Abstract
Ligninolytic and other oxidative enzymes have emerged as promising biocatalysts in several industries. Since their production at a low cost is necessary for any large-scale application, we demonstrate the use of rice bran (RB), an agricultural waste and agri-food wastes such as potato peelings (PP), banana peelings (BP), and green pea peelings (GPP) for their production. High activity of laccase (12 U/ml), manganese peroxidase (16.11 ± 1.43 U/ml), and aryl alcohol oxidase (1.25 U/ml) was obtained on the PP on the 12th day of growth and ~ 6 U/ml of lytic polysaccharide monooxygenase was obtained on the 14th day of growth demonstrating PP to be a good substrate for their production. RB served as the next best substrate for the production of these enzymes. While the GPP was effective for the production of laccase (9.2 U/ml), this and the BP were not good substrates for the production of other enzymes. Efficient (48–82%) decolorization of several azo-, triarylmethane- dyes, and real textile effluent, without the addition of any mediator, demonstrated the high oxidative ability of the crude culture filtrate produced on the PP (CF-PP), which was a significant improvement compared to the treatment given by the previously reported culture filtrate obtained on wheat bran (CF-WB). An extensive breakdown of Reactive Orange (RO) 16 was demonstrated using CF-PP resulting in the formation of a new product at m/z of 294.05 (6-acetamido-3,4-dioxo-3,4-dihydronapthalene-2-sulfonate), previously reported to be produced on ozonation/advanced oxidation of RO16. The predominant laccase and manganese peroxidase isoforms produced on the PP were also identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to increasing agricultural activities, about five million metric tonnes of biomass are produced annually (Bharathiraja et al. 2017) on a global level. Out of this, nearly one-third is lost or wasted as a consequence of the processing of agricultural products. The highest wastage is generated from fruits, vegetables, roots, and tubers (called agri-food wastes) leading to 40–50% of the food loss quantitated per year (Ravindran et al. 2018). The agri-food wastes are rich in nutrients and if not utilized properly pose considerable environmental hazards in terms of providing breeding grounds for pathogenic microbes. Although a considerable part can be used on the farm itself as animal feed, there has been growing interest in the use of the wastes for the production of second-generation biofuel, and valuable ligninolytic enzymes (Capanoglu et al. 2022). This is because the wastes contain high amounts of cellulose, hemicellulose, lignin, and other valuables such as pectins, proteins, polyphenols, and amino acids (Bharathiraja et al. 2017; Vats and Mishra 2021). While agricultural wastes, such as wheat bran (WB), rice straw (RS), and rice bran (RB) have been extensively used for the production of enzymes by white-rot fungi through solid-state fermentation (Rodríguez-Couto 2017; Vats and Mishra 2017; Ravindran et al. 2018; Tanruean et al. 2021), other agri-food wastes, such as potato peelings (PP), banana peelings (BP) and green pea peelings (GPP) have yet to be exploited for the production of enzymes. The use of PP has been recently reported for the production of fermentable sugars useful in bioethanol and bioproducts production (Liang and McDonald 2014; Pennacchio et al. 2021). According to the recent National Potato Council data [Knudson W and Miller SR (2023) Measuring the economic significance of the US potato industry. Annual-Potato-Yearbook-2023.pdf of the potatoes (nationalpotatocouncil.org), accessed 27 July 2023], the US ranks fifth among the potato-producing countries and produced more than 21.3 billion kg of potatoes in 2020. During the processing of the potatoes, ~ 6–10% of this amount accumulated as the PP, and further processing (such as defect removal, trimming, and cutting) led to the generation of additional 15% waste (total waste ~ 5.7 billion kg) (Mader et al. 2009) providing large quantities of nutritionally rich material that can be used for enzyme production. The global production of bananas was reported to be ~ 116 million tonnes in 2019 (FAO, 2020, http://faostat.fao.org), and given that 25% of this is the fruit, and, that 30% of this fruit is still left as the BP, the total amount of waste generated has been estimated at ~ 10 million tonnes. The BP has been used as animal food but there have been concerns about the effect of tannin (present in the husks) on animals’ health. India is reported to be the leading producer of green peas, based on world vegetable statistics, and produces ~ 3.56 million metric tons of green peas. The processing of the green peas’ leaves ~ 1 million tons of pea pod waste/yr. Thus, wastes resulting from the processing of potatoes, bananas, and green peas pose globally a significant waste management challenge, and, therefore, using them efficiently for the production of valuable enzymes would be a good solution.
Production of enzymes on the agri-food wastes, using solid-state fermentation, is a well-established and successful method as the physicochemical nature of these substrates allows the white-rot fungi to colonize and produce extracellular enzymes (Ravindran et al. 2018). A battery of core extracellular enzymes, such as laccase (Lac), manganese peroxidase (MnP), lignin peroxidase (LiP), and versatile peroxidases (VP) is produced on these substrates. These are accompanied by various auxiliary enzymes, such as aryl-alcohol oxidase (AAO) and lytic polysaccharide monooxygenase (LPMO) (Kumar and Chandra 2020) which facilitate the action of the core enzymes. AAOs oxidize lignin-derived phenolic aromatic acids to corresponding aldehydes and H2O2, the latter supporting the activity of various peroxidases. The fungal LPMOs belong to the family AA (auxiliary activity) 9 in the carbohydrate-active enzyme database (http://www.cazy.org) and modify cellulose oxidatively at the C-1 or C-4 position to form aldonic acids or gem-diols respectively (Koskela et al. 2019) resulting in a decrease in the crystallinity index of cellulose which enhances fibrillation. These extracellular enzymes also serve as a valuable resource and can be used in pulp and paper, food, textile, cosmetic, and chemical industries (Rodriguez-Couto 2017). Their cost of production can be kept low if the agricultural and agri‐food wastes can be used (Giacobbe et al. 2020).
