Abstract
In Grand Bay National Estuarine Research Reserve (Grand Bay NERR), Mississippi, we used quantitative drop sampling in three common shallow estuarine habitats—low profile oyster reef (oyster), vegetated marsh edge (VME), and nonvegetated bottom (NVB)—to address the dearth in research comparing nekton utilization of oyster relative to adjacent habitats. The three habitats were sampled at two distinct marsh complexes within Grand Bay NERR. We collected a total of 633 individual fishes representing 41 taxa in 22 families. The most diverse fish family was Gobiidae (seven species) followed by Blennidae and Poeciliidae (three species each). We collected a total of 2,734 invertebrates representing 24 taxa in 11 families. The most diverse invertebrate family was Xanthidae (six species) followed by Palaemonidae (five species). We used ordination techniques to examine variation in species relative abundance among habitats, seasons, and sampling areas, and to identify environmental gradients correlated with species relative abundances. Our resulted indicated that oyster provided a similarly complex and important function as the adjacent VME. We documented three basic trends related to the importance of oyster and VME habitats: 1) Oyster and VME provide habitat for significantly more species relative to NVB, 2) Oyster and VME provide habitat for rare species, and 3) Several species collected across multiple habitats occurred at higher abundances in oyster or VME habitat. We also found that salinity, temperature, and depth were associated with seasonal and spatial shifts in nekton communities. Lastly, we found that the relative location of the two marsh complexes we studied within the context of the whole estuary may also explain some of the temporal and spatial differences in communities. We conclude that oyster habitat supported a temporally diverse and spatially distinct nekton community and deserves further attention in research and estuarine conservation efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries along the Gulf of Mexico are characterized by a patchwork of shallow water estuarine habitats. Estuarine residents, such as grass shrimp (Palaemonidae), mud crabs (Xanthidae), gobies (Gobiidae), and toadfish (Opsanus spp.), depend on these shallow habitats for food resources, refuge from predation, and sites for reproduction (Breitburg et al. 2000; Kneib 1997; Shervette et al. 2004). Estuarine-dependent marine residents, including several of economic importance, such as blue crabs Callinectes sapidus, white shrimp Litopenaeus setiferus, brown shrimp Farfantopeneaus aztecus, and spot Leiostomus xanthurus, also utilize these habitats for food and refuge (Boesch and Turner 1984; Baltz et al. 1993; Howe and Wallace 2000; Harding and Mann 2001).
Common estuarine habitats such as vegetated marsh, oyster reef, and nonvegetated bottom are considered essential for a multitude of fishes and invertebrates. Many studies have documented the importance of structurally complex vegetated marsh habitats to fishes and invertebrates (see review in Minello et al. 2003). Juveniles of several species are dependent on vegetated marsh habitats as evidenced by studies reporting higher growth rates in Spartina marsh edge habitat when compared to adjacent habitats (Minello et al. 1989; Stunz et al. 2002). Other studies have demonstrated high survival rates of juveniles in salt marsh habitats (Minello and Zimmerman, 1983, 1985; Minello et al. 1989). Not as much literature substantiates the importance of oyster habitat (see review: Peterson et al. 2003) relative to adjacent habitats. Glancy et al. (2003) found that oyster reefs support distinct assemblages of decapod crustaceans and represent an important ecological component of estuarine habitats. Glancy et al. (2003) speculated that the mechanisms underlying the importance of oyster habitat may include increased survival or greater forage availability for decapods. Nonvegetated bottom habitats, often adjacent to vegetated marsh edge, sea grass, oyster, and other habitats, also support many estuarine species (Zimmerman et al. 1990; Minello et al. 1994; Rozas and Minello 1998; Castellanos and Rozas 2001). In fact, some species, such as Atlantic croaker Micropagonias undulatus and spot L. xanthurus, may select for open water habitat (including nonvegetated bottom areas) over vegetated marsh (Minello et al. 2003).
Several studies have characterized the inhabitants of oyster reef habitats along the coastal Gulf of Mexico and the Atlantic through various sampling strategies (Zimmerman et al. 1989; Larsen et al. 2001; Perry et al. 2001; Grabowski 2002; Glancy et al. 2003). However, few published studies have compared fish and macroinvertebrate communities, species abundances, and species richness between oyster and adjacent vegetated and unvegetated habitats (Zimmerman et al. 1989; Glancy et al. 2003; Hosack et al. 2006). In fact, no previously published research has compared the fish and invertebrate communities of oyster, marsh, and nonvegetated bottom habitats. Such comparisons are essential in determining relative habitat value and targeting conservation efforts within estuaries (Beck et al. 2001).
