Abstract
Purpose
To determine clinical safety and cardiovascular, cardiac autonomic and inflammatory responses to a single session of inspiratory muscle training (IMT) in obstructive sleep apnea (OSA) subjects.
Methods
In a randomized controlled trial individuals of both sexes, aged between 30 and 70 years old with diagnosis of moderate to severe OSA were enrolled. Volunteers with OSA (n = 40) performed an IMT session with three sets of 30 repetitions with a 1-min interval between them. The IMT group (n = 20) used a load of 70% of the maximum inspiratory pressure (MIP), and the placebo group (n = 20) performed the IMT without load. Measurements of systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), heart rate variability (HRV), and inflammatory markers were performed pre, post-immediate and 1 h after the IMT session.
Results
No differences were shown in SBP, DBP, HRV, or inflammatory markers at any of the intervals analyzed. However, HR in the IMT group was lower 1 h after the IMT session compared to the pre-session values (p = 0002). HR was higher in the placebo group when comparing pre × post-immediate (p < 0.001). HR decreased after the first hour in relation to the pre (p < 0.001) and post-immediate (p < 0.001) values.
Conclusion
IMT sessions promote discreet hemodynamic, cardiac autonomic and inflammatory responses. Therefore, IMT is considered clinically safe and can be performed at home, guided but unsupervised, with lower cost and greater adherence to exercise program for subjects with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a respiratory sleep disorder with repeated episodes of hypoxia and reoxygenation. The intermittent hypoxia triggers an increase in oxidative stress which promotes local and systemic inflammation, sympathetic hyperactivity, and pathological hemodynamic profile [1, 2].
Continuous positive airway pressure (CPAP) is the first-line intervention for treating moderate and severe OSA. However, physical training is a low-cost and easy-to-adopt supplementary therapeutic option to treat patients with sleep disorders [3, 4]. Inspiratory muscle training (IMT) emerges from different physical training modalities as a supportive alternative to treat patients with OSA. Studies reported that IMT improves the disease severity and quality of sleep and promotes adjustments in pulmonary function in subjects with OSA [5,6,7].
Although studies show the impact of IMT on OSA, little is known about the acute responses of this intervention to inflammatory, autonomic, and hemodynamic markers and therefore the clinical safety of this therapy. Adjustments in physiological systems such as activation of the sympathetic nervous system to regulate blood pressure and blood flow [8] and the increase in the inflammatory response during effort are necessary during physical exercise to meet the new muscle metabolic demand [9, 10]. In addition, IMT programs are carried out at home, guided but unsupervised and unmonitored. Thus, the physiological repercussions are important to assess the clinical safety of this modality of training in special populations.
Therefore, the objective of this study was to determine clinical safety and cardiovascular, cardiac autonomic and inflammatory responses to a single session of IMT in subjects with OSA.
Methods
Sample
In this randomized controlled clinical trial individuals of both sexes aged between 30 and 70 years with a diagnosis of moderate to severe OSA were included, being diagnosed by polysomnography or polygraphy and who were not under specific treatment for OSA for the last 2 months. The volunteers were asked about heart and lung diseases. Individuals with neuromuscular disorders, infectious diseases, immunological diseases, tumors, peripheral vascular diseases, coagulation disorders, pregnant women, with decompensated diabetes mellitus, uncontrolled arterial hypertension, thrombocytopenic patients, with a history of head or neck injury and/or neck surgery in the last 3 months, subjects using oral corticosteroids, central nervous system depressants, barbiturates and/or muscle relaxants, and individuals unable to understand the research stages were excluded.
This study was approved by the Ethics and Research Committee of the Otávio de Freitas Hospital (HOF) (no: 3,105,259), and all volunteers read and signed the free and informed consent form (ICF) before participating in the study. The volunteers were randomized by the website www.randomization.com, dividing them into two groups: IMT group and placebo group.
The sample calculation was performed using the G POWER software program, with inflammatory markers as the primary outcome, an effect size (f) of 0.3, a significance level of 95%, and study power of 80%, giving a size total sample of 36 subjects (18 in the IMT group and 18 in the placebo group).
Personal data collection, anthropometric assessment, and questionnaires were used in the initial evaluation to assess sleep quality (Pittsburg sleep quality index — PSQI) [11], excessive daytime sleepiness (Epworth sleepiness scale – ESS) [12], and the level of physical activity (International physical activity questionnaire — IPAQ-short version) [13].