Cyathus bulleri, belonging to the family Nidulariaceae, is an avid lignin degrader and grows rapidly on decaying wood and herbaceous stems. A vast array of lignocellulases and other auxiliary enzymes are produced by the fungus when cultivated on WB (Vats and Mishra 2021). The extracellular culture filtrate obtained by growing the fungus on WB and orange peelings (OP) effectively removed the color and degraded several azo and triarylmethane dyes (Vats and Mishra 2017) making this fungus to be a valuable resource in the bioremediation of xenobiotics. These dyes are toxic and mutagenic and persist in the water bodies as no single enzyme is effectively able to degrade them (Ayed et al. 2017; Mishra and Maiti 2018; Farhadian et al. 2022). Of the many azo dyes, Reactive Orange 16 (RO16) is a unique reactive dye that can link covalently to textile fibers and is resistant to degradation by conventional chemical and biological (including enzymatic) methods. However, it has been reported to be degraded by photo electrocatalytic (Luo et al. 2022) and by advanced oxidation (Tizaoui and Grima 2011) methods which are expensive as well as difficult to operate on a large scale. Among the biological methods, a combination of Lac (or crude culture filtrate) and mediators have shown promise in the decolorization of RO16 (Vats and Mishra 2017). Mediators are low molecular weight compounds (natural such as vanillin, syringaldehyde, acetosyringone or synthetic such as 1-hydroxy benzotriazole, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) or ABTS) that act as a shuttle between the Lac active site and the substrate to allow stereo-chemically unfit and large substrates or the ones with high redox potential to undergo oxidation (Zeng et al. 2017). The synthetic mediators while being effective are not economical and may add toxicity to the treated dyes (Blánquez et al. 2019) hence their use is not recommended. In this study, RB and other agri-food wastes, namely, PP, BP, and the GPP were used for the production of extracellular ligninolytic and other auxiliary enzymes by C. bulleri which was then evaluated for decolorization of azo and triarylmethane dyes. The long-term objective of this study is to better manage the various agri-food wastes for the production of valuable enzymes.
Materials and methods
Dyestuff and chemicals
The dyes used in the present study were Reactive Orange 16 (RO16), Reactive Violet 5 (RV5), Reactive Black 5 (RB 5), Acid Violet 17 (AV 17), Ketone Blue A (KBA) and these were procured from the Department of Textile and Fiber Engineering, IIT Delhi. RO16, RV5, and RB5 are azo dyes while AV17 and KBA are triarylmethane dyes. The structure of the dyes is shown in Table 1. ABTS, guaiacol, veratryl alcohol, and dimethoxy phenol (DMP) were from Sigma–Aldrich Chemicals Pvt. Ltd. All other chemicals were of high quality and procured from Merck or HiMedia Laboratories. The combined effluent was provided by a local textile company near Delhi.
Microorganism
The white rot fungus Cyathus bulleri (Brodie 195062) was from Canadian National Collection of Fungal Cultures, Ottawa, Ontario, Canada. It was maintained at 4 °C on malt-extract agar (MEA) plates and sub-cultivated at 28 °C on fresh medium every month for a period of 7–8 days.
Solid substrates and cultivation conditions
The solid substrates used for the cultivation of the fungus were WB, RB (procured from a local flour mill), PP, BP, and GPP. WB and RB were passed through a sieve (pore size ~ 5 mm × 5 mm) and used without further processing. The PP, BP and GPP were cut into small pieces (length∼5 mm) and washed with distilled water. The peelings were dried to constant weight at 37 °C. Five g of each substrate was taken in Erlenmeyer flasks, wetted with 100 ml distilled water, and autoclaved for 40 min at 121 °C. A total of 16 flasks were set up for each substrate and two flasks were removed at regular intervals (at the times shown in Figs. 1a–e). Flasks were inoculated with mycelial plugs (Ø ~ 5 mm from 5-day old culture grown on the MEA plates) and incubated at 28 °C under static conditions for 21 days. The mycelium-free supernatant was collected by centrifugation (8000g × 30 min) and then filtered through a 0.20 μm filter. The clear culture filtrate was used for biochemical assays. The clear centrifuged supernatant from the PP-grown fungus was also tested for decolorization of several dyes and the combined effluent obtained from a local dyeing mill.
Profile of ligninolytic enzyme activity in the culture filtrate obtained by cultivation of C. bulleri on different substrates. Extracellular laccase activity (a) Manganese peroxidase activity (b) Aryl alcohol oxidase activity (c) Lignin peroxidase activity (d) and Lytic polysaccharide mono-oxygenase activity (e)
Proteins were precipitated from the crude culture filtrates by the addition of trichloroacetic acid (TCA) as given under analytical methods. The solubilized proteins were run on SDS-PAGE to determine the general extracellular protein profile. The details of the electrophoretic method are given under the analytical methods section below.
Decolorization of synthetic dyes and monitoring degradation products of RO16
The decolorization of water-soluble reactive azo dyes, RB5, RV5, and RO16, and, two triarylmethane dyes AV17 and KBA, was investigated using culture filtrate obtained from PP-grown fungus. The 5 mL reaction mixture (in triplicate) consisted of the aqueous solution (50 ppm) of the dye in sodium citrate buffer (20 mM), pH 4. Culture filtrate, equivalent to 0.5 U/ml Lac activity, was added to the reaction mixtures and the samples were incubated with shaking at 100 rpm. The % decolorization was also monitored in the presence of ABTS (100 μM). Control samples were run for each dye in which no culture filtrate was added. Samples were incubated for 24 h and then quenched by placing the flasks in a boiling water bath for 10 min. The boiling also ensures the arrest of further oxidation of ABTS into the ABTS cation radical (Kenzom et al. 2014). Centrifugation was carried out to remove any precipitates and the clear supernatant was analyzed by UV–vis spectrophotometry. To monitor the rate of degradation of each dye, samples were removed every 4 h, and the residual dye concentration (in nmoles) was calculated from the optical density values. Decolorization (D) was measured as a percentage reduction in absorption maximum of RV5 (λmax 560 nm), KBA (λmax 640 nm), AV17 (λmax 542 nm), RB5 (λmax 600 nm), and RO16 (λmax 490 nm) as shown below.
where %D denotes percentage decolorization, IA is the initial absorbance, and FA is the final absorbance at the λmax of each dye.