The goal of our study was to evaluate the relationship between habitat and nekton community structure. In addition, we investigated relationships among physicochemical variables, habitats, and spatiotemporal variation in nekton community structure in a Mississippi estuary. We specifically characterized species composition, relative abundance, and richness of fishes and invertebrates occupying oyster reefs and oyster shell (oyster), vegetated marsh edge (VME), and nonvegetated bottom (NVB) habitats in Grand Bay National Estuarine Research Reserve (Grand Bay NERR). To address the current lack of quantitative studies comparing oyster and adjacent habitats, we designed our study to determine if oyster, vegetated marsh edge, and nonvegetated bottom support distinct nekton communities and if observed patterns vary seasonally and spatially within Grand Bay NERR.
Materials and Methods
Study Areas
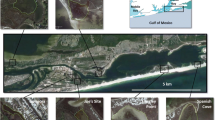
Grand Bay NERR is located on the Mississippi coast in the north central Gulf of Mexico (Fig. 1). It is a productive and diverse estuary occupying a total area of 74.5 km2 and is bordered on the west by the heavily industrialized Pascagoula estuary and on the east by another heavily industrialized estuary, Mobile Bay. The Grand Bay estuary is microtidal with a typical tidal range of 30–60 cm. For this study, we focused sampling in two main marsh complexes within Grand Bay NERR: Bayou Heron and Crooked Bayou (Figs. 1 and 2). Bayou Heron is located in the upper zone of the estuary and is characterized by oligohaline salinities. Common shallow habitats included vegetated Spartina alterniflora marsh edge and inner marsh, low profile Crassostrea virginica oyster shell from oyster midden deposits (with no live oyster), and shallow nonvegetated bottom. In addition, Bayou Heron had small amounts of subtidal Ruppia maritima that occurred in small, sparse, patchy beds. Crooked Bayou, located closer to the outer zone of the estuary, is approximately 6 km southwest of Bayou Heron. Crooked Bayou is characterized by polyhaline salinities and is connected directly with Mississippi Sound. Common shallow habitats in the Crooked Bayou marsh complex included vegetated Spartina alterniflora marsh edge and inner marsh, low profile C. virginica oyster reefs and oyster midden deposits, and shallow nonvegetated bottom. No subtidal seagrasses were observed in Crooked Bayou.
Quantitative Nekton Sampling
To determine nekton community composition of vegetated marsh edge (VME), oyster, and nonvegetated bottom (NVB) habitats of Grand Bay NERR, we sampled in Crooked Bayou and Bayou Heron, two marsh complexes that had all three of these habitats (Figs. 1 and 2). For both marsh complexes, sampling occurred in Fall 2003 (4–10 October), Spring 2004 (13–20 May), and Summer 2004 (16–28 July) within 2 hours of high tide when all target habitats were completely inundated. To determine where to sample within each habitat, we created a rough map of each sampling area on a numbered grid. We used a random numbers table with the map to determine where in each habitat to sample each replicate. We repeated this procedure each season for both sampling areas.
We chose drop sampling for assessing nekton communities of the three habitats because the catch efficiency does not appear to vary significantly with habitat characteristics (Rozas and Minello 1997). We randomly collected four replicates in each of the three habitats present at the two sampling areas with a 1.17-m2 drop sampler according to the procedures of Zimmerman et al. (1984). A total of 72 drop samples (two sampling areas × three habitats × four replicates × three seasons) were collected by dropping a 1.4-m diameter × 1.5-m-tall cylinder from a boom mounted on the bow of a 5.1-m boat with the boom extending an additional 2.4 m. Two people positioned the cylinder over the randomly selected site by slowly pushing the skiff by the stern. Once the cylinder was in place it was released from the boom and rapidly enclosed a 1.17-m2 area.
In each drop sample, we measured temperature (°C), salinity (Practical Salinity Units: PSU), and dissolved oxygen (DO: mg/L) using a YSI 85 meter and water depth (cm) using measuring tape. After collecting these data, we used a pump and hose with plastic mesh (1 mm) fixed to the intake nozzle to pump out water from within the sampler. In the VME habitat, we removed marsh vegetation from the sampler and recorded the number of stems present. Stems were pulled apart over a 3-mm sieve and rinsed thoroughly so any organisms hiding within the roots were collected. In oyster habitat, percent oyster cover was recorded after water was removed. All oysters were removed from the sampler and washed over 3-mm plastic mesh sieve, and the organisms recovered were collected. Oyster clusters were always broken apart and rinsed thoroughly so organisms hiding in the interstitial spaces were collected. If any oyster was found in VME or NVB samples, percent oyster was recorded and oyster was processed as described previously. Percent oyster was always assessed by V. Shervette for consistency. While the water was being pumped out of the drop sampler, we collected nekton from the drop sampler with dipnets (3.2-mm mesh) until each of the two dipnetters had five consecutive no-catches once the water was completely removed. In addition, we inspected the bottom of the sampled area for organisms missed by nets after water was pumped out. All organisms collected were euthanized with MS-222, preserved in 10% buffered formalin for at least 4 days then stored in 70% ethanol. Fish and invertebrates were identified to species and measured with digital calipers: fish were measured to 0.1 mm standard length (SL), crabs to 0.1 mm carapace length (CL), and shrimp to 0.1 mm total length (TL).