Respiratory muscle strength
The recommendations proposed by the Thoracic Society/European Respiratory Society [14] were followed to assess respiratory muscle strength considering the reference values for the Brazilian population [15]. An analog manovacuometer (M120, Globalmed, Brazil) was used.
Pulmonary function
Pulmonary function was evaluated using spirometry according to the guidelines of the Thoracic Society/European Respiratory Society [14] using a Minispir® spirometer considering the reference values for the Brazilian population [16]. Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), and FEV1/FVC (%) were evaluated.
Cardiovascular variables
Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured before, immediately after and 1 h after the IMT session. Three initial blood pressure (BP) measurements were performed on the right arm (auscultatory method), with an interval of 2 min between each one, using an aneroid sphygmomanometer (Premium) and a Littmann Classic III stethoscope. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) evaluations were performed after a 10-min rest period with the participants seated. The Polar® RS800CX HR frequency meter (Polar Electro öy, Kempele, Finland) was used for HR assessment, as well as the G-TECH digital finger oximeter.
Heart rate variability (HRV)
In the autonomic control evaluation, the volunteers were in a supine position with the stretcher head at 45° inclination and were instructed to remain calm and without movement. The recording was performed after 10 min of rest to stabilize the heart rate before the IMT session (duration: 10 min), immediately after the IMT session (duration: 10 min) and 1 h after the IMT session (duration: 10 min). The autonomic control evaluation was performed with a Polar® RS800CX frequency meter (Polar Electro öy, Kempele, Finland). The sensor belt was positioned on the participant’s chest, and the signal was automatically stored in the RR interval.
After acquisition, the signals were transferred to the Polar Pro Trainer Software. The data were processed using Kubios HRV analysis software (MATLAB, version 3.1 beta, Kuopio, Finland). HRV was analyzed using the FFT (fast Fourier transformation) method, and the frequency domain with the components of the low frequency (LF: 0.04 to 0.15 Hz) and high frequency (HF: 0.15 to 0.4 Hz) was used as sympathetic/parasympathetic and parasympathetic indices, respectively. The LF and HF spectral components were expressed in normalized values (nu), and the cardiac autonomic balance was calculated by the ratio between the absolute components of the LF and HF bands (LF/HF) [17].
Inflammatory markers
Next, 5 ml of peripheral venous blood was collected in the antecubital region to analyze inflammatory markers. The first collection was performed before the IMT session (pre); the second, 10 min after the end of the IMT session; and the third 1 h after the end of the IMT session. The blood samples were centrifuged for 10 min at 3000 rpm, the supernatant was extracted, and the serum fraction was stored in a refrigerated container at − 80 °C.
The cytokine quantification procedure was performed by flow cytometry (cytometric bead array — CBA). The BD TM CBA uses a series of particles (microspheres or beads) with different fluorescent intensities to simultaneously detect the various soluble cytokines through a captured surface. The following cytokines were analyzed: IL-2, interleukin 2; IL-4, interleukin-4; IL-6, interleukin 6; IL-10, interleukin 10; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma.
Experimental protocol
The volunteers attended the rehabilitation center and received all the necessary instructions on how to carry out the experimental protocol. POWERbreathe®classiclight was used to perform the inspiratory muscle training session, which is a device capable of providing linear resistance in the inspiratory phase.
Prior to performing the experimental protocol, subjects had access to the POWERbreathe®classiclight to familiarize themselves with the device and to clarify all doubts. Participants were instructed to perform the experimental protocol while sitting in a chair with a back but without arm support, so that their feet were resting on the floor and the joints of the hip and knee remained at a 90° angle.
The MIP measurement was performed to define the training load. Thus, the IMT group performed inspiratory efforts using a load equivalent to 70% of MIP, while the placebo group performed the same protocol, but without the use of load. All volunteers used a nose clip and were instructed to perform the following protocol: three sets of 30 breaths with an interval of one minute between sets. The IMT protocol was based on the previously published data by Souza et al. [6].
Statistical analysis
Statistical analysis was performed with SigmaPlot 12.0 (Systat Software, Inc., Germany), GraphPad Prism 4.0 (GraphPad Software Inc., USA), and IBM SPSS Statistics Software v. 25.0 (SPSS, Inc. IBM Company, New York, USA) implementing a 95% significance level (p < 0.05). The Shapiro-Wilk normality test was performed for data distribution analysis. Continuous variables were expressed as mean and standard deviation, and categorical variables were expressed as number of cases and frequency (%). The two-way ANOVA test was used for comparison of means for repeated measures (time [pre, post-immediate, and 1-h post] versus groups [placebo and IMT]), or the Friedman test. Lastly, the Chi-squared or Fisher’s exact tests were performed to compare categorical variables.
