Abstract
Besides the major and widespread use of laccases in bioremediation of dyes and many other toxic chemical wastes, there is an increasing interest towards their exploitation in the valorization of lignocellulosic biomass in frame with the biorefinery concept. Biochemical conversion of lignocelluloses includes three major steps: pretreatment, enzymatic hydrolysis, and fermentation. Laccases find application in the pretreatment phase since they are effective both in removing/modifying the lignin polymer and in reducing the amount of phenolic compounds of already pretreated lignocellulosic materials. As a fact, pretreatment steps often release some biomass-derived products inhibiting both enzymes involved in saccharification and microorganisms used for the fermentation. Together with detoxification processes, laccases can be used for delignification of lignocellulosic biomasses thanks to their direct action on phenolic units of lignin polymer. Laccase-based pretreatments avoid sugars degradation, thanks to the mild conditions of reaction. Conversely, the hard conditions usually adopted for chemical-physical pretreatments, cause sugars degradation. Furthermore, laccase effect in delignification is improved by the use of mediator compounds which allow enzyme to oxidize both phenolic and non-phenolic component of lignin moieties, producing an extensive cleavage of covalent bonds in lignin. A survey of uses of laccase in lignocellulosic waste valorization is offered in this chapter with the aim to underline their potential. Indeed, applications of laccases in the valuable conversion of different wastes have been deeply described, focusing on available examples of laccase integration in biorefineries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
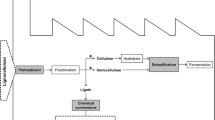
The constant increase in the world energy consumption needs for effective alternatives to gradually replace the fossil fuels, as encouraged by the EU Bioeconomy Strategy. In this context, biomass and most particularly lignocellulosics can offer a concrete option for the production of chemicals and energy in future biorefineries, alternative to current oil refineries. Lignocellulosic raw materials, including forest residues, agricultural wastes, and agrofood wastes (AFW) (Fig. 1), are the most abundant source of organic material in the world: their production is estimated to be around 1.3 billion tons per year (Baruah et al. 2018).
Hence, lignocellulosic biomass represents a promising platform for the production of bio-based products, even if its potential is still untapped.
Albeit composition of lignocellulosic materials depends on many factors, such as plant species, age and growth conditions, and the eventual undergone process, generally these biomasses consist of 15–35% lignin, 40–50% cellulose, and 25–30% hemicelluloses (Pleissner et al. 2016). Very complex inter- and intramolecular network (covalent or noncovalent) exists between these components, rendering the utilization of these residues very challenging. Therefore, a pretreatment step is required to both destroy the layer of lignin that limits enzyme accessibility to cellulose and disassemble the crystalline cellulose structure.
The typical process for the valorization of lignocellulosic materials conventionally includes three primary steps (Fig. 2):
-
1.
Pretreatment, aimed at disrupting the fibrous matrix and remove lignin
-
2.
Hydrolysis, aimed at breaking down (hemi)cellulose and cellulose into monomers
-
3.
