairment in COHO salmon exposed to CHLORPYRIFOS. Environ Toxicol Chem 24(1):136–145. https://doi.org/10.1897/04-195r.1
Scaps P, Demuynck S, Descamps M, Dhainaut A (1996) Biochemical and enzymatic characterization of an Acetylcholinesterase from Nereis diversicolor (Annelida, Polychaeta): comparison with the Cholinesterases of Eisenia fetida (Annelida, Oligochaeta). Biol Bull 190(3):396–402
Sturm A, da Silva de Assis H, Hansen P-D (1999) Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination. Mar Environ Res 47(4):389–398
Talesa V, Contenti S, Mangiabene C, Pascolini R, Rosi G, Principato GB (1990) Propionylcholinesterase from Murex brandaris: comparison with other invertebrate cholinesterases. Comp Biochem Physiol C Comp Pharmacol 96(1):39–43. https://doi.org/10.1016/0742-8413(90)90041-7
Thompson H, Walker C (1992) Blood esterases as indicators of exposure to organophosphorus and carbamate insecticides. In: Fossi MC, Leonzio C (eds) Nondestructive biomarkers in vertebrates. Boca Raton, Lewis Publishers, pp 37–62
Valbonesi P, Bruneli F, Mattioli M, Rossi T, Fabbri E (2011) Cholinesterase activities and sensitivity to pesticides in different tissues of silver European eel, Anguilla anguilla. Comp Biochem Physiol C Toxicol Pharmacol 154(4):353–359
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149
Van Dolah RF, Maier PP, Fulton MH, Scott GI (1997) Comparison of azinphosmethyl toxicity to juvenile red drum (Sciaenops ocellatus) and the mummichog (Fundulus heteroclitus). Environ Toxicol Chem 16(7):1488–1493
Varó I, Navarro JC, Amat F, Guilhermino L (2002) Characterisation of cholinesterases and evaluation of the inhibitory potential of chlorpyrifos and dichlorvos to Artemia salina and Artemia parthenogenetica. Chemosphere 48(6):563–569
Varo I, Nunes B, Amat F, Torreblanca A, Guilhermino L, Navarro JC (2007) Effect of sublethal concentrations of copper sulphate on seabream Sparus aurata fingerlings. Aquat Living Resour 20(3):263–270
Vieira LR, Gravato C, Soares AMVM, Morgado F, Guilhermino L (2009) Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: linking biomarkers to behaviour. Chemosphere 76(10):1416–1427
Wheelock CE, Eder KJ, Werner I, Huang H, Jones PD, Brammell BF, Elskus AA, Hammock BD (2005) Individual variability in esterase activity and CYP1A levels in Chinook salmon (Oncorhynchus tshawytscha) exposed to esfenvalerate and chlorpyrifos. Aquat Toxicol 74(2):172–192
Whitehead A, Anderson SL, Ramirez A, Wilson BW (2005) Cholinesterases in aquatic biomonitoring: assay optimization and species-specific characterization for a California native fish. Ecotoxicology 14(6):597–606
Xuereb B, Noury P, Felten V, Garric J, Geffard O (2007) Cholinesterase activity in Gammarus pulex (Crustacea Amphipoda): characterization and effects of chlorpyrifos. Toxicology 236(3):178–189
Acknowledgements
Bruno Nunes is hired by “ECO-R-pharmplast—Ecotoxicity of realistic combinations of pharmaceutical drugs and microplastics in marine ecosystems”, Fundação para a Ciência e a Tecnologia, FCT (reference POCI-01-0145-FEDER-029203). This research was financially supported by CESAM (UIDB/50017/2020 + UIDP/50017/2020), by FCT/MCTES through national funds (PIDDAC) and by the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia, FCT (project ECO-R-pharmplast—Ecotoxicity of realistic combinations of pharmaceutical drugs and microplastics in marine ecosystems, reference POCI-01-0145-FEDER-029203). FCT also funded the research centre CESAM, in which the research was conducted (UIDB/50017/2020 + UIDP/50017/2020); CESAM was also co-funded by ERDF, within the PT2020 Partnership Agreement and Compete 2020. None of these sources of funding had any role in the design of the study and collection, analysis and interpretation of data and in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Madalena Vieira was involved in formal analysis; investigation; methodology; and writing of the original draft.
Bruno Nunes was involved in conceptualization; data curation; formal analysis; funding acquisition; project administration; resources; supervision; validation; and in writing, namely, reviewing and editing the manuscript.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vieira, M., Nunes, B. Cholinesterases of marine fish: characterization and sensitivity towards specific chemicals. Environ Sci Pollut Res 28, 48595–48609 (2021). https://doi.org/10.1007/s11356-021-13748-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13748-2