Abstract
Purpose
Obstructive sleep apnea (OSA) is frequently accompanied by hypertension, resulting in cardiovascular comorbidities. Continuous positive airway pressure is a standard therapy for OSA but has poor adherence. Inspiratory muscle training (IMT) may reduce airway collapsibility and sympathetic output, which may decrease OSA severity and blood pressure. In this meta-analysis of randomized controlled trials (RCTs), we evaluated the efficacy of IMT in patients with OSA.
Methods
We searched PubMed, EMBASE, Cochrane Library, Web of Science, and ClinicalTrials.gov databases for relevant RCTs published before November 2022.
Results
Seven RCTs with a total of 160 patients with OSA were included. Compared with the control group, the IMT group exhibited significantly lower systolic and diastolic blood pressure (mean difference [MD]: − 10.77 and − 4.58 mmHg, respectively), plasma catecholamine levels (MD: − 128.64 pg/mL), Pittsburgh Sleep Quality Index (MD: − 3.06), and Epworth Sleepiness Scale score (MD: − 4.37). No significant between-group differences were observed in the apnea–hypopnea index, forced vital capacity (FVC), ratio of forced expiratory volume in 1 s to FVC, or adverse effects. The data indicate comprehensive evidence regarding the efficacy of IMT for OSA. However, the level of certainty (LOC) remains low.
Conclusion
IMT improved blood pressure- and sleep-related outcomes without causing adverse effects and may thus be a reasonable option for lowering blood pressure in patients with OSA. However, additional studies with larger sample sizes and rigorous study designs are warranted to increase the LOC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent upper airway obstruction (apnea) during sleep, resulting in intermittent hypoxia [1]. It has been demonstrated that approximately 50% of OSA patients have underlying hypertension [2]. A recent meta-analysis found that OSA raises the risk of hypertension in a dose-dependent manner [3]. Patients with OSA and hypertension are more likely to develop cardiovascular disease [4]. The pathogenesis of hypertension in OSA is complicated and remains unclear. Intermittent hypoxia, sympathetic hyperactivity, systemic inflammation, and activation of the renin–angiotensin–aldosterone (RAA) system may all play a role in elevated blood pressure [5].
Continuous positive airway pressure (CPAP), the standard treatment for OSA, stents and stabilizes the upper airway [1]. A meta-analysis of randomized controlled trials (RCTs) on OSA with CPAP treatment found a low reduction in blood pressure (2.3 mmHg for systolic blood pressure and 1.98 mmHg for diastolic blood pressure) and revealed that better CPAP compliance may be a positive predictor of greater blood pressure reduction [6]. However, 25 to 50% of patients failed to adhere to treatment due to discomfort from wearing a mask and head gear at night [7], resulting in no improvement in hypertension or OSA severity [8]. Consequently, other interventions, in addition to CPAP, are frequently required in clinical practice to control blood pressure.
Aerobic exercise and physical training are non-pharmacological therapies that have well-documented benefits for blood pressure and may help with OSA [9, 10]. However, most patients with OSA have a limited exercise capacity due to obesity or lethargy [10]. Inspiratory muscle training (IMT) is a novel form of exercise that has emerged as an easy-to-adopt daytime treatment program that can strengthen the lower respiratory muscles and be performed at home [11]. It decreases sympathetic activity through respiratory muscle metaboreflex attenuation, which improves systolic and diastolic blood pressure as well as autonomic cardiovascular control in patients with hypertension [12]. According to an MRI study, inspiratory phasic activity increases the active tone and passive stiffness of upper airway dilator muscles [13], which may reduce the tendency for upper airway collapse during sleep. As a result, IMT may help reduce blood pressure in patients with OSA and potentially relieve OSA-related symptoms.
Although some randomized controlled trials (RCTs) have reported some beneficial effects of IMT in patients with OSA, their results are inconsistent and controversial [1, 11, 14,15,16,17,18]. Previous meta-analyses pooled the results of various training interventions, including inspiratory and expiratory muscle training, which may have resulted in heterogeneity, and they did not assess the effects on blood pressure–related outcomes [19, 20]. Since there was no formal synthesis of this evidence, the objective of our study was to conduct a meta-analysis of RCTs to assess the effectiveness of IMT in patients with OSA. Our primary outcomes were blood pressure–related outcomes. Secondary outcomes were sleep-related outcomes, respiration-related outcomes, and the adverse effects. Furthermore, we performed a subgroup analysis to distinguish the efficacy of low- and high-intensity IMT, providing clearer clinical guidance.