Fermentation, aimed at transforming the obtained sugars into value-added chemicals, according to the metabolic abilities of the used yeasts
The pretreatment step is still a matter of investigation, since an effective method that combines environmental sustainability, sugar recovery, and cost-effectiveness is still missing (Fillat et al. 2017). A number of physical and chemical pretreatment methods have been tested; however they are often high-energy demanding and cause loss of carbohydrates and/or formation of by-products (furans, phenols, and weak acids) that may inhibit the following hydrolysis and/or fermentation steps. Therefore, biological approaches able to modify lignin and to reduce the amount of inhibitors represent interesting and greener alternatives to improve the efficiency of the bioconversion processes. These methods rely on the ability of some microorganisms to grow on ligninocellulosic substrates thanks to the secretion of ligninolytic enzymes, such as lignin peroxidases, manganese peroxidases, versatile peroxidase, and laccases (Faraco et al. 2009). On the other hand, the exploitation of ligninolytic enzymes over whole cells includes the possibility of using temperatures which are not necessarily optimal for the growth of the microorganisms. In this context, laccases have been widely investigated, and the obtained results recently reviewed (Kudanga et al. 2010; Roth and Spiess 2015; Fillat et al. 2017). Laccases have been proven able to contribute in different ways to the process of waste valorization. Indeed, they degrade the lignin, modify the structure of the lignocelluloses microfibre, and reduce the phenolic content of pretreated lignocellulosic materials, thus decreasing their inhibitory effect. Laccases have been used alone, or in mixture with other ligninolytic enzymes (Wang et al. 2013; Asgher et al. 2013; Ji et al. 2014). In this latter case, it is not easy to ascribe a defined role to laccase, since the different enzymes/molecules present in the mix can synergistically cooperate in lignin modification/degradation. Laccase delignification is often carried out in combination with mediators, easily oxidizable phenolic units that enable enzyme to indirectly oxidize large molecules and even non-phenolic substrates (Cañas and Camarero 2010). Nonetheless, the use of mediators also delivers various drawbacks, such as their high cost, the possible formation of toxic species, and the occurrence of grafting side reactions (Oliva-Taravilla et al. 2015a; Moreno et al. 2016b; Fillat et al. 2017). Natural mediators, such as lignin-derived soluble phenols (e.g. vanillin, acetosyringone, p-hydroxycinnamic acids), offer environmental and economic advantages compared to chemical mediators (Giacobbe et al. 2018). Nonetheless, the phenoxy radicals generated by laccase can be grafted onto pretreated material, with consequent increase of lignin content and decrease of sugar hydrolysis, depending on specific unpredictable combination among laccase, ligninolytic source, and mediator (Oliva-Taravilla et al. 2015a; Moreno et al. 2016b).
Very often the laccase treatment has been combined with other physical and chemical treatment methods, resulting in higher yields than the individual methods alone (see below).
2 Laccases in Biorefinery: Delignification and Detoxification of Lignocellulosic Biomasses
In this chapter, the laccase action in lignocellulosic valorization has been organized according to the different residues tested: softwood and hardwood forest residues, agricultural residues, and agrofood wastes (Fig. 1).
It is important to highlight that it is difficult to compare laccase efficiencies among the different reports. Indeed, not all phenolic compounds are susceptible to oxidation by laccase enzymes at the same extent, and lignin structure is highly dependent on biomass feedstock and/or on pretreatment conditions.
The aim of this chapter is to give a comprehensive picture of the potentiality of laccase enzymes in this interesting field.
2.1 Softwood and Hardwood Forest Residues
Among lignocellulosic biomasses, woody biomasses represent an interesting nonedible biomass for biofuels and biomaterials production. However their resistance to delignification hinders their sustainable transformation.
The lignin content of softwood (25–35% weight) is typically higher than that of hardwood (18–25% weight) (Azadi et al. 2013). Softwood’s lignin is mainly composed by guaiacyl units (G), while hardwood contains also syringyl units (S). The G units reduce accessibility of hydrolytic enzymes; hence the first wood biomass is more difficult to be hydrolysed (Palonen and Viikari 2004; Jönsson et al. 2013). Hemicellulose in softwood is composed by galactoglucomannans (15–20%), and xylans represent only a small percentage (7–10%), while in hardwoods it is mainly composed by xylan (15–30%) (Palonen and Viikari 2004).
Several works demonstrated the ability of laccases and laccase mediator system (LMS) in delignification of woody biomasses, also in combination with other pretreatments. Laccases alone are not able to oxidize softwood biomasses; therefore these complex lignocellulosic substrates need to be pretreated with other methods. Two-step pretreatment of softwood has some attractive advantages, such as high ethanol yield and low consumption of enzymes (Galbe and Zacchi 2002). As summarized in Table 1, often LMS is required to improve delignification and saccharification yields from softwood since laccase is too large a molecule to penetrate into the fibre wall of native wood.
In 1999, Larsson’s group (Larsson et al. 2003) demonstrated the ability of T. versicolour laccase to remove furans and phenolic compounds from steam-pretreated spruce with consequently improvement of ethanol production. Palonen and Viikari (2004) demonstrated that the lignin from pretreated spruce binds cellulase enzymes, but the action of the laccases, before the hydrolysis, improves the enzymatic hydrolysis of 13% by using laccase alone and by 21% by applying LMS. More recently, Moilanen et al. (2014) explored the action of different mediators to reduce hydrolytic enzymes adsorption with consequent increase in the enzymatic hydrolysis yields.