Due to the high rate of oxidation of RO16 by the culture filtrate obtained on PP, relative to the rates obtained with other culture filtrates, the mode of the breakdown of this dye was investigated. The degradation products of RO16 (obtained in the absence of ABTS) were determined by electron spray ionization-mass spectrometry (ESI–MS) of the treated dye and compared with the spectrum obtained from the untreated dye. The details of the operation of the ESI–MS are given under analytical methods.
Decolorization of real effluent and monitoring of the spectrum of the treated effluent
Decolorization was also monitored of the real effluent under similar conditions as used for the individual dyes. For this, 25 mL of the effluent was taken in a conical flask (250 mL) and the pH was adjusted to 4.0. Culture filtrate obtained on PP was added so that the final Lac activity was 0.5 U/ml of the effluent. The flasks were incubated at room temperature (28 °C) with shaking at 100 rpm. The %decolorization was also monitored in the presence of ABTS (100 μM). Control samples were run for the effluent to which no culture filtrate was added (abiotic treatment). Samples were incubated for 1, 6, 24, 48, and 72 h. After removal, the samples were quenched in a boiling water bath for 10 min. Centrifugation was carried out to remove any precipitates and the absorbance spectrum of the clear supernatant was monitored by UV–Vis spectrophotometry in the visible spectrum (380–700 nm). The %decolorization was calculated by measuring the area under the curve for the untreated and the treated effluent.
The untreated and the treated effluent (in the absence of ABTS) were subjected to ESI–MS to evaluate the profile of the degraded products. The details of the operation of the MS are described under analytical methods.
Total RNA extraction, cDNA synthesis, and gene expression analysis using qPCR
The PP-grown fungal culture was harvested on the 12th day when the maximum activity of Lac was obtained, and the total RNA was extracted with Trizol according to the manufacturer’s instructions. The mRNA was next prepared and converted to cDNA through an iScriptTM cDNA synthesis kit according to the manufacturer's protocol. SsoFast TM EvaGreenR Supermix was used to perform quantitative real-time PCR (qPCR). The transcription pattern of genes encoding for the Lac and MnP isozymes was determined using qPCR analysis. Primers were designed for the eight Lac genes (Lcc1-8), five MnP genes (MnP 1–5) (Vats and Mishra 2018), and the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, GAPDH (Table 2). The qPCR was performed on an Applied BioSystems 7500 Real-Time PCR system (USA). The 2 − ΔΔCt relative quantification strategy was used to measure the relative quantities of the Lcc (encoding Lac) and the MnP (encoding the MnP activity) transcripts (Schmittgen and Livak 2008).
Analytical methods
In order to remove the H2O2 formed due to the presence of other oxidases in the culture filtrate, catalase (1000 U/ml; Sigma–Aldrich) was added to the assay solution and the samples were incubated for 1 h at 37 °C. Lac activity was assayed using ABTS as a substrate as described earlier by monitoring an increase in the ABTS* radical at 420 nm (Eggert et al. 1996). The activity of MnP was measured using DMP and H2O2 as substrates as described previously (Kuwahara et al. 1984). LiP activity was measured by monitoring the H2O2-dependent oxidation of veratryl alcohol to veratraldehyde at 25 °C as described (Tien and Kirk 1984). AAO activity was assayed at 30 °C using veratryl alcohol as a substrate (Okamoto and Yanase 2002). LPMO activity was assayed at 30 °C using DMP as a substrate (Wang et al. 2018). For all ligninolytic enzyme activities, 1 U was defined as the amount of enzyme that catalyzed 1 μmol substrate/min.
For SDS-PAGE analysis of the extracellular protein produced on different substrates, these were first precipitated by the addition of 12% TCA-acetone to the cell-free culture supernatant in the ratio of 2:1. The solutions were incubated at − 20 °C overnight. The protein pellet was obtained by centrifugation at 4 °C at 15,000 g. The pellet was washed with 100 ml of acetone and then air-dried. The pellet was solubilized in a solubilization buffer [1 M Tris–Cl, pH 6.8, SDS (1%) and β-mercaptoethanol (2.5%)]. Protein was measured using the Bradford method using bovine serum albumin as the standard. Electrophoresis was performed using a Tris–glycine buffer system at 100 V for 3 h. The gels were stained with Coomassie brilliant blue R-250 and the image was captured by Gel DocTM XR + Imaging (Bio-Rad) system.
The ESI–MS was carried out using a hybrid Q-TOF detection system (AB Sciex, USA). Both the RO16 (untreated and treated) and the effluent (untreated and treated) samples (20 μL) were diluted with methanol–water (1:1, vol/vol) and injected into the ESI–MS system. The spectrum was monitored in positive ion mode with the following conditions: ion spray voltage, 5500 V; nebulizer gas, 20 lb/in2; curtain gas, 25 lb/in2; declustering potential, 60 V; focusing potential, 265 V; and flow rate, 5 μL/min (Kenzom et al. 2014). The spectra were acquired using a mass range (m/z) of 100 to 1000 atomic mass units.