Statistical Analyses
Seasonal data were analyzed separately, unless otherwise indicated, because many species occurred during one season only. We calculated species diversity using Shannon’s Index of Diversity (H1),
where p i is the proportion of the density comprised by the ith taxa. We also assessed species richness (number of species collected) for each habitat, season, and the two sampling areas.
We used randomized block analysis of variance (ANOVA) to test for significant differences in total density (fish and macroinvertebrates combined) among habitats. Assumptions for the ANOVA test included normality and homogeneity of variance. Density data were log(x + 1) transformed to meet the assumptions. Density was the dependent factor, habitat was the independent factor, and sampling area was the blocking factor. We conducted additional separate randomized block ANOVA to determine significant differences among habitats and between sampling areas for the following dependent variables: salinity, temperature, DO, depth, and species richness. If needed, data were log(x + 1) or square-root transformed to meet assumptions of tests.
Correspondence analysis (CA) of the species-by-replicate matrix was used to examine variation in species relative abundance among habitats, seasons, and between sampling areas. Density data were log(x + 1) transformed. Multi-response permutation procedures (MRPP) were performed to test the null hypothesis of no difference in species relative abundance among the three seasons within and among the two sampling areas and three habitats. MRPP is a nonparametric technique used to test the significance of a priori sample groupings when the data violate the assumptions of parametric procedures such as multivariate analysis of variance. When significant sample groupings were detected, comparisons were made using Bonferroni corrected p values.
Canonical correspondence analysis (CCA) was used to identify environmental gradients correlated with species relative abundance. CCA is a weighted averaging method, which directly relates community data to environmental variables by constraining species ordination patterns that correlate maximally with the environmental variables. Inter-set correlations between environmental variables (salinity, temperature, depth, stem density, and percent oyster) were used to determine each variable’s contribution. Monte Carlo permutation analysis simulation was used to test the significance (p = 0.05) of the contribution of each variable to the CCA axes. Only significant, non-redundant variables were retained for interpretation. Both CA and CCA (including the Monte Carlo permutation analysis) were performed using CANOCO (Version 4, Microcomputer Power) and MRPP was performed using PC-ORD version 4 (McCune and Mefford 1999).
Results
Environmental Data
Salinity did not vary significantly across habitat types during any of the sampling periods (randomized block ANOVA: p > 0.05; Table 1) but was consistently higher at Crooked Bayou compared to Bayou Heron (for all three seasons randomized block ANOVA: p < 0.001). Mean temperature varied seasonally (Table 1). Consistent for the three sampling periods, temperature did not vary significantly among habitats or between sampling areas (randomized block ANOVA: p > 0.05). Depth was significantly less in intertidal VME habitat relative to the other two habitats during the three sampling periods at both sampling areas (Table 1).
Species Richness and Abundance
A total of 633 individual fishes representing 41 taxa in 22 families were collected with the drop sampler (Table 2). Twenty-eight species were collected in VME, 13 exclusively; 13 species were collected from NVB, none exclusively; and 27 species were collected in oyster, nine exclusively. In Fall 2003, Spring 2004, and Summer 2004, we collected 27, 17, and 25 fish species, respectively. Ten species were collected exclusively in Fall 2003, four species were collected exclusively in Spring 2004, and five species were collected exclusively in Summer 2004.
A total of 2,734 individual invertebrates representing 24 taxa in 11 families were collected with the drop sampler. Twenty-two species were collected in VME habitat with three species exclusively; 10 species were collected in NVB habitat with none exclusively; 20 species were collected in oyster, two species exclusively. Fall 2003 samples had 17 species of invertebrates with one species collected exclusively during that season. Spring 2004 samples had 16 species of invertebrates with two species exclusively. Summer 2004 samples had 19 species of invertebrates with four exclusively (Table 2).
Nonvegetated bottom habitat had the highest overall diversity compared to the other two habitats, but only 24 species were collected in NVB (Table 3). Oyster habitat had the second highest diversity and a total of 49 species. VME habitat had the highest number of species overall (52), but the lowest diversity. Among seasons, Spring 2004 had the lowest overall species richness (34 species collected), but the highest diversity. Fall 2003 had the second highest diversity. Both Fall 2003 and Summer 2004 had a species richness of 46. Summer 2004 had the lowest diversity. Bayou Heron had lower diversity and richness than Crooked Bayou (Table 3).
For each of the three sampling periods, mean total richness among habitats was significantly different (Fig. 3a). Within each season, mean total richness was significantly greater in VME and oyster habitat relative to nonvegetated habitat (Bonferroni post hoc comparisons p < 0.005; Fig. 3a).