Results
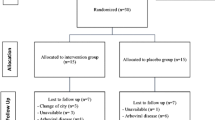
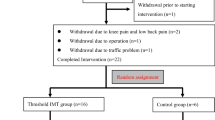
The sample consisted of 40 subjects and the flowchart of capturing, randomizing, and allocating patients is shown in Fig. 1.
Table 1 shows that the IMT, and placebo groups were similar in terms of anthropometric and clinical characteristics.
Regarding cardiovascular variables, systolic (SBP) and diastolic (DBP) blood pressure did not show intra- or intergroup differences when comparing the pre, post-immediate, and 1 h after the IMT session (p > 0.05 for all comparisons). However, heart rate (HR) was lower in the IMT group 1 h after the session compared to pre values (p = 0.002). HR was higher in the placebo group in the post-immediate period compared to pre-values (p < 0.001). HR decreased after the first hour in relation to the pre (p < 0.001) and post-immediate (p < 0.001) values. There was no difference in HR levels in the intergroup comparison (Table 2).
Figure 2 shows the variation percentage (∆%) of cardiovascular variables in the pre vs. post-immediate and pre vs. 1 h after the IMT session between the IMT and placebo groups. There were no significant differences between groups in SBP ∆% (Fig. 2a: pre vs. post-immediate, p = 0.670; pre vs. post 1 h, p = 0.702), DBP ∆% (Fig. 2b: pre vs. post-immediate, p = 0.438; pre vs. post 1 h, p = 0.574), and HR ∆% (Fig. 2c: pre vs. post-immediate, p = 0.973; pre vs. post 1 h, p = 0.265).
Comparison of the percentage variation of the pre-post-immediate and pre-post 1 h values of the IMT session in the IMT and placebo groups for: 2a: ∆SBP (%); 2b: ∆DBP (%), and 2c: ∆HR (%) ∆(%): percentage difference between pre and post-intervention values; IMT: inspiratory muscle training; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate
Table 3 shows a similar result in heart rate variability, as there were no differences between the groups in the LF, HF bands, and in the cardiac autonomic balance in comparing the pre-immediate and 1 h after the IMT session. However, increases in the RR interval were observed in the IMT group in comparing pre × post-immediate (p < 0.001), pre × 1 h after (p < 0.001) and post-immediate × 1 h after the IMT session (p < 0.001).
In relation to the placebo group, an increase in RR intervals was also observed when comparing the three moments; pre vs. post-immediate (p = 0.005), pre vs. post 1 h (p = 0.005), and post-immediate vs. after 1 h (p = 0.005). There was no difference in the RR interval in the intergroup comparison (Table 3).
Fig. 3 presents the heart rate variability variation percentage (∆%) between the pre- and post-immediate moments and 1 h after the IMT session. There was no difference between groups for RR intervals (Fig. 3a: pre × post-immediate: p = 0.280; pre × post 1 h: p = 0.215), the LF bands (Fig. 3b: pre vs. post-immediate: p = 0.989; pre × post 1 h, p = 0.759), HF (Fig. 3c: pre × immediate, p = 0.944; pre × post 1 h, p = 0.737) or in the cardiac autonomic balance (Fig. 3d: pre × post-immediate, p = 0.744; pre × post 1 h, p = 0.575)
Comparison of the percentage variation of the pre-post-immediate and pre-post 1 h values of the IMT session in the IMT and placebo groups for: 3a: ∆RR (%); 3b: ∆LF (%); 3c: ∆LF/HF (%), and 3d: ∆HF (%) ∆ (%): percentage difference between pre and post-intervention values; LF: low frequency; HF: high frequency; RR: RR intervals; LF/HF: low frequency/high frequency ratio; IMT: inspiratory muscle training
The analysis of inflammatory markers is shown in Fig. 4. It can be seen that there were no significant differences at any time between the IMT and placebo groups for IL-2 interleukins (Fig. 4a: p = 0.968), IL-4 (Fig. 4b: p = 0.894), IL-6 (Fig. 4c: p = 0.949), IL-10 (Fig. 4d: p = 0.978), IFN-γ (Fig. 4e: p = 0.874) or TNF-α (Fig. 4f: p = 0.959).