Methods
Study guidelines and registration
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 guidelines (Supplemental Table 1) [21]. This study is registered with PROSPERO, the International Prospective Register of Systematic Reviews of the National Institute for Health Research (CRD42020176166).
Selection criteria
We included RCTs investigating the effects of IMT in patients with OSA. The following inclusion criteria were applied: (1) description of patient inclusion and exclusion criteria, including OSA recruitment severity; (2) diagnoses of OSA without heart disease (chronic heart failure, myocardial infarction, etc.), neurological and neuromuscular disease; (3) IMT as the primary intervention, with description of the pressure setting and training protocol; and (4) adequate description of the evaluation of clinical outcomes, including the method and timing of outcome assessments. We excluded trials with unclear clinical outcomes or duplicate reporting of patient cohorts.
Search strategy and study selection
We searched full-text studies freely available on PubMed, EMBASE, the Cochrane Library, Web of Science, and ClinicalTrials.gov (http://ClinicalTrials.gov) by using the following keywords and Boolean operators as MeSH terms: (“sleep disordered breathing” OR “apnea, sleep” OR “nocturnal apnea” OR “nocturnal apnoea” OR “obstructive sleep apnea” OR “obstructive sleep apnea hypopnea syndrome” OR “obstructive sleep apnea syndrome” OR “obstructive sleep apnoea” OR “obstructive sleep apnoea hypopnoea syndrome” OR “obstructive sleep apnoea syndrome” OR “obstructive sleep-disordered breathing” OR “sleep apnea” OR “sleep apnea syndrome” OR “sleep apnea syndromes” OR “sleep apnea, obstructive” OR “sleep apnoea” OR “sleep apnoea syndrome” OR “sleep apnoea syndromes” OR “sleep apnoea, obstructive”) AND (“inspiratory muscle training”).
The “Related Articles” option in PubMed was used to broaden the search. We applied no language restrictions. Furthermore, we attempted to identify additional studies by manually searching the reference sections of relevant papers and contacting experts in the field. The final search was performed in November 2022.
Data extraction
Two authors (STM and TAC) independently selected RCTs by screening titles and abstracts and extracted relevant data regarding the study design, sample size, patient characteristics, inclusion and exclusion criteria, treatment duration and frequency, outcomes assessed, and assessment time points. If sufficient information was not available for any study, we contacted the authors to obtain additional data. Any discrepancies were resolved by a third reviewer (YCK).
Methodological quality appraisal
Two authors (STM and TAC) independently assessed the methodological quality of each study by using the Cochrane risk of bias tool (RoB 2.0) (Cochrane Collaboration, Oxford, England). Studies were categorized as having a high, some concern, or a low risk of bias. Any disagreement was resolved by a third reviewer (YCK). We determined the risk of bias by assessing 5 domains: bias arising from the randomization process, bias owing to deviations from intended interventions, bias due to missing outcome data, bias in outcome measurement, and bias in the selection of reported results. If necessary, a sensitivity analysis was performed after studies with a high risk of bias were excluded.
Outcome assessment
IMT may decrease sympathetic outflow and have a positive effect on blood pressure. IMT may also reduce the tendency for upper airway collapse during sleep through inspiratory phasic activity and improved sleep-related outcomes. Therefore, our primary outcome was blood pressure–related outcomes such as changes in systolic and diastolic blood pressure and plasma catecholamine levels. Secondary outcomes were sleep-related outcomes (including changes in apnea-hypopnea index (AHI), sleep quality, and excessive daytime sleepiness), respiration-related outcomes (including changes in maximal inspiratory pressure (MIP), forced expiratory volume in 1 s to forced vital capacity (FEV1.0/FVC) and forced vital capacity (FVC)), and the adverse effects.