In Fall 2003, mean total density was significantly different among habitats (randomized block ANOVA: F 4, 2 = 20.8, p < 0.001) and not significantly different between sampling areas (F 0 .2, 1 = 0.9, p = 0.4). Bonferroni post hoc tests indicated the following relationship for mean total density among the habitats (when significant p < 0.001): VME = oyster > NVB (Fig. 3b). In Spring 2004 sampling, mean total density was significantly different among habitats (F 1.7, 2 = 48.3, p < 0.001) and between sampling areas (F 11, 1 = 6.1, p = 0.02), with Crooked Bayou having the higher mean density. Post hoc tests indicated the following relationships among habitats for mean density: VME > oyster > NVB (p < 0.03). Mean densities in Summer 2004 sampling differed significantly among habitats (F 4.1, 2 = 25.3, p < 0.001) and did not differ significantly between sampling areas (F 0.3, 1 = 1.8, p = 0.2). Post hoc tests indicated the following relationship among habitats for mean density: VME = oyster > NVB (p < 0.001).
Multivariate Community Analyses
Community structure differed significantly among the three seasons and between the two sampling areas (Fig. 4; Table 4). The CA produced two axes that explained 84% of the variation in species relative abundances. Samples collected in Spring 2004 generally had higher scores on Axis 1 associated with more Callinectes similis, L. xanthurus, and F. aztecus. Fall and Summer samples generally had lower scores on Axis 1 associated with more Alpheus sp., Eucinostomus melanopterus, and Orthopristis chrysoura. Spring VME and most of summer VME samples had higher scores on Axis 2 associated with more Fundulus grandis, Cyprinodon variegates, and Menidia berrylina.
Within Fall 2003 sampling season, community structure differed significantly between sampling areas and among the site-habitat combinations (Fig. 5a; Table 4). The CA produced two axes that explained 61% of the variation in species relative abundances. Bayou Heron samples generally had positive values on Axis 1 associated with the presence of Palaemonetes pugio, M. berrylina, and Evorthodus minutus. Crooked Bayou samples generally had negative values on Axis 1 associated with the presence of two of the gastropod species Clibanarius vittatus and Stramonita haemastoma. Community structure during Spring 2004 sampling differed significantly between sampling areas, among habitats, among habitats within Crooked Bayou, and among site–habitat combinations (Fig. 5b; Table 4). The CA produced two axes that explained 89% of the variation in species assemblages. Crooked Bayou samples generally had more positive values on Axis 1 and were characterized by higher abundances of Ctenogobius bolesoma, Panopeus obesus, and S. haemastoma. Bayou Heron samples were characterized by higher abundances of Gobiosoma bosc, Litopanaeus setiferus, and Neritina usnea. During this season, major habitat differences were characterized by higher abundances of Littorina irrorata and L. setiferus in VME and L. xanthurus and Symphurus plagiusa in NVB (Fig. 5b). Community structure during Summer 2004 differed significantly between sampling areas, among habitats, among habitats within Bayou Heron, and among site-habitat combinations (Fig. 5c; Table 4). The CA produced two axes that explained 67% of the variation in species assemblages. Along Axis 1, Bayou Heron samples were characterized by higher abundances of C. variegates and F. grandis, whereas Crooked Bayou samples were characterized by higher abundances of Alpheus sp. and Eurypanopeus depressus. Along Axis 2, oyster habitat was characterized by higher abundances of Gobiesox stromosus and C. variegates.
Canonical Correspondence Analysis (CCA) resulted in a total model inertia of 3.35. Eigenvalues for the first four multivariate axes were 0.306 for CCA axis 1, 0.143 for CCA axis 2, 0.105 for CCA axis 3, and 0.078 for CCA axis 4. Cumulative percent variance of species-environmental relationship for all four CCA axes was 91.5%. Correlations between five of the six environmental variable and the first four axes were statistically significant (p < 0.03 for percent oyster, salinity, depth, marsh stem density, and temperature). All environmental variables were retained except for DO (Fig. 6). Salinity, percent oyster, and depth were strongly correlated with Axis 1 (Fig. 6). Axis 1 explained 44.2% of the species environmental relationship. Depth, marsh stem density, and temperature were strongly correlated with Axis 2, which explained 20.7% of the species–environment relationship. Axis 1 models a salinity-oyster gradient that distinguishes species associated with the higher salinities of Crooked Bayou and some of the oyster-associated species. Axis 2 represents a more seasonal gradient with species collected mainly in Spring 2004 (high scores) separated from species collected through the study (scores close to the origin) and species collected in seasons other than Spring 2004 (low scores).
Some of the more notable species environmental relationships include: 1) Alpheus sp., P. simpsoni, Panopeus obesus, and E. depressus were strongly associated with samples in which oyster was present, 2) C. vittatus and S. haemostoma were associated with higher salinities, and 3) P. pugio was correlated with stem density (Fig. 6).