Statistical analysis
The experiments were conducted in triplicate (for the dye samples) and in duplicate (for the effluent sample) and the results are reported as an average of this ± standard deviation. ANOVA was carried out to assess significant differences among the means using Graph Pad Prism version-6.1 (Graph Pad Software, San Diago, CA, USA).
Results
Ligninolytic and auxiliary enzyme production on solid substrates
Extracellular protein profile on different substrates, as determined by SDS-PAGE, is shown in Supplementary Fig. S1. As seen, the extracellular protein profile was different on these substrates. A prominent band around 58 kDa was observed in all lanes indicating the presence of Lac. Another band at ~ 38 kDa was observed in culture filtrates obtained from WB- and PP-grown fungus and was proposed to be that of the MnP. A smaller protein of ~ 25 kDa was prominent among the proteins seen for the PP-grown fungus and was proposed to be that of LPMO. The enzyme activity of prominent ligninolytic and a few auxiliary enzymes was measured and the results are shown in Fig. 1a–e. As seen in Fig. 1a, Lac activity reached its maximum value (13.8 U/ml or ~ 280 U/g dry substrate) on the 12th day on WB. Among the agri-wastes, maximum Lac activity of 12.2 U/ml was produced on PP, slightly more than ~ 10 U/ml on GPP. A decrease in Lac activity was observed on all the substrates after 12 days of incubation. RB, as seen, was potentially a good substrate for Lac production. Unlike the Lac activity, MnP was produced to a high level of 16.5 U/ml (or 1288.31 U/g dry substrate) on PP on the 12th day, which was more than twofold of the activity obtained on WB and RB. This was attributed to the presence of high lignin and suberin content (20%) in PP. Low activity (0.2–0.75 U/ml) of AAO was detected in all the substrates, except on PP and WB where ~ 1.3 U/ml and 1.1 U/ml of AAO respectively were detected after 10 days of growth. As opposed to this, low activity (~ 0.75 U/ml) of LiP was obtained on all the substrates (Fig. 1d). Figure 1e shows the production of LPMO and the data indicated maximum activity of 5.6 U/ml on PP on the 14th as opposed to 5.1 U/ml on WB on the 12th day. RB also served as a good substrate for the production of this enzyme. It was also observed that high levels of extracellular protein were obtained when the fungus was cultivated on PP and GPP and a thick mat of mycelium was obtained.
Decolorization of synthetic dyes and monitoring degradation products of RO16 using the crude culture filtrate obtained on PP
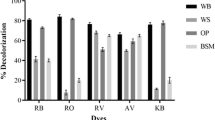
Based on the observation that high Lac and MnP activities were obtained on the PP, the crude culture filtrate produced on PP was evaluated for the removal of color from several dyes. As shown in Table 3, the culture filtrate produced on PP was effective (30–50%) in the decolorization of all the dyes, without the aid of a mediator, suggesting high amounts of Lac and MnP (and possibly other enzymes) to collectively display high oxidative ability. The actual color of the dyes after treatment with the culture filtrate obtained from the PP-grown fungus is shown in Supplementary Fig. S2. When compared to the decolorization carried out with the culture filtrate obtained on WB, this was significant as very little (< 20%) decolorization was carried out by it in the absence of ABTS (Vats and Mishra 2017). In the presence of ABTS, these values were increased from 35% (for AV17) to 68% for RV5 (Table 3). Decolorization of RO16, a very recalcitrant dye, was nearly 90% in 96 h in the absence of ABTS (data not shown). Measurement of the rate of oxidation (Table 1) indicated the rates to be nearly similar for RV5, RB5, and RO16 (in the absence or presence of ABTS). For AV17 and KBA, faster rates (1.5-fold more) were obtained in the presence of ABTS. Since nearly 90% decolorization was obtained for RO16 (in the absence of ABTS) in 96 h with the culture filtrate produced on PP, the products of degradation of RO16 were monitored through ESI–MS. Figure 2a shows the presence of a major peak at 619.45 m/z, corresponding to intact RO16. Treatment with the crude culture filtrate of PP-grown fungus decreased the intensity of the peak at 619.45 m/z and several degradation products were obtained (Fig. 2b). Several smaller peaks (m/z 316.95, 294.05, 284, 201, 187.06, 158.96) were detected in the spectrum. The peak at m/z of 472.94 could not be assigned any structure and could be a new product.
Decolorization of real effluent using the crude culture filtrate obtained on PP and monitoring the degradation products by ESI–MS
The effectiveness of the culture filtrate obtained on PP, in removing the color of the real effluent, was monitored and the data indicated the removal of color by 56% within 48 h (in the absence of ABTS) (Fig. 3). This number was lower in the presence of ABTS, unlike the data obtained with the dyes in the presence of ABTS. An increase in the UV-absorbing molecules was also seen during the treatment (data not shown). Figure 4a shows the mass spectrum of the untreated textile effluent. As seen, the effluent contained a mixture of dyes and peaks at m/z values between 900–1200 suggesting the presence of several high molecular weight compounds. The peak at m/z value of 991.29 showed the presence of RB5 and the presence of a peak at m/z of 619.45 corresponded to the presence of RO16 in the effluent. Figure 4b shows that the treatment of the effluent (in the absence of ABTS) resulted in a reduction of the peak at 900 m/z and the appearance of several metabolites with lower m/z values indicating that the dyes in the effluent were efficiently degraded by the enzymes present in the culture filtrate obtained on the PP.