Statistical analysis
Statistical analysis was performed using Review Manager (Version 5.3, Cochrane Collaboration, Oxford, England). Mean differences (MDs) for all these continuous outcomes were extracted or calculated using either the change from baseline or posttreatment values based on randomly allocated trials without significant difference in the baseline. When necessary, standard deviations were estimated according to reported CI limits, standard errors, or ranges [22]. A pooled estimate of MDs was calculated using the DerSimonian and Laird random-effects model [23] to alleviate possible heterogeneity. A result with a P value of < 0.05 or a 95% CI not including zero in the weighted MD was considered statistically significant. Statistical heterogeneity was assessed using the I2 test and the Cochrane Q test (chi2). I2 quantifies the proportion of total outcome variability that was attributable to the variability among the studies. Sensitivity analysis will be performed if necessary.
Subgroup analysis
Studies have classified a training threshold of > 60% MIP as high-intensity training and approximately 30% MIP as low-intensity training [24, 25]. A training threshold of < 30% MIP is considered insufficient to produce respiratory muscle training and may be labeled placebo [26]. As a result, we divided the participants into low-intensity (30% MIP) and high-intensity (> 60% MIP) training groups and compared them with the control group (< 30% MIP).
Quality of evidence
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to determine the quality of evidence for the following domains: risk of bias, consistency, precision, directness, and publication bias [27].
Results
Study selection and characteristics of the included studies
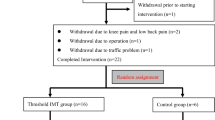
Figure 1 presents the PRISMA 2020 flow diagram of the study selection process. The initial search yielded 126 citations, of which 63 were duplicates and 28 were deemed ineligible after title and abstract screening. The full text of 35 studies was retrieved, 28 of which were excluded for the following reasons: 12 were conference papers, 6 were on a different topic, 2 were review articles, 1 was a commentary on previous publications, 5 were registered only on ClinicalTrials.gov and did not contain actual data, and 2 included a different study population. The remaining 7 eligible trials were included in our study.
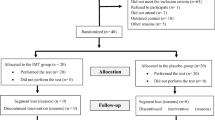
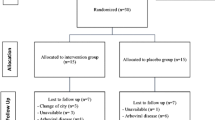
Table 1 summarizes the characteristics of the 7 RCTs. They were published between 2016 and 2021 and included a total of 160 patients with OSA [1, 11, 14,15,16,17,18]. The inclusion criteria of these RCTs were OSA with an AHI of ≥ 5/h (two RCTs) and an AHI of ≥ 15/h (five RCTs) based on type 1 and 3 sleep study (five RCTs conducted type 1 sleep study [11, 14,15,16,17], while two RCTs performed type 3 sleep study [1, 18]). Within current evidence, there was one RCT that described their diagnostic criteria in accordance with the AASM [17], whereas the others simply mentioned their inclusion AHI level [1, 11, 14,15,16, 18]. Four studies excluded uncontrolled hypertension patients [1, 11, 15, 16], whereas the remaining studies did not mention hypertension in their recruiting criteria. According to the baseline characteristics of our included studies, there were four studies that enrolled a portion of OSA patients (59%, 60/102) who had hypertension as their underlying disease yet did not specify the diagnostic criteria for hypertension [1, 11, 16, 18]. Five studies involved patients who had never used CPAP or had stopped using it and were then treated solely with IMT [11, 14,15,16,17]. The use of CPAP was not mentioned in 1 study [18]. Ramos-Barrera et al. evaluated the effect of IMT on blood pressure and included 20% of patients using CPAP in the IMT group and 30% of patients using CPAP and 10% using an oral appliance (OA) in the control group [1]. Table 2 presents preintervention and postintervention blood pressure- and sleep-related outcome parameters. Regarding overall baseline values, the neck circumference was 35.5 to 43.5 cm; BMI was 26.2 to 34.0 kg/m2 (Souza et al. did not report BMI [16]); systolic blood pressure (SBP) and diastolic blood pressure (DBP) were 126.5 to 141.6 mmHg and 73.2 to 81.6 mmHg, respectively (Lin et al. [15], Erturk et al. [14], and Nobrega-Junior et al. [18] did not report blood pressure); and AHI was 21.9 to 38.7 (Ramos et al. did not report AHI [1]). None of these parameters were significantly different between the intervention and control groups. The following IMT devices were used: POWER-breathe (Warwickshire, UK) [1, 11, 16,17,18], Healthscan Products (Cedar Grove, NJ, USA) [15], and Respironics (Murrysville, PA, USA) [14]. Five studies examined high-intensity IMT at 60 to 75% MIP [1, 11, 16,17,18], and 2 evaluated low-intensity IMT at 30% MIP [14, 15]. In six studies, IMT was performed 5 to 7 days a week for 6 to 12 weeks, and one study investigated clinical safety by examining the acute effect on hemodynamic parameters after a single session of IMT [11]. One study was supervised [15], one was home-based [16], and others did not mention supervision. All studies documented adherence to the intervention, and no dropout due to side effects was reported.