Discussion
Habitat-specific Trends in Nekton Abundances and Communities
When we examined nekton abundances, oyster, both spatially and temporally, supported similarly high densities of nekton as VME. Also, oyster consistently equated VME in species richness. Both oyster and VME supported significantly higher densities and species richness compared with adjacent NVB. Several studies have documented higher organism abundances and densities in structured habitats, such as marsh or oyster, relative to unstructured habitat (Glancy et al. 2003; Heck et al. 2003; Lehnert and Allen 2002; Zimmerman et al. 1989). In addition, some research has demonstrated higher species richness in structured habitats (Heck et al. 2003; Lehnert and Allen 2002). Our study supports the idea that oyster habitat, when compared with adjacent VME and NVB, is occupied by a distinct community of fishes and invertebrates and supports high densities of these residents and estuarine-dependent species.
Direct comparisons of fish and invertebrate communities between adjacent VME and oyster habitats are lacking in current literature. To our knowledge, ours is the first peer-reviewed study to directly compare communities of adjacent oyster, VME, and NVB habitats. One study, Glancy et al. (2003), examined the invertebrate communities of adjacent sea grass, nonvegetated marsh edge, and oyster habitats and documented habitat-specific communities. Many studies have examined fish and invertebrate use of oyster habitat in general (Glancy et al. 2003; Harding and Mann 2001, 1999 Coen et al. 1999; Wenner et al. 1996) and these studies have contributed greatly to the current understanding of oyster habitat. However, few studies that utilized enclosure sampling included oyster in habitat comparisons (Minello et al. 2003; but see Glancy et al. 2003; Zimmerman et al. 1989). This deficit has resulted in the low value ranking of oyster habitat compared to other salt marsh estuarine habitats (Minello et al. 2003). Our study and Glancy et al. (2003) do not support such findings. Both studies clearly demonstrate that oyster supports high nekton abundances relative to other shallow estuarine habitats.
The occurrence and prevalence of several species in our study appeared to be related to the presence of live oyster clusters and oyster shell within the three habitats. A similar relationship was documented in a Texas estuary (Zeug et al. 2007). In our study, sampling was conducted in a random design within a turbid environment, and on several occasions small clusters of oyster were discovered in and collected from VME and NVB habitats (Table 1). Mud crabs (P. obesus, P. simpsoni, E. depressus) and snapping shrimp (Alpheus sp.) were highly correlated with percent oyster present in samples according to CCA results (Fig. 4). These species were collected in the non-oyster habitats, but only when oyster was also collected in the samples. Eurypanopeus depressus and Panopeus spp. are common oyster reef residents (Glancy et al. 2003; Shervette et al. 2004) and Alpheus spp. have also been collected in habitats where oyster was present (Zimmerman et al. 1989; Lehnert and Allen 2002; Glancy et al. 2003; Shervette et al. 2004; Zeug et al. 2007). Differences in habitat-specific communities were not always strong because many species occurred in multiple habitats. If we had sampled exclusive NVB and VME habitats, we may have documented stronger differences in communities among habitats (Rozas and Minello 1997). In addition, the two sampling areas, Bayou Heron and Crooked Bayou, had a distinct difference between their oyster habitats. The Bayou Heron oyster habitat was comprised of shell hash with no live oyster (their settlement and growth inhibited by the lower salinities), whereas the Crooked Bayou oyster habitat was comprised of a combination of shell hash and live oyster. The differences in the oyster habitat between the two areas may have contributed to the overall variability within the community of organisms utilizing oyster.
Oyster and VME may provide habitats for relatively rare species. Nine fish species and two invertebrate species were collected exclusively in oyster habitat. Similarly for VME habitat, 13 fish species and three invertebrate species were collected exclusively in VME. Our results indicate that at least some of the species we collected exclusively in one habitat may prefer that habitat over the others. In addition, these species can be considered relatively rare because they occur naturally in relatively low abundances, especially outside of their peak recruitment periods. The two blenny species collected exclusively in oyster habitat (Hypsoblennius hentzi and H. ionthas) are commonly associated with oyster reefs (Coen et al. 1999). Fundulus jenkinsi was collected exclusively in summer VME and is considered an uncommon species that occurs in Grand Bay NERR (M. Woodrey, research coordinator, Grand Bay NERR, personal communication). The two Sygnathus spp. were collected exclusively in VME in the current study and in a similar unpublished study (Zimmerman et al. 1989). The lyre goby Evorthodus lyricus also appears to prefer VME habitat and occurs at lower abundance than other estuarine gobies (V. Shervette, unpublished data). In addition, toadfish Opsanus beta, an oyster-associated fish (Shervette et al. 2004), was collected exclusively in marsh habitat, but only in samples with oyster. So, oyster and VME may provide important habitat for some of the less abundant fish species.