Plasma concentration of the: 4a: IL-2; 4b: IL-4; 4c: IL-6; 4d: IL-10; 4e: TNF-α and 4f: IFN-γ inflammatory markers in the IMT and placebo groups in the pre-IMT, post-immediate, and 1-h training periods IMT, inspiratory muscle training; IL-2: interleukin 2; IL-4: interleukin 4; IL-6: interleukin 6; IL-10: interleukin 10; TNF-α: tumor necrosis factor alpha; IFN-γ: interferon gamma
Discussion
In our knowledge, this is the first study to assess clinical safety and acute effect of an IMT session on hemodynamic parameters, cardiac autonomic activity, and inflammatory markers in OSA subjects. It was verified the clinical safety of the IMT session and the mild cardiovascular, cardiac autonomic and inflammatory responses to this training session in OSA patients.
Cardiovascular control during exercise is regulated by the command center in the central nervous system and by the ergoreflex control, which involves mechanoreceptors (myelinated nerve fibers in group III) and metaboreceptors (amyelinic nerve fibers in group IV or C). The receptors respond to the mechanical and chemical changes induced by exercise [18]. The increase in HR is associated with the muscle mass recruited for performing physical exercise and consequently with the afferent impulses coming from the muscular mechanoreceptors [19]. In the present study, the heart rate and the blood pressure responses to the IMT session were clinically irrelevant, and we suggest that the small muscle mass involved in performing the IMT could justify these milder hemodynamic changes, making safe to perform this unsupervised and unmonitored training.
Ramos et al. [20] evaluated the clinical safety of implementing IMT in an older adult population inserted in a rehabilitation program. They indicated no significant clinical or hemodynamic changes during an IMT session performed with two sets of 15 repetitions and using 30% of MIP, thus guaranteeing the clinical safety of unsupervised IMT in this specific population.
OSA patients have a high prevalence of cardiovascular comorbidities [21] and the supervision of exercise session can be necessary. The adoption of clinically safe training methodologies to use at home without supervision contributes to improve the adherence to the physical exercise program and, consequently, to achieve the adaptations resulting from training.
Regarding the heart rate variability, the present study showed no differences in the HF and LF components or in the relationship between them when comparing the IMT and placebo groups. On the other hand, in evaluating healthy subjects who underwent IMT using 60% of MIP, Plentz et al. [22] demonstrated that IMT increased sympathetic modulation concomitant to a reduction of parasympathetic modulation during the acute and subacute phases of exercise. However, the results in the time domain were less pronounced compared to the frequency domain.
The pathophysiology of OSA triggers disordered respiratory events, which are characterized by increasing efforts against an occluded airway during sleep. Thus, the result is an accentuated sympathetic response due to the activation of chemoreflexors by hypoxemia, increasing the sympathetic neural flow [23, 24]. Even the IMT performed in the present study, with a load of 70% of the MIP, cause a significant change in the autonomic modulation. We suggest that a less demanding muscular metabolic demand may not exacerbate the already high basal sympathetic activity showed in subjects with moderate and severe OSA.
Regarding the inflammatory response, no differences in the analyzed inflammatory markers (IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ) are showed between the evaluated groups. Exercise, diet, disease states, drug use, socioeconomic status, psychological stress, body composition, and alcohol use alter the inflammatory state. Therefore, type, duration, and intensity of the exercise can influence the acute inflammatory response by intensifying or attenuating the inflammatory cascade [25].
Windsor et al. [26] evaluated the concentration of plasma cytokines in healthy older adult subjects. The findings indicated an acute increase in plasma IL-6 and IL-10 concentrations of the 3 groups analyzed: moderate intensity continuous exercise, high intensity interval exercise, and a control group. One study assessed young, active, healthy adults who completed three exercise sessions with different intensities and volumes. The results showed that 35 min of aerobic exercise induces a slight increase in the circulating IL-6 concentration but are insufficient to induce an increase in the anti-inflammatory cytokine IL-10 [27].
The inflammatory response is also sensitive to the exercising muscle mass. The skeletal muscle contraction is an important source of IL-6 in plasma and exercises involving reduced muscle mass, such as in IMT, may be insufficient to increase plasma IL-6 above the pre-exercise level. In addition, long-duration exercises promote higher post-exercise plasma IL-6 concentrations [28]. Subjects already have an elevated inflammatory state in OSA due to repeated hypoxia and reoxygenation episodes during sleep, triggering the increase in oxidative stress and inflammatory cytokines [29, 30].