Methodological quality appraisal
Figure 2 presents the methodological quality of the RCTs. All RCTs reported acceptable methods of randomization, but 4 RCTs did not mention the process of allocation concealment [1, 11, 15, 17]. Five RCTs [1, 11, 16,17,18] indicated that patients were blinded through the application of a placebo or sham exercise. Although the other 2 RCTs [14, 15] did not provide adequate blinding information, the patients were asked to visit the hospital every week to determine their compliance to prevent bias resulting from deviation from intended interventions. Moreover, 4 of 7 studies had a low risk of bias from missing outcome data; among the remaining 3, 1 study [1] reported a 40% loss to follow-up because of failure to complete the outcome measurement, 1 study [16] indicated a 47% loss to follow-up due to participants’ change of residence area and the presence of arboviral diseases in both groups, and 1 study [18] revealed a 45% loss to follow-up due to unavailability or surgery. All trials had a low risk of bias in outcome measurement and the selection of reported results [1, 11, 14,15,16,17,18]. Therefore, the overall risk of bias was low in one study [14] and some concerns in other six studies. No study has high risk of bias.
Methodological quality assessment of the included randomized controlled trials by using the Cochrane risk of bias tool (RoB 2.0). aNo description of allocation concealment. b47% of the participants were lost to follow-up because of change in residence or the presence of arboviral disease. c40% of the participants were lost to follow-up because they failed to complete the outcome measurement. d45% of the participants were lost to follow-up because of unavailability or surgery
Primary outcomes
Blood pressure–related outcomes
Three RCTs investigated resting blood pressure measured after 6-week [1, 17] or 12-week [16] training in the high-intensity IMT and control groups. Two studies measured resting blood pressure in accordance with American Heart Association guidelines [1, 17]. Compared with the control group, the IMT group exhibited significantly lower systolic blood pressure (pooled MD: − 10.77, 95% CI: − 18.33 to − 3.22, mmHg, I2: 56%; Fig. 3a) and diastolic blood pressure (pooled MD: − 4.58, 95% CI: − 8.71 to − 0.44, mmHg, I2: 0%; Fig. 3b). Two RCTs examined plasma catecholamine levels [1, 17] measured after 6-week training and found significantly lower plasma catecholamine levels in the high-intensity IMT group than in the control group (pooled MD: − 128.64, 95% CI: − 255.92 to − 1.37, pg/mL, I2: 0%; Fig. 3c).
Secondary outcomes
Sleep-related outcomes
Four RCTs examined the posttreatment AHI measured after 6-week [17] or 8-week [18] or 12-week training [14, 15], and the meta-analysis indicated no significant difference between the high-intensity [17, 18] (pooled MD: − 0.63, 95% CI: − 14.45 to 13.19, I2: 0%; Fig. 4a) or low-intensity IMT group [14, 15] (pooled MD: − 6.87, 95% CI: − 15.95 to 2.20, I2: 0%; Fig. 4a) and the control group. Regarding sleep-associated symptoms, compared with the control group, both high-intensity [16,17,18] (pooled MD: − 2.72, 95% CI: − 4.42 to − 1.03 I2: 0%; Fig. 4b) and low-intensity IMT [14, 15] groups (pooled MD: − 3.68, 95% CI: − 5.96 to − 1.39, I2: 0%; Fig. 4b) had significantly better sleep quality, as assessed using the 21-point Pittsburgh Sleep Quality Index (PSQI), measured after 6-week [17] or 8-week [18] or 12-week [14,15,16] training. However, only low-intensity IMT was associated with significantly less daytime sleepiness evaluated using the Epworth Sleepiness Scale (ESS) after 12-week [14,15,16] training (pooled MD: − 5.52, 95% CI: − 7.76 to − 3.27, I2: 0%; Fig. 4c).