Transcription profiling of genes encoding for Lac and MnP isozymes produced on PP
High activities of Lac and MnP were found to be produced in the culture filtrate obtained from PP-grown fungus. Since both Lac and MnP occur in several isoforms, it is important to link the effectiveness of the degradation of dyes to the isoforms being produced. Among the 8 Lac genes, Lcc1 was the most predominantly expressed gene, relative to other isoforms, followed by Lcc8 and Lcc4 (Fig. 5a). Among the 5 MnP isoforms, MnP5 and MnP4 encoding transcripts were the most expressed form followed by MnP2, MnP1, and MnP3 (Fig. 5b). Based on the transcription data, Lcc1 and MnP5 were identified as the major contributor to the Lac and MnP activity in the culture supernatant.
Discussion
Large amounts of agricultural and agri-food wastes are available which pose a general health hazard and apart from their use as animal feed, these have not been used for the production of valuable enzymes. WB has been most extensively used for the production of ligninolytic enzymes using the white-rot fungi Cerrena unicolor T71 (Osma et al. 2011), C. bulleri (Vats and Mishra 2017; Vats and Mishra 2018), Penicillium chrysosporium (Siva and Banu, 2011), Pleurotus ostreatus DSM11131, Trametes pubescens MB89, and Trametes versicolor K120 (Osma et al. 2011). The wheat straw and the wheat straw extract have also been used for the production of Lac in solid-state as well as submerged fermentation (González-Rodríguez et al. 2022). While PP, BP, and GPP have been used for bioethanol production (Oberoi et al. 2011; Hossain et al. 2018; Nimbalkar et al. 2018) there are very few reports on their usage for the production of ligninolytic and other auxiliary enzymes involved in the degradation of biomass-based residues. Production of polyphenol oxidase was reported on the PP and the enzyme, rather than the crude culture filtrate, was used for the decolorization of dyes (Loncara et al. 2012). In the present study, a systematic investigation was carried out on the type of major ligninolytic and a few other auxiliary enzymes produced on RB and some agri-wastes.
Among the different agri-wastes screened, high production of both Lac and MnP occurred on PP. Maximum Lac activity of 12.2 U/ml was produced on PP, slightly more than ~ 10 U/ml on GPP. The production of Lac was comparable to that produced by WB. High activity of Lac was also produced on RB and this substrate has been reported to support high production of other carbohydrate-degrading activities (Oliveira et al. 2010). Due to the utility of RB for the production of nutraceuticals, nutrients (γ-oryzanol, α-tocopherol, polyphenols, ubiquinone-10), and oil (Vallabha et al. 2015), its availability is limited for the production of ligninolytic enzymes. BP didn’t support high production of Lac and this was observed in earlier studies also (Osma et al. 2007; Wehaidy et al. 2018). The high level of MnP production in PP was attributed to the presence of high lignin and suberin content (~ 20%) (Camire et al. 1997) in PP (Liang and McDonald 2014). A further increase in lignin and suberin components (to 37%) has been reported after microbial fermentation. Suberin is a major constituent of the potato cell walls and comprises glycerol, alkanoic acids, α,ω-diacids, and ω-hydroxyacids. Minor amounts of alkan-1-ols, hydroxycinnamic acids, and amino acids have also been reported to be the constituents of the suberin (Liang and McDonald 2014). While the lignin-derived components and hydrocinnamic acids were found to be good inducers of Lac in C. bulleri (Salony et al. 2006) it is not known if these also induce high MnP activity. In general, while the high activity of Lac has been reported on several agri-wastes, few reports appear on the production of MnP on such substrates. Recently, MnP production (0.34 U/ml) was reported by Irpex lacteus on wheat-straw extract which was less than 40-fold of that produced on PP, used in the present study. The drawback of wheat straw and its extract was that low Lac activity was produced on this substrate (González-Rodríguez et al. 2022) and hence the crude culture filtrate may not have strong oxidizing activity.
Among other oxidative enzymes, a high level (~ 1300 U/L) of AAO was obtained on the PP. Good (1021 U/L) production of AAO has also been demonstrated, but in a genetically modified strain of Aspergillus nidulans, in submerged fermentation using corn steep liquor (Liu et al. 2020) but the availability of higher levels (1300 U/L) on PP makes the latter process more economical. Apart from exhibiting broad substrate specificity, the availability of AAO at low cost is important as these enzymes require only oxygen for their oxidative function. The second important use for the AAO is generating hydrogen peroxide for peroxidase- and peroxygenase-dependent reactions. AAOs have also been used in the synthesis of aromas and other high-value-added compounds (Urlacher and Koschorreck 2021). These also have a high potential in dye decolorization and pulp bio-bleaching. LiP, in general, is produced at low levels on agri-wastes. There is only one study (Ergun and Urek 2017) on the use of PP for the production of ligninolytic enzymes; however, low enzyme activities were reported compared to the activities obtained in the present study. LPMOs are a new category of enzymes that have been discovered recently and cleave a broad range of oligo- and polysaccharides oxidatively. It is reported to significantly improve the activity of Trichoderma reesei CBHI on cellulosic substrates (Tandrup et al. 2018). Relatively high production of LPMO on PP also favors the use of this material for the production of LPMO. Since both PP and GPP have high starch content, it is proposed that these will be efficiently degraded by the LPMO into simple sugars which can support growth and enzyme production on these substrates.