Many fish and invertebrate species found in two or more habitats occurred at higher densities in one specific habitat, either oyster or VME. For example, F. grandis was collected in all three habitats, but occurred at higher abundances in marsh samples. The goby Gobiosoma bosc was also collected in all three habitats, but more were collected in marsh samples. For the invertebrates, white shrimp Litopenaeus setiferus, the mud crabs Panopeus simpsoni, P. obesus, E. depressus, and Rhithropanopeus harrisoni, were collected in multiple habitats, but consistently occurred at higher abundances in oyster. The grass shrimp P. pugio occurred in VME and oyster habitats, but more P. pugio were consistently collected in VME. Other studies have found similar relationships with one or more of these species. During fall sampling, Minello and Webb (1997) collected a higher mean density of Ctenogobius bolesoma in natural VME (3.3 individuals per 2.6 m2) relative to NVB (0.9 individuals per 2.6 m2). That study also documented a higher mean density of P. pugio in VME (234.5 individuals per 2.6 m2) relative to NVB (0.6 individuals per 2.6 m2) for the same season. Rozas and Reed (1993) found that F. grandis used structured habitat (intact vegetated marsh) over non-structured habitat (deteriorated hummocky Spartina marsh).
Temporal and Spatial Trends in Nekton Abundances and Communities
Differences in fish and invertebrate abundances and community structure may be related to observed differences in environmental variables. Many studies have observed a relationship between temporal and spatial shifts in community structure and changes in environmental factors such as temperature, salinity, and DO (Rakocinski et al. 1996; Gelwick et al. 2001; Akin et al. 2003). In the current study we found that salinity, temperature, and depth were associated with seasonal and spatial shifts in nekton communities. The CA and MRPP results demonstrated that with each season and over the course of the whole study the fish and invertebrate community of Crooked Bayou differed from that of Bayou Heron (Table 4). We also found that salinity varied temporally and was consistently higher in Crooked Bayou. Temperature also increased temporally, but did not vary between the areas. Salinity is often cited as important in the organization of estuarine communities (Rakocinski et al. 1992; Baltz et al. 1998; Gelwick et al. 2001; Kupschus and Tremain 2001; Akin et al. 2003). In fact, salinity zones are commonly identified within an estuary and utilized in long-term monitoring of community dynamics as a measure of ecosystem health (Bulger et al. 1993). In our study common polyhaline species, such as the hermit crab Clibanarius vittatus and the oyster drill, S. haemasroma, occurred only in samples from Crooked Bayou, where salinity was within the polyhaline range. Results from the CCA confirmed the strong relationship between the abundances of many of the species we collected and salinity.
The relative location of the two marsh complexes within the context of the whole estuary may also explain some of the temporal and spatial differences in communities. Location also explains the differences in salinities between the two areas. Bayou Heron is situated in the upper zone of Grand Bay NERR within 1 km of an underground freshwater source. Crooked Bayou, although receiving some freshwater from rain events, is located in a lower zone of the estuary and is directly connected to Mississippi Sound (Fig. 1). These different locations may vary in their proximity to marine larval and freshwater larval supplies. Proximity to larval sources has been documented as an important factor in determining community composition and organismal abundances (Heck and Thoman 1984). Timing of larval recruitment also plays a role in temporal fish and invertebrate community composition and abundance patterns (Akin et al. 2003) and our study demonstrated through the seasonal occurrence of several species how temporal recruitment affects nektonic communities. For example, in our study we collected the majority of brown shrimp in Spring 2004, which coincided with the timing of their recruitment period into Mississippi estuaries.
Conclusions
The goal of our study was to determine the relationship between three common shallow estuarine habitats (oyster, VME, and NVB) and nekton community structure to address the lack of research comparing oyster with adjacent habitats. In obtaining that goal, we documented three basic trends related to the importance of oyster and VME habitats: 1) Oyster and VME provide habitat for significantly more species relative to NVB; 2) Oyster and VME provide habitat for uncommon and rare species; and 3) Several species collected across multiple habitats occurred at higher abundances in oyster or VME habitat. We also found that contrary to the current low-value ranking of oyster habitat relative to other estuarine habitats (Minello et al. 2003), oyster provides high-quality habitat for many species. As a structured habitat, oyster, similar to VME and submerged aquatic vegetation, may provide higher growth rates for some species and refuge from predation for others. As documented in studies concerning other habitats, high abundances of certain species in oyster may be indicative of higher growth rates in oyster, greater refuge from predation in oyster, or both. Further research comparing habitat-specific growth and survival is essential in verifying the overall importance of oyster habitat for resident and nursery species. Oyster appears to support a temporally diverse and spatially distinct nekton community and deserves further attention in research and conservation. Our study also documented that differences in fish and invertebrate abundances and community structure were related to differences in environmental variables and site location within the estuary. Lastly, we found that the relative location of the marsh complexes within the context of the whole estuary may explain some of the temporal and spatial differences in communities.
References
Akin, S., K.O. Winemiller, and F.P. Gelwick. 2003. Seasonal and spatial variation in fish and macrocrustacean assemblage structure in Mad Island Marsh estuary, Texas. Estuarine Coastal and Shelf Science 56: 1–14.
Baltz, D.M., C. Rakocinski, and C. Fleeger. 1993. Microhabitat use by marsh-edge fishes in a Louisiana estuary. Environmental Biology of Fishes 36: 109–126.