Respiration-related outcomes
Three RCTs compared the inspiratory muscle strength between the high-intensity IMT and control groups by examining MIP measured after 6-week [1] or 8-week [18] or 12-week [16] training. The meta-analysis indicated that MIP was significantly improved in the high-intensity IMT group compared with the control group (pooled MD: − 18.53 cmH2O, 95% CI: − 27.75 to − 9.32, I2: 0%; Fig. 5a). Neither low-intensity [15] nor high-intensity IMT [16] affected the ratio of FEV1.0/FVC% and FVC measured after 12-week training [15, 16] (Fig. 5b and c).
Sensitivity analysis
Because one of the included studies recruited a proportion of patients with CPAP and OA use [1], we performed a sensitivity analysis by excluding that study. The results still indicated significant decreases in SBP (pooled MD: − 8.67 mmHg, 95% CI: − 15.30 to − 2.04, I2 = 53%, Supplemental Fig. 1a) and plasma catecholamine levels (pooled MD: − 149.50, 95% CI: − 289.33 to − 9.67 pg/mL, Supplemental Fig. 1c) in the IMT group compared with the control group. However, diastolic blood pressure and MIP were no longer significantly different (DBP: pooled MD: − 4.81, 95% CI: − 10.69 to 1.06, mmHg, I2 = 38%, Supplemental Fig. 1b) (MIP: pooled MD: − 20.17 cmH2O, 95% CI: − 46.27 to 5.93, I2 = 0%, Supplemental Fig. 1d).
Adverse effects
No adverse effects related to IMT were reported in any of the included RCTs. Ferreira et al. indicated that the acute responses of heart rate and blood pressure to a single IMT session were clinically irrelevant [11].
GRADE
Currently, the certainty of the evidence of all above outcomes was low due to small sample sizes and some concerns of risk of bias from not mentioning the allocation concealment or loss of follow-up (Fig. 6).
Discussion
This is the first meta-analysis to evaluate the efficacy of IMT for patients with OSA and the effects on blood pressure–related outcomes. In the present study, we observed that IMT reduced blood pressure and plasma catecholamine levels. IMT enhanced inspiratory muscle strength but not lung function. In addition, IMT improved sleep quality and reduced daytime sleepiness but did not decrease the AHI. The absence of adverse events and clinically irrelevant effects on acute hemodynamic parameters indicated that IMT may be a clinically safe treatment modality for patients with OSA [11].
Our results revealed that MIP was significantly improved in the IMT group, likely due to favorable changes in thickness, strength, and fatigue resistance of the inspiratory muscles. Less muscle fatigue during apnea decreases metabolite accumulation, which may decrease the muscle metaboreflex that lowers sympathetic output [12]. This hypothesis may be supported by Ramos-Barrera et al. [1], who observed that IMT reduced muscular sympathetic nerve activity. This phenomenon may explain the significant decrease in blood pressure (SBP: − 10.77 mmHg; DBP: − 4.58 mmHg) and plasma catecholamine levels (− 128.64 pg/mL) observed in our meta-analysis.
Patients with OSA experience significant decreases in plasma catecholamine levels after CPAP [28] as well as in BP (CPAP [6], SBP: − 2.3 mmHg; DBP: − 1.98 mmHg; OA [29] SBP: − 2.7 mmHg; DBP: − 2.7 mmHg). Although the mean decrease in blood pressure was more obvious after IMT, no head-to-head comparison of their antihypertensive effect has been performed. According to the existing evidence, our meta-analysis revealed a significant reduction in blood pressure in patients with OSA after IMT, but a proportion of normotensive patients were enrolled in our included studies. There was no existing study that investigated the role of IMT in blood pressure regulation in patients with OSA and concurrent hypertension; therefore, further clinical research is required in the future.