While several physical, chemical, and advanced oxidation methods or a combination of these have been proposed for the decolorization of dyes and effluent, the physical and the chemical methods are not applicable on a large scale as the physical methods lead to the transfer of dyes from one phase to another while the chemical methods (such as the advanced oxidation methods) are expensive to operate on a large scale. This is particularly applicable to water-soluble azo dyes, RO16, and RB5, to name a few. Since the culture filtrate obtained on PP contained high levels of Lac and MnP, it was evaluated for the decolorization of dyes. Both Lac and peroxidases have been shown to have a high potential for the degradation of several aromatics (Heinzkill et al. 1998), including dyes. Importantly, MnP is a key enzyme in the oxidation of polycyclic aromatic hydrocarbons, chlorophenols, and other effluents due to its high oxidative potential (Saroj et al. 2013). The data on the rates of oxidation indicated the primary role of Lac in the degradation of KBA and AV17 as the addition of ABTS led to higher rates of oxidation. It has been reported that degradation of AV17 and KBA occurred through the formation of the carbinol form of the dyes, in the presence of ABTS (Chhabra et al. 2009; Vats and Mishra 2017). The purified Lac and MnP from white rot fungi have been previously reported to decolorize some of these dyes within a few hours (Harazono et al. 2003; Salony et al. 2006) but, the use of the crude culture filtrate, as shown in the present study, makes it a highly effective method for large scale applications. The high oxidative ability of the PP-obtained culture filtrate was tested for decolorization and degradation of RO16. The peaks at m/z 201 and 284 were concluded to be 4-(2-hydroxyethyl sulfonyl) phenolate (m/z 201.02) and (E)-2-(4-acetamidophenyl)-1-carboxy ethene sulfonate (m/z 284). The same metabolites were also reported to be produced after the degradation of RO16 by the laccase of I. lacteus. These were proposed to be formed due to asymmetrical cleavage of the azo bond (Svobodova et al. 2006) by the crude culture filtrate obtained on wheat straw which was enriched for the presence of MnP. The peaks at m/z 201.02, m/z of 284.03 (Fig. 2b) were also reported in the previous study (Vats and Mishra 2017) except that the latter was presented as a sodium adduct (m/z 316.95). The presence of this sodium adduct was also seen in the present study (Fig. 2b). It is also important to note that the products at m/z of 284 and 294.05 were also detected when RO16 was subjected to degradation by growing I. lacteus in an aqueous solution of this dye (Svobodová et al. 2007) and separation of the compounds by chromatography followed by identification of the structures through product ion spectrum. The product with m/z of 294.05 was identified as 6-acetamido-3,4-dioxo-3,4-dihydronapthalene-2-sulfonate. The product of m/z of 294.05 was also detected when RO16 was subjected to ozonation (Tizaoui and Grima 2011) or to advanced oxidation using chloramine-T (CAT)/IrCl3/HClO4 redox system (Sukhdev and Manjunadha 2017). This product was not detected when RO16 was treated with the culture filtrate obtained from the WB-grown fungus indicating the superior oxidative ability of the culture filtrate obtained on PP. The peaks at m/z of 187.06, and 158.96 (Fig. 2b) were also observed and are likely to have arisen from the removal of a methyl chain (–CH2) from 4-(2-hydroxyethyl sulfonyl) phenolate (m/z 201.02) or removal of an oxygen atom from a product of m/z 172.99. The latter was detected in small amounts in the spectrum and resulted from the removal of a methyl group (–CH2) of a compound with m/z of 187.06. The products of Pathway 2, as proposed earlier (Vats and Mishra 2017) were not detected in the present study. The peak at m/z of 472.94 could not be assigned any structure and could be a new product. While the real effluent was decolorized well in the absence of ABTS, decolorization decreased in its presence. Polymerization of the small fragments into colored products was proposed as a reason for decreased decolorization in the presence of ABTS. Many of the mediators have been reported to enhance the laccase-catalyzed polymerization reactions (Agustin et al. 2021). Smaller fragments of the dyes were also seen confirming the high oxidative ability of the culture filtrate. However, a complete degradation was not achieved which might be due to the presence of detergent and other inhibitory compounds in the real textile effluent.
An attempt was also made to identify the predominant Lac and the MnP encoding genes as C. bulleri produces several isoforms of these enzymes (Ahlawat and Mishra 2022). A study on laccase isozymes of Trametes versicolor suggested that the proportion of laccase isoenzymes varied with the nature of the lignocellulosic material (barley straw and barley bran, grape stalks, grape seeds, and corn cob) added as an inducer to the culture medium (Moldes and Sanromán 2006). The occurrence of isozymes, apparently allows the fungus to use a variety of lignocellulosic material in nature. The predominance of transcription of Lcc1 confirmed its major role in the decolorization process. This form was also the highly transcribed isoform when C. bulleri was grown on WB (Vats and Mishra 2018). The culture filtrate from the WB-grown fungus was highly effective in the decolorization of crystal violet as well as highly derivatized molecules of this dye (Ahlawat and Mishra 2023). While relatively higher transcription of Mn5 and MnP4 encoding genes was observed, no conclusions could be made as little is known about the properties of these isoforms. Cloning and expression of these specific genes would allow large-scale production and trial on a bigger scale.
Conclusions
This study indicated the use of PP as a low-cost substrate for the semi-solid-state cultivation of C. bulleri for the production of ligninolytic and other auxiliary enzymes. The presence of high levels of MnP activity enhanced the degradation capability of the crude culture filtrate towards RO16 leading to the formation of products that were reported to be produced when the dye was subjected to advanced oxidation treatment. The ability to remove color and break down compounds in the effluent into smaller fragments, even in the absence of any mediator, makes this an effective formulation for the treatment of dye-containing effluents. Besides the use of PP as a raw material for the production of enzymes, this will also minimize the generation of waste, reduce the carbon footprint and lower the greenhouse gases. However, large-scale studies are further required for this to be implemented in the industry.
Data availability
Data will be made available on request.