The present study has limitations. Despite being a randomized clinical trial, a cross-over and double-blinded study may have been more appropriate, thus minimizing the potential for confusion, especially in relation to measuring inflammatory markers.
Conclusion
IMT sessions promote discreet cardiovascular, cardiac autonomic and inflammatory responses and appear to be clinically safe and well tolerated in subjects with OSA.
The option for a type of guided training carried out at home, while unsupervised and unmonitored, may improve adherence by resolving issues such as difficult access to the rehabilitation center.
IMT may become an option as a training modality for patients with OSA and comorbidities by including this exercise in the daily routines of these subjects.
References
Choi JH, Thomas RJ, Suh SY, Park IH, Kim TH, Lee SH, Lee HM, Yun CH, Lee SH (2015) Sleep quality change after upper airway surgery in obstructive sleep apnea: electrocardiogram-based cardiopulmonary coupling analysis. Laryngoscope 125:1737–1742. https://doi.org/10.1002/lary.25101
Lavie L, Lavie P (2009) Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 33:1467–1484. https://doi.org/10.1183/09031936.00086608
Andrade FMD, Pedrosa RP (2016) O papel do exercício físico na apneia obstrutiva do sono. J Bras Pneumol 42:457–464. https://doi.org/10.1590/S1806-37562016000000156
Mendelson M, Bailly S, Marillier M, Flore P, Borel JC, Vivodtzev I, Doutreleau S, Verges S, Tamisier R, Pépin JL (2018) Obstructive sleep apnea syndrome, objectively measured physical activity and exercise training interventions: a systematic review and meta-analysis. Front Neurol 9:73. https://doi.org/10.3389/fneur.2018.00073
Herkenrath SD, Treml M, Priegnitz C, Galetke W, Randerath WJ (2017) Effects of respiratory muscle training (RMT) in patients with mild to moderate obstructive sleep apnea (OSA). Sleep Breath 22:323–328. https://doi.org/10.1007/s11325-017-1582-6
Souza AKF, Andrade AD, Medeiros AIC, Aguiar MIR, Rocha TDS, Pedrosa RP, Lima AMJ (2018) Effectiveness of inspiratory muscle training on sleep and functional capacity to exercise in obstructive sleep apnea: a randomized controlled trial. Sleep Breath 22:631–639. https://doi.org/10.1007/s11325-017-1591-5
Vranish JR, Bailey EF (2016) Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in OSA. SLEEP 39:1179–1185. https://doi.org/10.5665/sleep.5826
Katayama K, Saito M (2019) Muscle sympathetic nerve activity during exercise. J Physiol Sci 69:589–598. https://doi.org/10.1007/s12576-019-00669-6
Benini R, Nunes PRP, Orsatti CL, Portari GV, Orsatti FL (2015) Influence of sex on cytokines, heat shock protein and oxidative stress markers in response to an acute total body resistance exercise protocol. J Exerc Sci Fit 13:1–7. https://doi.org/10.1016/j.jesf.2014.10.002
Cwikiel J, Seljeflot I, Berge E, Njerve IU, Ulsaker H, Arnesen H, Flaa A (2018) Cytokine effect of strenuous exercise on mediators of inflammation in patients with coronary artery disease. Cytokine 105:17–22. https://doi.org/10.1016/j.cyto.2018.02.006
Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, Barba ME, Barreto SS (2011) Validation of the Brazilian Portuguese version of the Pittsburgh sleep quality index. J Clin Sleep Med 2:70–75. https://doi.org/10.1016/j.sleep.2010.04.020
Bertolazi AN, Fagondes SC, Hoff LS, Pedro VD, Barreto SSM, Johns MW (2009) Validação da escala de sonolência de Epworth em português para uso no Brasil. J Bras Pneumol 35(9):877–883. https://doi.org/10.1590/S1806-37132009000900009
Matsudo S, Araújo T, Matsudo V, Andrade D, Andrade E, Oliveira LC, Braggion G (2001) International physical activity questionnaire (IPAQ): study of validity and reability in Brazil. Rev Bras Ativ Fís Saúde 6(2):05–18. https://doi.org/10.12820/rbafs.v.6n2p5-18
American Thoracic Society/ European Respiratory Society (2002) ATS/ERS statement on respiratory muscle testing. Am J Resp Crit Care 166:518–624. https://doi.org/10.1164/rccm.166.4.518
Pessoa IMBS, Neto MH, Montemezzo D, Silva LAM, Andrade AD, Parreira VF (2014) Predictive equations for respiratory muscle strength according to international and Brazilian guidelines. Braz J Phys Ther 18:410–418. https://doi.org/10.1590/bjpt-rbf.2014.0044