As regards hardwood biomasses, laccase action is more pronounced (Table 2). Kuila et al. (2011a, b) reported the ability of laccase alone to delignify unpretreated bamboo wood with consequent efficient saccharification. More recently, Kumar et al. (2017) set up a simultaneous pretreatment and saccharification process by utilizing laccases with cellulases in order to obtain sugars for biobutanol production from bamboo. Thanks to its rapid growth and high biomass-producing woody, eucalyptus is an interesting hardwood biomass for biofuel production and was successfully pretreated by laccase (Rico et al. 2014) obtaining a delignification of about 20%. The action of the laccase was enhanced by using methyl syringate as mediator (50% of delignification) with a consequent increment in saccharification yield (about 40%). Laccases were also applied on pretreated eucalypt wood with or without LMS. In all reported studies (Table 2), the effect of laccases was not only on delignification but also on detoxification of pretreated biomasses. Noteworthy are the results reported by Gutiérrez and co-workers (Gutiérrez et al. 2012). The authors observed up to 48% lignin removal by using a combination of enzymatic pretreatment and alkaline extraction. The high delignification yield was linked to high glucose yield (61%) and ethanol production (over 4 g/L). To the best of our knowledge, only one paper reports the application of bacterial laccase to pretreated hardwood biomasses. Singh and co-workers (Singh et al. 2017) highlighted the biocatalytic potential of bacterial enzymes in delignification of steam-pretreated poplar. The small laccase (SLAC) from Amycolatopsis sp. 75iv3 allowed to reduce acid-soluble lignin of about 15% through oxidation of syringyl units of lignin, acting synergistically with commercial cellulases with a consequent increment of glucose production (about 8%).
2.2 Agricultural Residues
The broad term ‘agricultural waste’ is generally referred to any lignocellulosic residue produced by agrofood industries. Nevertheless, these residues can be further divided into two main categories depending if they are generated during the harvesting (primary biomass residues) and the processing (secondary biomass residues) of agricultural crops. Agricultural residues are produced during harvesting and are those considered in this paragraph. The residues most frequently tested are represented by wheat, and only few papers utilize different residues (corn, cotton, barley, rice, and pineapple).
When considering the laccase-mediated valorization of agricultural residues (Table 3), the most of examples rely on the exploitation of laccases as detoxification agent, rather than as delignifying one. Furthermore, even when laccases are used to delignify residues, they are very frequently used in combination with other pretreatment techniques and seldom alone (Hyeon et al. 2014; Davidi et al. 2016). Depending on laccases source, the treatments have been carried out at a wide range of pH, temperature, treatment time, and enzyme loading. Different phenol removal efficiencies have been described with different laccases, and reduced laccase effectiveness in detoxification has been reported at increasing viscosity of the medium (Moreno et al. 2013a). Moreno et al. tested the efficiency of laccase treatment before or after enzymatic hydrolysis, obtaining the highest phenol reduction (93–94%) when the laccase treatment was carried out before the enzymatic hydrolysis. Laccase action improved the fermentation performance of the thermotolerant yeast Kluyveromyces marxianus strain used, shortening its lag phase and enhancing the ethanol yields (Moreno et al. 2012). The ability of laccases in the detoxification of pretreated biomasses enabled working at high-substrate loading, resulting in the possibility to reduce freshwater and energy consumption without affecting the yields of the final product (Moreno et al. 2013b, c).
A unique example of laccase treatment with immobilized enzyme is present in the recent literature (Ludwig et al. 2013). The immobilized T. versicolour laccase efficiently removed phenolic compounds from a wheat straw organosolv fraction, with a consequence increase of the fermentation performance of the yeast Pichia stipitis. The immobilized laccase was efficiently reused, and an in situ product removal was achieved since insoluble products precipitated onto the carrier surface.