Baltz, D.M., J.W. Fleeger, C.F. Rakocinski, and J.N. McCall. 1998. Food, density, and microhabitat: factors affecting growth and recruitment potential of juvenile saltmarsh fishes. Environmental Biology of Fishes 53: 89–103.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.P. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51: 633–641.
Boesch, D.F., and R.E. Turner. 1984. Dependence of fishery species on salt marshes: the role of food and refuge. Estuaries 7: 460–468.
Breitburg, D.L., L.D. Coen, M.W. Luckenbach, R. Mann, M. Posey, and J.A. Wesson. 2000. Oyster reef restoration: Convergence of harvest and conservation strategies. Journal of Shellfish Research 19: 371–377.
Bulger, A.J., B.P. Hayden, M.E. Monaco, D.M. Nelson, and M.G. McCormick-Ray. 1993. Biologically based estuarine salinity zones derived from a multivariate analysis. Estuaries 16: 311–322.
Castellanos, D.L., and L.P. Rozas. 2001. Nekton use of submerged aquatic vegetation, marsh, and shallow unvegetated bottom in the Atchafalaya River delta, a Louisiana tidal freshwater ecosystem. Estuaries 242: 184–197.
Coen, L.D., M.W. Luckenbach, and D.L. Breitburg. 1999. The role of oyster reefs as essential fish habitat: a review of current knowledge and some new perspectives. American Fisheries Society Symposium 22: 438–454.
Gelwick, F., S. Akin, D. Arrington, and K. Winemiller. 2001. Fish assemblage structure in relation to environmental variation in a Texas Gulf coastal wetland. Estuaries 242: 277–284.
Glancy, T.P., T.K. Frazer, C.E. Cichra, and W.J. Lindberg. 2003. Comparative patterns of occupancy by decapod crustaceans in seagrass, oyster, and marsh-edge habitats in a northeast Gulf of Mexico estuary. Estuaries 26: 1291–1301.
Grabowski, J.H. 2002. The influence of trophic interactions, habitat complexity, and landscape setting on community dynamics and restoration of oyster reefs. Ph.D. Dissertation, University of North Carolina at Chapel Hill, North Carolina, USA.
Harding, J.M., and R. Mann. 1999. Observations on the biology of the veined rapa whelk, Rapana venosa (Valenciennes, 1846) in the Chesapeake Bay. Journal of Shellfish Research 181: 9–17.
Harding, J.M., and R. Mann. 2001. Oyster reefs as fish habitat: Opportunistic use of restored reefs by transient fishes. Journal of Shellfish Research 203: 951–959.
Heck, K.L., and T.A. Thoman. 1984. Experiments on predator–prey interactions in vegetated aquatic habitats. Journal of Experimental Marine Biology and Ecology 53: 125–134.
Heck, K.L., G. Hays Jr., and R.J. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136.
Hosack, G.R., B.R. Dumbauld, J.L. Ruesink, and D.A. Armstrong. 2006. Habitat associations of estuarine species: comparisons of intertidal mudflat, seagrass (Zostera marina), and oyster (Crassostrea gigas) habitats. Estuaries and Coasts 29: 1150–1160.
Howe, J.C., and R.K. Wallace. 2000. Relative abundance of postlarval and juvenile penaeid shrimps in submerged aquatic vegetation and emergent marsh habitats. Gulf of Mexico Science 2000: 130–137.
Kneib, R.T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanographic Marine Biology Annual Review 35: 163–220.
Kupschus, S., and D. Tremain. 2001. Associations between fish assemblages and environmental factors in nearshore habitats of a subtropical estuary. Journal of Fish Biology 58: 1383–1403.
Larsen, K.M., H.M. Perry, J.S. Warren, and C.B. Trigg. 2001. Seasonal colonization of low profile artificial reefs in Mississippi coastal waters: Vertebrates. Proceedings of the Gulf and Caribbean Fisheries Institute 52: 488–497.
Lehnert, R.L., and D.M. Allen. 2002. Nekton use of subtidal oyster shell habitat in a southeastern US estuary. Estuaries 25: 1015–1024.
McCune, B., and M.J. Mefford. 1999. PC-ORD. Multivariate analysis of ecological data. Version 4.0. MjM Software, Gleneden Beach, Oregon.
Minello, T.J., and J.W. Webb Jr. 1997. Use of natural and created Spartina alterniflora salt marshes by fishery species and other aquatic fauna in Galveston Bay, Texas, USA. Marine Ecology Progress Series 151: 165–179.
Minello, T.J., and R.J. Zimmerman. 1983. Fish predation on juvenile brown shrimp, Penaeus aztecus Ives: the effect of simulated Spartina structure on predation rates. Journal of Experimental Marine Biology and Ecology 72: 211–231.
Minello, T.J., and R.J. Zimmerman. 1985. Differential selection for vegetative structure between juvenile brown shrimp (Penaeus aztecus) and white shrimp (P. setiferus), and implications in predator–prey relationships. Estuarine, Coastal and Shelf Science 20: 707–716.