In our study, the I2 of nearly all outcomes was < 25% indicating low statistical heterogeneity except for SBP (Fig. 3a; I2 = 56%). Because all trials evaluating the SBP have reported a decrease after IMT, the heterogeneity results mainly from the different degrees of the improvement of SBP between studies instead of whether it is effective or not. This difference may be attributed to clinical heterogeneity. For example, Ramos-Barreara et al. [1] enrolled some participants using CPAP or OA in both groups. A sensitivity analysis conducted after that study was excluded yielded similar results (Supplemental Fig. 1). Another possibility is that the improvement of SBP was less in the study by Souza et al., which used a lower training threshold of IMT (50–60% of MIP) than that used by the other 2 studies (75% of MIP), despite the fact that all three studies were characterized as high-intensity IMT (> 60% of MIP). Current RCTs examining blood pressure–related outcomes used high-intensity IMT, so the effect of low-intensity IMT remains unclear. A study indicated that a low-intensity IMT program reduced blood pressure in patients with hypertension [12]; however, future studies must investigate whether similar benefits can be observed in patients with concomitant OSA.
Our meta-analysis was unable to demonstrate a significantly positive effect of IMT on AHI, which may be attributed to small sample size and heterogeneity. There were varying degrees of OSA severity, different training intensity, times, and duration among our included studies. According to the subgroup analysis, low-intensity IMT studies result in superior performance. In those studies, they enrolled patients with AHI greater than 29 and performed 12 weeks of low-intensity IMT [14, 15]. Although the exact mechanisms of IMT on the upper airway is unknown, upper airway patency may deteriorate if the negative pressure generated by the inspiratory muscle exceeds the pressure produced by the upper airway dilating muscle to maintain upper airway patency, which may explain why high-intensity IMT results in an unfavorable result in our subgroup analysis [30]. However, this is not the case in majority of patients with OSA. In most situations, sleep-related decreases in dilating muscle activity of upper airway and anatomical predisposition to airway closure would narrow or obstruct the airway in OSA [30]. Further research is required to validate the underlying mechanisms and to determine the optimal training settings to improve OSA based on current findings. Furthermore, our result indicated that IMT is not influencing outcomes by addressing upper airway collapse, so it may benefit other populations through improving sympathetic overactivation, such as for the patients with chronic heart failure [31] or hypertension [12], rather than OSA in particular.
Clinically, many patients have difficulty tolerating CPAP due to air leakage, dryness, and general discomfort from the mask and headgear, which may negatively affect sleep comfort. However, IMT is performed while awake, and our meta-analysis revealed that it significantly improved sleep quality and reduced daytime sleepiness compared with the control group. According to Vranish et al., patients with OSA who underwent IMT had fewer nighttime arousals and periodic limb movements, which may be associated with improved sleep quality [17]. In addition, because periodic limb movements are accompanied by blood pressure surges and increased sympathetic activation, their reduction may decrease the sympathetic activity. On the other hand, daytime sleepiness in patients with OSA was associated with decreased baroreflex sensitivity and increased sympathetic cardiac modulation throughout the night. Furthermore, a vicious cycle of increased sympathetic nerve activity and decreased baroreflex sensitivity existed, and baroreceptor afferents may influence alertness during wakefulness [32]. Taken together, the results indicate that IMT may improve daytime sleepiness not only due to less arousal and better sleep quality but also due to decreased sympathetic output.
Our review has several strengths. First, our study is the first comprehensive meta-analysis for all relevant studies focusing on the efficacy of only one intervention, namely IMT. Previous review has pooled the results from various training interventions and did not evaluate the effects on blood pressure and respiratory function [19, 20]. Second, the inclusion criteria were systematically and explicitly applied, research quality was carefully considered, and rigorous analytical methods were used. Third, we evaluated not only sleep-related outcomes but also blood pressure and respiratory outcomes and performed a subgroup analysis to distinguish between the efficacy of low-intensity and high-intensity IMT.
However, this study has some limitations. First, the level of certainty was low mainly due to some risk of bias and small sample sizes. The considerable loss to follow-up may have resulted in some bias, limiting the statistical power and precision of our results. Second, some clinical heterogeneity was noted across trials in relation to patient characteristics (e.g., severity of OSA, with or without hypertension, or with or without other treatment for OSA), protocols of the intervention and control groups, and follow-up duration. Third, the benefits on blood pressure were evaluated only with high-intensity IMT and not low-intensity IMT; therefore, whether or not low-intensity IMT also helps reduce blood pressure remains unclear. Finally, most participants enrolled in our included trials had moderate or severe OSA (mean AHI: approximately 30/h) without uncontrolled hypertension. Future studies must evaluate whether IMT is effective in individuals with mild OSA or uncontrolled hypertension.