Few examples of residue valorization using bacterial laccases are present in the literature (Moreno et al. 2016a; De La Torre et al. 2017; Rocha-Martín et al. 2018), mainly due to the lower redox potential of these enzymes with respect to the fungal laccases. Moreno et al. (2016a) assayed the use of a novel bacterial laccase (MetZyme®) for enhancing the hydrolysability and fermentability of steam-exploded wheat straw. Albeit a low phenol reduction was measured, it was sufficient to improve the fermentation performance during a simultaneous saccharification and fermentation (SSF) process. De La Torre et al. (2017) compared the efficiencies of a commercial fungal laccase from T. villosa with a bacterial laccase from S. ipomoeae for delignification and detoxification of steam-exploded wheat straw. When the reduction of lignin was considered, a slight reduction in lignin content and a consequent increase in both glucose and xylose production was detected with the bacterial laccase, while no effect was produced by the T. villosa laccase. Conversely, a higher efficiency in the decrease of the phenol content was measured using the fungal laccase with respect to the treatment performed with the S. ipomoeae laccase.
With the aim to get insights into lignin modifications, some authors carried out detailed analyses (Lu et al. 2010; Li et al. 2012; Qiu and Chen 2012; Wang et al. 2013; Rencoret et al. 2016; Banerjee et al. 2019). When the Sclerotium sp. laccase was employed in combination with steam explosion, the authors suggested that the laccase could oxidize the aromatic ring of lignin resulting in the formation of micropores which contributed to loosening the compact wrap of lignin-carbohydrate complex and consequently enhancing the enzymatic hydrolysis efficiency of cellulose (Qiu and Chen 2012). Rencoret et al. (2016) performed two-dimensional nuclear magnetic resonance (2D NMR) analysis of the whole wheat straw material pretreated with a P. cinnabarinus laccase in the presence of HBT as mediator. The authors highlighted the formation of Cα-oxidized lignin units during the enzymatic treatment, showing the selective action of laccase-mediator on the lignin moiety, with respect to the polysaccharide signals. Avanthi and Banerjee (2016) performed detailed analysis to verify the existence of a correlation between biomass composition and laccase-mediated lignin degradation. According to their results, G-type lignin has synergistic effect on laccase-mediated delignification, while the presence of S type lignin showed antagonistic relationship with laccase adsorption and delignification.
Contradictory effects due to laccase action have also been described due to the formation of laccase-derived compounds from phenols inhibiting cellulolytic enzymes and/or to the occurrence of grafting phenomena. For example, Oliva-Taravilla et al. (2015b) showed a decrease in the yield of the enzymatic hydrolysis, due to oligomeric products derived from the oxidative polymerization of vanillin and syringaldehyde by M. thermophila laccase. Interestingly, Rocha-Martin et al. (2018) highlighted the existence of a lower inhibiting effect of the enzymatic hydrolysis by bacterial laccase, when comparing the performance of commercial bacterial (S. ipomoeae) and fungal (M. thermophila) laccases on pretreated corn stover and sugarcane straw. As for grafting, lignin-derived phenols resulting from pretreatment of biomass can be oxidized by laccase to phenoxy radicals, which can undergo polymerization by radical coupling onto materials, resulting in an increment of the lignin content (Oliva-Taravilla et al. 2015a).
Particularly interesting are those works designed to obtain better processes integration through the simultaneous action of laccases and hydrolytic enzymes by genetic engineering (Zhang et al. 2012; Hyeon et al. 2014; Davidi et al. 2016) or by mixing extracellular products (Wang et al. 2017). Zhang et al. (2012) heterologously expressed a laccase from Trametes sp. AH28-2 in the cellulolytic fungus Trichoderma reesei and obtained an increased enzymatic saccharification of cellulosic materials by the laccase producing strains. The integration of laccase activity into cellulosome systems has been successfully obtained (Hyeon et al. 2014; Davidi et al. 2016). In particular, Davidi et al. (2016) incorporated the laccase from the aerobic bacterium T. fusca into a cellulase- and xylanase-containing cellulosome. The resulting complex allowed doubling the amount of reducing sugars released from untreated wheat straw compared with the same system lacking the laccase. Inspired by a similar idea, Wang and co-workers (Wang et al. 2017) built a composite enzymatic system mixing the extracellular products of the white-rot fungus E. taxodii with cellulolytic enzymes. A synergistic action between the extracellular products and cellulases was observed during the valorization of acid-pretreated and raw maize stovers.