References
Agustin MB, de Carvalho DM, Lahtinen MH, Hilden K, Lundell T, Mikkonen KS (2021) Laccase as a tool in building advanced lignin-based materials. Chemsuschem 14:4615
Ahlawat A, Mishra S (2022) Structural organization of laccase and manganese peroxidase genes in the white-rot fungus Cyathus bulleri, their regulation and role in lignocellulose degradation. Fuel 314:122716
Ahlawat A, Mishra S (2023) Complex laccase-oxidoreductase mixture: effective for oxidative degradation and detoxification of dyes and complex effluent from textile mill. Water Air Soil Pollut 234:135
Ayed L, Bakir K, Mansour HB, Hammami S, Cheref A, Bakhrouf A (2017) In vitro mutagenicity, NMR metabolite characterization of azo and triphenylmethanes dyes by adherents bacteria and the role of the “cna” adhesion gene in activated sludge. Microb Pathogen 103:29–39
Bharathiraja S, Suriya J, Krishnan M, Manivasagan P, Kim S-K (2017) Production of enzymes from agricultural wastes and their potential industrial applications. Adv Food Nutr Res 280:125–148
Blánquez A, Rodríguez J, Brissos V, Mendes S, Martins LO, Ball AS, Arias ME, Hernández M (2019) Decolorization and detoxification of textile dyes using a versatile Streptomyces laccase-natural mediator system. Saudi J Biol Sci 26:913–920
Camire ME, Violette D, Dougherty MP, McLaughlin MA (1997) Potato peel dietary fiber composition: effects of peeling and extrusion cooking processes. J Agric Food Chem 45:1404–1408
Capanoglu E, Nemli E, Tomas-Barberan F (2022) Novel approaches in the valorization of agricultural wastes and their applications. J Agric Food Chem 70:6787–6804
Chhabra M, Mishra S, Sreekrishnan TR (2009) Degradation and detoxification of triarylmethane dyes by laccase/mediator-assisted laccase of Cyathus bulleri. J Biotechnol 143:69–78
Eggert C, Temp U, Eriksson KEL (1996) The ligninolytic system of white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Ergun SO, Urek RO (2017) Production of ligninolytic enzymes by solid-state fermentation using Pleurotus ostreatus. Ann Agrar Sci 15:273–277
Farhadian S, Hashemi-Shahraki F, Amirifar S, Asadpour S, Shareghi B, Heidari E et al (2022) Malachite Green, the hazardous materials that can bind to Apo-transferrin and change the iron transfer. Intl J Biol Macromol 194:790–799
Giacobbe S, Pezzella C, Sannia G, Piscitelli A (2020) Old enzymes at the forefront of lignocellulosic waste valorization. In: Schlosser D (ed) Laccases in bioremediation and waste valorization. Springer, Cham, pp 57–78
González-Rodríguez S, Lu-Chau TA, Trueba-Santiso A, Eibes G, Moreira MT (2022) Bundling the removal of emerging contaminants with the production of ligninolytic enzymes from residual streams. Appl Microbiol Biotechnol 106(3):1299–1311. https://doi.org/10.1007/s00253-022-11776-7
Harazono K, Watanabe Y, Nakamura K (2003) Decolorization of azo dye by the white-rot basidiomycete Phanerochaete sordida and by its manganese peroxidase. J Biosci Bioeng 95:455–459
Heinzkill M, Bech L, Halkier T et al (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microb 64:1601–1606
Hossain T, Miah AB, Mahmud SA et al (2018) Enhanced bioethanol production from potato peel waste via consolidated bioprocessing with statistically optimized medium. Appl Biochem Biotechnol 186:425–442
Koskela S, Wang S, Xu D, Yang X, Kai Li K et al (2019) Lytic polysaccharide monooxygenase (LPMO) mediated the production of ultra-fine cellulose nanofibres from delignified softwood fibres. Green Chem 21:5924–5933
Kenzom T, Srivastava P, Mishra S (2014) Structural insights into 2,2- azino-bis (3 ethylbenzothiazoline-6-sulfonic acid) (ABTS)-mediated degradation of reactive blue 21 by engineered Cyathus bulleri laccase and characterization of degradation products. Appl Environ Microbiol 80:7484–7495
Kumar A, Chandra R (2020) Ligninolytic enzymes and their mechanisms for degradation of lignocellulosic waste in the environment. Heliyon 6:e0317070
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Liang S, McDonald AG (2014) Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J Agric Food Chem 62:8421–8429
Liu E, Li M, Abdella A, Wilkins MR, MR, (2020) Development of a cost-effective medium for submerged production of fungal aryl alcohol oxidase using a genetically modified Aspergillus nidulans strain. Bioresour Technol 305:123038
Loncara N, Janovica B, Vujcicb M, Vujcica Z (2012) Decolorization of textile dyes and effluents using potato (Solanum tuberosum) phenoloxidase. Inter Biodeter Biodegrad 72:42–45
Luo C, Yao W, Gao X (2022) Degradation of reactive orange 16 in textile wastewater treatment using CuO/ZnO nanocomposite as photocatalyst. Int J Electrochem Sci 17:220732
Mader J, Rawel H, Kroh L (2009) Composition of phenolic compounds and glycoalkaloids α-solanine and α-chaconine during commercial potato processing. J Agric Food Chem 57:6292–6297
Mishra S, Maiti A (2018) The efficacy of bacterial species to decolorize reactive azo, anthraquinone and triphenylmethane dyes from wastewater: a review. Environ Sci Pollut Res 25:8286–8314
Moldes D, Sanromán MÁ (2006) Amelioration of the ability to decolorize dyes by laccase:relationship between redox mediators and laccase isoenzymes in Trametes versicolor. World J Microbiol Biotechnol 22:1197–1204
Nimbalkar PR, Khedkar MA, Chavan PV, Bankar SB (2018) Biobutanol production using pea pod waste as substrate: impact of drying on saccharification and fermentation. Renewable Ener 117:520–529
Oberoi HS, Vadlani PV, Saida L, Bansal S, Hughes JD (2011) Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manag 31:1576–1584