Conclusion
Although the level of certainty was low, our study is the first comprehensive meta-analysis focusing on IMT for patients with OSA. IMT lowered blood pressure, improved sleep quality, and reduced daytime sleepiness, but did not affect AHI, in patients with mostly moderate or severe OSA. Although CPAP is the first-line therapy in patients with moderate to severe OSA, who may also have concomitant hypertension, intolerance or poor adherence to CPAP is common, which reduces its benefits. In these patients, IMT may serve as a daytime treatment option to lower blood pressure and improve sleep quality. Additional studies with larger sample sizes, optimal training intensity, and long-term follow-up and those enrolling patients with mild OSA and/or uncontrolled hypertension are required to comprehensively understand the efficacy of IMT on OSA.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary materials.
References
Ramos-Barrera GE, DeLucia CM, Bailey EF (2020) Inspiratory muscle strength training lowers blood pressure and sympathetic activity in older adults with OSA: a randomized controlled pilot trial. J Appl Physiol 1985 129:449–458. https://doi.org/10.1152/japplphysiol.00024.2020
Ahmad M, Makati D, Akbar S (2017) Review of and updates on hypertension in obstructive sleep apnea. Int J Hypertens 2017:1848375. https://doi.org/10.1155/2017/1848375
Hou H et al (2018) Association of obstructive sleep apnea with hypertension: a systematic review and meta-analysis. J Glob Health 8:010405. https://doi.org/10.7189/jogh.08.010405
Virani SS et al (2021) Heart Disease and Stroke Statistics-2021 update: a report from the American Heart Association. Circulation 143:e254–e743. https://doi.org/10.1161/cir.0000000000000950
Martynowicz H et al (2021) Renalase and hypertension-demographic and clinical correlates in obstructive sleep apnea. Sleep Breath 25:669–675. https://doi.org/10.1007/s11325-020-02157-3
Fava C et al (2014) Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 145:762–771. https://doi.org/10.1378/chest.13-1115
Bratton DJ et al (2015) CPAP vs Mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA 314:2280–2293. https://doi.org/10.1001/jama.2015.16303
McEvoy RD et al (2016) CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 375:919–931. https://doi.org/10.1056/NEJMoa1606599
Whelton PK et al (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71:e13–e115. https://doi.org/10.1161/HYP.0000000000000065
Kline CE et al (2011) The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep 34:1631–1640. https://doi.org/10.5665/sleep.1422
Ferreira S et al (2021) Clinical safety and hemodynamic, cardiac autonomic and inflammatory responses to a single session of inspiratory muscle training in obstructive sleep apnea. Sleep Breath 26(1):99–108. https://doi.org/10.1007/s11325-021-02364-6
Ferreira JB et al (2013) Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol 166:61–67. https://doi.org/10.1016/j.ijcard.2011.09.069
Cheng S et al (2014) Healthy humans with a narrow upper airway maintain patency during quiet breathing by dilating the airway during inspiration. J Physiol 592:4763–4774. https://doi.org/10.1113/jphysiol.2014.279240
Erturk N et al (2020) The effectiveness of oropharyngeal exercises compared to inspiratory muscle training in obstructive sleep apnea: a randomized controlled trial. Heart Lung 49(6):940–948. https://doi.org/10.1016/j.hrtlng.2020.07.014
Lin HC et al (2019) The effects of threshold inspiratory muscle training in patients with obstructive sleep apnea: a randomized experimental study. Sleep Breath 24(1):201–209. https://doi.org/10.1007/s11325-019-01862-y
Souza AKF et al (2018) Effectiveness of inspiratory muscle training on sleep and functional capacity to exercise in obstructive sleep apnea: a randomized controlled trial. Sleep Breath 22:631–639. https://doi.org/10.1007/s11325-017-1591-5
Vranish JR, Bailey EF (2016) Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep 39:1179–1185. https://doi.org/10.5665/sleep.5826