2.3 Agrofood Wastes
The food supply chain is characterized by huge waste losses, whose volume and composition depend on the phase they are produced at (harvesting, processing, distribution, and consumption). Postharvest losses are very variable, being a function of the technology available in a country, the extent to which markets have developed for a specific crop, as well as of the crop perishability (Parfitt et al. 2010). Apart from harvesting, according to European Commission, up to 42% of food waste (FW) derives from households, 39% is produced by food manufacturing industry, 14% pertains to food service sector (catering, restaurants), and only the remaining 5% is a surplus of market chain. Despite the strong push towards the prevention of FW in all the stages of food life cycle, FW production is expected to reach about 126 Mt by 2020 (Mirabella et al. 2014). Besides representing an economic and environmental issue, most of waste generated from the food industry is lignocellulosic in nature, being potential substrate for the production of high-added value products (Ravindran and Jaiswal 2016).
Laccases were applied to valorization of different types of AFWs. Most of the reported examples refer to wastes derived from sugarcane biomass, only a few of them concern other sources (Tables 4, 5, and 6). In the frame of AFW valorization, laccases were exploited in two main processes: (1) delignification followed by saccharification (sequential process) and (2) concomitant delignification and saccharification (simultaneous process) (Tables 4 and 5).
In most of the described processes, laccases act directly on the biomass as the only delignificant agent (Table 4). Pretreatment conditions differ in operating parameters, such as, pH, temperature, enzyme loading, enzyme/biomass ratio, and incubation time and are strictly dependent on the biochemical properties of the tested laccases. Although fungal laccases are the catalysts of choice, the synergistic action of the whole ligninolytic enzymatic systems produced by selected fungi was effectively exploited for pretreatment of sugarcane bagasse (Asgher et al. 2013) and Jerusalem artichoke (Ji et al. 2014). In the latter case, the authors formulated a new enzymatic cocktail for simultaneous delignification and hydrolysis, based on a psychrophilic lignocellulosic enzymatic mixture from C. cladosporioides and a commercial xylanase. The wide thermal adaptability in mesophilic and low temperatures of the characterized enzymatic mixture provided consistency of hydrolysis and further fermentation at ambient temperatures (Ji et al. 2014). Bacterial laccases were applied only in limited cases (Chen et al. 2013). When the laccase from the moderately thermophilic T. fusca was added to the enzymatic cocktail for sugarcane bagasse hydrolysis, it significantly enhanced the saccharification yield. The enzyme was recombinantly produced in E. coli, thus supporting the cost-effectiveness of the process (Piscitelli et al. 2010). Statistical optimization of process parameters was applied to laccase-promoted delignification process (Rashid Shah et al. 2016; Sherpa et al. 2018). Delignification of sugarcane tops by P. djamor laccase was optimized through Response surface methodology and its effectiveness substantiated by the consequent improvement in saccharification yield (Sherpa et al. 2018). The depolymerization effect of laccase on the substrate was also confirmed by structural analyses. Interestingly, low-molecular compounds formed during delignification were proposed to act as natural laccase mediators, contributing to process economy and sustainability (Sherpa et al. 2018). Particularly interesting is the process proposed by Giacobbe et al. for the treatment of different kinds of AFWs (Giacobbe et al. 2018, 2019). The authors tested different combinations of P. ostreatus laccases with and without the addition of a natural phenolic mediator, designing a sequential protocol in which delignification and following saccharification occur in the same bioreactor without any washing step. The two enzymatic steps are separated only by a short thermal deactivation phase, in order to overcome the observed inhibition of cellulase activity caused by laccases. It is worth to note that significant differences emerged in the way of action of the tested enzymes. The native mix from P. ostreatus displayed a tendency to graft phenols, causing increase in the lignin content, conversely the main action of the recombinant POXA1b laccase was in lignin degradation (Giacobbe et al. 2018, 2019).