Pereira CAC, Duarte AAO, Gimenez A, Soares MR (2014) Comparação entre os valores de referência para CVF, VEF1 e relação VEF1/CVF em brasileiros caucasianos adultos e aqueles sugeridos pela Global Lung Function Initiative 2012. J Bras Pneumol 40:397–402. https://doi.org/10.1590/S1806-37132014000400007
(1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Circulation 93(5):1043–1065
Mitchell JH (2012) Neural control of the circulation during exercise: insights from the 1970-1971 Oxford studies. Exp Physiol 97:14–19. https://doi.org/10.1113/expphysiol.2011.058156
Vianna LC, Oliveira RB, Ramos PS, Ricardo DR, Araújo CG (2010) Effect of muscle mass on muscle mechanoreflex-mediated heart rate increase at the onset of dynamic exercise. Eur J Appl Physiol 108:429–434. https://doi.org/10.1007/s00421-009-1237-9
Ramos PS, Silva BC, Silva LOG, Araújo CG (2015) Acute hemodynamic and electrocardiographic responses to a session of inspiratory muscle training in cardiopulmonary rehabilitation. Eur J Phys Rehabil Med 51:773–779
Bonsignore MR, Baiamonte P, Mazzuca E, Castrogiovanni A, Marrone O (2019) Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med 14:1–12. https://doi.org/10.1186/s40248-019-0172-9
Plentz RDM, Silva VGS, Dipp T, Macagnan FE, Lemos LC, Tartari JLL, Sbruzzi G (2014) Inspiratory muscle training in autonomic control in healthy individuals. Salud (i) Ciencia 21:28–34
Abboud F, Kumar R (2014) Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 124:1454–1457. https://doi.org/10.1172/JCI70420
Mansukhani MP, Kara T, Caples S, Somers VK (2014) Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep 16:476. https://doi.org/10.1007/s11906-014-0476-2
Silva FOC, Macedo DV (2011) Exercício físico, processo inflamatório e adaptação: uma visão geral. Rev Bras de Cineantropom Desempenho Hum 13:320–328. https://doi.org/10.5007/1980-0037.2011v13n4p32
Windsor MT, Bailey TG, Perissiou M, Meital L, Golledge J, Russel FD, Askew CD (2018) Cytokine responses to acute exercise in healthy older adults: the effect of cardiorespiratory fitness. Front Physiol 9:1–8. https://doi.org/10.3389/fphys.2018.00203
Cullen MT, Thomas AW, Webb R, Hughes MG (2016) Interleukin-6 and associated cytokine responses to an acute bout of high intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab 41:803–808. https://doi.org/10.1139/apnm-2015-0640
Steensberg A, Van Hall G, Osada T, Sacchetti BS, Pedersen BK (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242. https://doi.org/10.1111/j.1469-7793.2000.00237.x
Kheirandish-Gozal L, Gozal D (2019) Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci 20:459. https://doi.org/10.3390/ijms20030459
Li L, Ren F, Qi C, Xu L, Fang Y, Liang M, Feng J, Chen B, Ning W, Cao J (2018) Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir Res 19:1–9. https://doi.org/10.1186/s12931-018-0727-x
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001
Author information
Authors and Affiliations
Contributions
Sílvia Thamilis Barbosa Pessoa Ferreira — acquisition, analysis and interpretation of data and drafting the manuscript
Juliana Baptista Teixeira and Thayse Neves Santos Silva — acquisition of data
Michelle Christiane da Silva Rabello, Virgínia Maria Barros de Lorena, and Breno Quintella Farah — analysis and/or interpretation of data
Maria do Socorro Brasileiro-Santos — revising the manuscript critically for important intellectual content
Anna Lima Jaguaribe de Lima — conception and design of study, analysis, and/or interpretation of data and revising the manuscript critically for important intellectual content. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent to participate for publication was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interests; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ReBEC: RBR – 74497t 23/03/2020
Rights and permissions
About this article
Cite this article
Ferreira, S.T.B.P., do Socorro Brasileiro-Santos, M., Teixeira, J.B. et al. Clinical safety and hemodynamic, cardiac autonomic and inflammatory responses to a single session of inspiratory muscle training in obstructive sleep apnea. Sleep Breath 26, 99–108 (2022). https://doi.org/10.1007/s11325-021-02364-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02364-6