Nóbrega-Júnior JCN et al (2020) Inspiratory muscle training in the severity of obstructive sleep apnea, sleep quality and excessive daytime sleepiness: a placebo-controlled, randomized trial. Nat Sci Sleep 12:1105–1113. https://doi.org/10.2147/NSS.S269360
Hsu B et al (2020) Effects of respiratory muscle therapy on obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med 16:785–801. https://doi.org/10.5664/jcsm.8318
Torres-Castro R et al (2022) Respiratory muscle training in patients with obstructive sleep apnoea: a systematic review and meta-analysis. Clocks Sleep 4:219–229. https://doi.org/10.3390/clockssleep4020020
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Verges S (2019) Respiratory muscle training. In: Cogo A, Bonini M, Onorati P (eds) exercise and sports pulmonology: pathophysiological adaptations and rehabilitation. Springer International Publishing, Cham, pp 143–151
de Abreu RM et al (2017) Effects of inspiratory muscle training on cardiovascular autonomic control: a systematic review. Auton Neurosci 208:29–35. https://doi.org/10.1016/j.autneu.2017.09.002
Hill K et al (2010) Inspiratory muscle training for patients with chronic obstructive pulmonary disease: a practical guide for clinicians. Arch Phys Med Rehabil 91:1466–1470. https://doi.org/10.1016/j.apmr.2010.06.010
Guyatt G et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Green M et al (2021) Meta-analysis of changes in the levels of catecholamines and blood pressure with continuous positive airway pressure therapy in obstructive sleep apnea. J Clin Hypertens (Greenwich) 23:12–20. https://doi.org/10.1111/jch.14061
Iftikhar IH et al (2013) Effect of oral appliances on blood pressure in obstructive sleep apnea: a systematic review and meta-analysis. J Clin Sleep Med : JCSM : Off Publ Am Acad Sleep Med 9:165–174. https://doi.org/10.5664/jcsm.2420
Tan SN, Yang HC, Lim SC (2021) Anatomy and pathophysiology of upper airway obstructive sleep apnoea: review of the current literature. Sleep Med Res 12:1–8. https://doi.org/10.17241/smr.2020.00829
Mello PR et al (2012) Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J Cardiopulm Rehabil Prev 32:255–261. https://doi.org/10.1097/HCR.0b013e31825828da
Cortelli P et al (2012) Baroreflex modulation during sleep and in obstructive sleep apnea syndrome. Auton Neurosci 169:7–11. https://doi.org/10.1016/j.autneu.2012.02.005
Acknowledgements
This study was supported by Taipei Medical University, Shuang Ho Hospital, and the Center for Evidence-Based Health Care (Department of Medical Research, Shuang Ho Hospital). In addition, this manuscript was edited by Wallace Academic Editing. We thank Professor E. Fiona Bailey, University of Arizona, for providing raw data to perform our meta-analysis.
Author information
Authors and Affiliations
Contributions
Tzu-Ang Chen and Sheng-Ting Mao contributed equally.
Conceptualization: Sheng-Ting Mao, Tzu-Ang Chen; data curation: Sheng-Ting Mao, Tzu-Ang Chen; formal analysis: Sheng-Ting Mao, Tzu-Ang Chen, Yi-Chun Kuan; investigation: Sheng-Ting Mao, Tzu-Ang Chen, Yi-Chun Kuan; methodology: Sheng-Ting Mao, Tzu-Ang Chen; project administration: Sheng-Ting Mao, Tzu-Ang Chen, Yi-Chun Kuan; resources: Ka-Wai Tam, Yi-Chun Kuan; supervision: Yi-Chun Kuan; validation: Huei-Chen Lin, Wen-Te Liu, Ka-Wai Tam, Cheng-Yu Tsai, Yi-Chun Kuan; roles/writing — original draft: Sheng-Ting Mao, Tzu-Ang Chen; writing — review and editing: Huei-Chen Lin, Wen-Te Liu, Ka-Wai Tam, Cheng-Yu Tsai, Yi-Chun Kuan.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. No human participants included. For this type of study, formal consent is not required.
Consent to participate
This article does not contain any studies with human participants performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, TA., Mao, ST., Lin, HC. et al. Effects of inspiratory muscle training on blood pressure- and sleep-related outcomes in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath 27, 1953–1966 (2023). https://doi.org/10.1007/s11325-022-02773-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02773-1