Minello, T.J., R.J. Zimmerman, and E.X. Martinez. 1989. Mortality of young brown shrimp Penaeus aztecus in estuarine nurseries. Transactions of the American Fisheries Society 118: 693–708.
Minello, T.J., R.J. Zimmerman, and R. Medina. 1994. The importance of edge for natant macrofauna in a created salt marsh. Wetlands 14: 184–198.
Minello, T.J., K.W. Able, M.P. Weinstein, and C.G. Hays. 2003. Salt marshes as nurseries for nekton: testing hypotheses on density, growth and survival through meta-analysis. Marine Ecology Progress Series 246: 39–59.
Perry, H.M., K. Larsen, J. Warren, and C. Trigg. 2001. Seasonal colonization of low profile artificial reefs in Mississippi coastal waters: Invertebrates. Proceedings of the Gulf and Caribbean Fisheries Institute 52: 498–511.
Peterson, C.H., J.H. Grabowski, and S.P. Powers. 2003. Estimated enhancement of fish production resulting from restoring oyster reef habitat: quantitative valuation. Marine Ecology Progress Series 264: 249–264.
Rakocinski, C.F., D.M. Baltz, and J.W. Fleeger. 1992. Correspondence between environmental gradients and the community structure of marsh-edge fishes in a Louisiana estuary. Marine Ecology Progress Series 80: 135–148.
Rakocinski, C.F., J. Lyczkowski-Shultz, and S.L. Richardson. 1996. Ichthyoplankton assemblage structure in Mississippi Sound as revealed by canonical correspondence analysis. Estuarine, Coastal and Shelf Science 43: 237–257.
Rozas, L.P., and T.J. Minello. 1997. Estimating densities of small fishes and decapod crustaceans in shallow estuarine habitats: A review of sampling design with focus on gear selection. Estuaries 20: 199–213.
Rozas, L.P., and T.J. Minello. 1998. Nekton use of salt marsh, seagrass, and nonvegetated habitats in a south Texas (USA) estuary. Bulletin of Marine Sciences 63: 481–501.
Rozas, L.P., and D.J. Reed. 1993. Nekton use of marsh-surface habitats in Louisiana (USA) deltaic salt marshes undergoing submergence. Marine Ecology Progress Series 96: 147–157.
Shervette, V.R., H.M. Perry, C.F. Rakocinski, and P.M. Biesiot. 2004. Recruitment of post-settlement stone crab Menippe adina juveniles in Mississippi Sound: Factors influencing refuge occupation. Journal of Crustacean Biology 244: 652–665.
Stunz, G.W., T.J. Minello, and P.S. Levin. 2002. Growth of newly settled red drum Sciaenops ocellatus in different estuarine habitat types. Marine Ecology Progress Series 238: 227–236.
Zeug, S.C., V.R. Shervette, D.J. Hoeinghaus, and S.E. Davis. 2007. Nekton assemblage structure in natural and created marsh-edge habitats of the Guadalupe Estuary, Texas, USA. Estuarine, Coastal and Shelf Science 71: 457–466.
Zimmerman, R.J., T.J. Minello, and G. Zamora. 1984. Selection of vegetated habitat by brown shrimp, Penaeus aztecus, in Galveston Bay salt marsh. US National Marine Fishery Service Bulletin 82: 325–336.
Zimmerman, R.J., T.J. Minello, T. Baumer, and M. Castiglione. 1989. Oyster reef as habitat for estuarine macrofauna. NOAA Technical Memorandum. NMFS-SEFC-249.
Zimmerman, R.J., T.J. Minello, D. Smith, and J. Kostera. 1990. The use of Juncus and Spartina marshes by fishery species in Lavaca Bay, Texas, with reference to the effects of floods. NOAA Technical Memorandum, NMFS- SEFC-251.
Acknowledgments
This research is from the dissertation of VRS. It would not have been possible without the field assistance of Stuart Ralston. We thank S. Zeug, T. Minello, L. Rozas for helpful comments improving this study and S. Walker for providing the imagery for Fig. 2. The majority of funding was provided by a National Estuarine Research Reserve Fellowship. We are grateful for additional funding from the following: Tom Slick Fellowship, Susan Arseven Award, Graduate Women in Science Vessa Notchev Fellowship, Texas Water Resources Institute Mills Scholarship, Seaspace Scholarship, Ryan Kelley Memorial Scholarship from the International Women’s Fishing Association, Coastal Conservation Association, and NOAA. Much thanks to Grand Bay NERR, Grand Bay NWR, and Weeks Bay NERR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shervette, V.R., Gelwick, F. Seasonal and Spatial Variations in Fish and Macroinvertebrate Communities of Oyster and Adjacent Habitats in a Mississippi Estuary. Estuaries and Coasts 31, 584–596 (2008). https://doi.org/10.1007/s12237-008-9049-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-008-9049-4