Okamoto K, Yanase H (2002) Aryl alcohol oxidases from the white-rot basidiomycete Pleurotus ostreatus. Mycosci 43:391–395
Oliveira MD, Feddern V, Kupski L, Cipolatti EP, Badiale-Furlong E, de Souza-Soares LA (2010) Physico-chemical characterization of fermented rice bran biomass Caracterización fisico-química de la biomasa del salvado de arroz fermentado. CyTA–J Food 8:229-236
Osma JF, Herrera JLT, Rodriguez-Couto SR (2007) Banana skin: a novel waste for laccase production by Trametes pubescens under solid-state conditions. application to synthetic dye decolouration. Dyes Pigm 75:32–37
Osma JF, Moilanen U, Toca-Herrera JL, Rodríguez-Couto S (2011) Morphology and laccase production of white-rot fungi grown on wheat bran flakes under semi-solid-state fermentation conditions. FEMS Microbiol Lett 318:27–34
Pennacchio A, Pitocchi R, Varese GC, Giardina P, Piscitelli A (2021) Trichoderma harzianum cerato-plantain enhances the hydrolysis of lignocellulosic materials. Microb Biotechnol 14:1699–1706
Ravindran R, Hassan SS, Williams GA, Jaiswal AK (2018) A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengg (basel) 5:93
Rodríguez-Couto S (2017) Industrial and environmental applications of white-rot fungi. Mycosphere 3:456–466
Salony MS, Bisaria VS (2006) Production and characterization of laccase from Cyathus bulleri and its use in decolorization of recalcitrant textile dyes. Appl Microbiol Biotechnol 71:646–653
Saroj S, Agarwal P, Dubey S, Singh RP (2013) Manganese peroxidases: molecular diversity, heterologous expression, and applications. In: Pratyoosh S, Brett IP (eds) Advances in enzyme biotechnology. Springer, New Delhi, pp 67–87
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Siva RS, Banu N (2011) Screening of manganese peroxidase production through solid state fermentation and textile dyes decolorization by Phenarochaete chrysosporium. Research J Sci Tech 3:33–43
Sukhdev A, Manjunatha AS (2017) Decolorization of reactive orange 16 azo dye in wastewater using CAT/IrCl3/HClO4 redox system: delineation of kinetic modeling and mechanistic approaches. J Taiwan Inst Chem Engg 70:150–160
Svobodová K, Erbanová P, Sklenář J, Novotný C (2006) The role of Mn-dependent peroxidase in dye decolorization by static and agitated cultures of Irpex lacteus. Folia Microbiol 51:573–578
Svobodová K, Senholdt M, Novotný Č, Rehorek A (2007) Mechanism of Reactive Orange 16 degradation with the white rot fungus Irpex lacteus. Proc Biochem 42:1279-1284
Tandrup T, Frandsen KEH, Johansen KS, Berrin J, Leggio LL (2018) Recent insights into lytic polysaccharide monooxygenases (LPMOs). Biochem Soc Trans 46:1431–1447
Tanruean K, Penkhrue W, Kumla J, Suwannarach N, Lumyong S (2021) Valorization of lignocellulosic wastes to produce phytase and cellulolytic enzymes from a thermophilic fungus, Thermoascus aurantiacus sl16w, under semi-solid-state fermentation. J Fungi 7:286
Tien M, Kirk TK (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci 81:2280–2284
Tizaoui C, Grima N (2011) Kinetics of the ozone oxidation of reactive orange 16 azo-dye in aqueous solution. Chem Engg J 173:463–473
Urlacher VB, Koschorreck K (2021) Peculiarities and applications of aryl-alcohol oxidases from fungi. Appl Microbiol Biotechnol 105:4111–4126
Vallabha V, Indira TN, Jyothi-Lakshmi A, Radha C, Tiku PK (2015) Enzymatic process of rice bran: a stabilized functional food with nutraceuticals and nutrients. J Food Sci Technol 52:8252–8259
Vats A, Mishra S (2017) Decolorization of complex dyes and textile effluent by extracellular enzymes of Cyathus bulleri cultivated on agro-residues/domestic wastes and proposed pathway of degradation of kiton blue A and reactive orange 16. Environ Sci Pollut Res 24:11650–11662
Vats A, Mishra S (2018) Identification and evaluation of bioremediation potential of laccase isoforms produced by Cyathus bulleri on wheat bran. J Hazard Material 344:466–479
Vats A, Mishra S (2021) An insight into transcriptome of Cyathus bulleri for lignocellulase expression on wheat bran. Arch Microbiol 203:3727–3736
Wang D, Li J, Wong ACY et al (2018) A colorimetric assay to rapidly determine the activities of lytic polysaccharide monooxygenases. Biotechnol Biofuels 11:215
Wehaidy HR, El-Hennawi HM, Ahmed SA, Abdel-Naby MA (2018) Comparative study on crude and partially purified laccase from Polyporus durus ATCC 26726 in the decolorization of textile dyes and wastewater treatment. Egypt Pharma J 17:94–103
Zeng S, Quin X, Xia L (2017) Degradation of the herbicide isoproturon by laccase-mediator system. Biochem Engg J 119:92–100
Acknowledgements
This work was supported by a grant from the Science and Engineering Research Board (SERB), Department of Science and Technology (Government of India). SERB is also thanked for providing National Postdoctoral Fellowship to SA (PDF/2017/001057).
Funding
This work was supported by Department of Science and Technology, Govt. of India.
Author information
Authors and Affiliations
Contributions
SA performed all the experiments. Mass spectrometry and data interpretation were carried out by SA and SM. The first draft of the MS was written by SA.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afreen, S., Mishra, S. Production of high-value oxidative enzymes by Cyathus bulleri on agricultural and agri-food wastes for application in the textile sector. World J Microbiol Biotechnol 39, 329 (2023). https://doi.org/10.1007/s11274-023-03769-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03769-z