Examples of AFW pretreatment based on the combination of laccases together with chemical/physical methods are listed in Table 5. The processes are aimed at improving biomass delignification (Thangavelu et al. 2018), hemicellulose extraction (Nie 2019), or saccharification yield (Sitarz et al. 2013; Rencoret et al. 2017; Rizal et al. 2018; Sánchez-Ramírez et al. 2018). Thangavelu and co-workers coupled T. versicolour laccase to hydrodynamic cavitation and applied the combined method to the pretreatment of corncobs. Both biomass and biocatalyst were circulated continuously throughout the reactor, leading to high delignification with low energy requirement. Morphological studies confirmed the deconstruction of biomass without modification of the cellulose structure (Thangavelu et al. 2018). A two-step process in which laccase pretreatment was followed by microwave-assisted alkaline extraction of hemicellulose was applied to sugarcane bagasse. Besides reducing lignin content, laccase treatment structurally altered the bagasse, increasing its specific surface area and porosity, thus enhancing the following hemicellulose extraction (Nie 2019).
In most of the reported examples, the efficiency of laccase-combined pretreatments was evaluated in terms of improvement of following saccharification step. A survey of reported results indicates that the effect of the applied treatment is strongly influenced by the choice of proper laccase as well as by the lignin composition of the AFW of interest. For example, Sitarz et al. (2013) verified that the addition of the laccase-rich G. lucidum broth to a state-of-art cellulase preparation significantly increased the cellulolytic yield of steam-exploded sugarcane bagasse. In contrast, the spiking with T. versicolour laccase produced an inhibitory effect on the same cellulase mixture. The authors hypothesized that the higher Kcat/KM or redox potential of G. lucidum laccase with respect to T. versicolour one caused a faster and more extensive solubilization of lignin degradation products, promoting cellulase adhesion. Alternatively, the enzymes may display different selectivity towards potential cellulase inhibitors derived from lignin hydrolysis (Sitarz et al. 2013). When the same T. versicolour laccase was combined to superheated steam (SHS) for the pretreatment of oil palm biomass, an enhancement of subsequent enzymatic hydrolysis was observed, as a consequence of the structural rearrangement occurring on lignin (Rizal et al. 2018). However, in this case, a sequential process was applied by introducing a washing step between the delignification and saccharification, in order to prevent any inhibitory effect on cellulases (Rizal et al. 2018). Rencoret et al. (2017) pointed attention on the lignin structural features that may influence the mechanism of laccase action. The LMS composed of the high-redox potential laccase from P. cinnabarinus and 1HBT as mediator was exploited to remove and/or modify the lignins in the sugarcane bagasse (S-rich lignin) and straw residues (G-rich lignin). The LMS pretreatment, followed by alkaline peroxide extraction, produced a rather similar lignin removal from both materials despite their different lignin composition (H/G/S ratio), suggesting that other features, i.e. the presence of p-coumarates acylating the γ-OH of the lignin side chains, may adversely affect laccase action (Rencoret et al. 2017). This scenario entirely changed when the treatment was carried out in the absence of mediator. In this case, the difference in lignin composition between the two materials resulted in a more pronounced lignin degradation in the straw with respect to bagasse (Rencoret et al. 2017). The overall economy of laccase-based process would benefit from enzyme immobilization on solid support, facilitating its recovery and reuse. However, when free and immobilized T. versicolour laccases were tested on previously autoclaved agave biomass waste, only the free enzyme led to raise the subsequent saccharification yield, while immobilized samples strongly impaired it. It was proposed that the formation of a complex between the nanocomposite, reaction products and biomass may hinder the interaction of cellulose with hydrolytic enzymes, preventing its hydrolysis (Sánchez-Ramírez et al. 2018). Conversely, in a more recent report, Kumar et al. (2019) succeeded in setting up an immobilized enzyme cocktail comprising cellulases and the T. versicolour laccase for the simultaneous detoxification and saccharification of pretreated rice straw. The reusability of the enzyme cocktail in the simultaneous process was verified up to eight cycles, conferring an economic advantage to the process. The effectiveness of the strategy was further confirmed by the improvement in yeast performance and enhanced ethanol production in subsequent fermentation step (Kumar et al. 2019).
Finally, several examples of laccase-promoted detoxification of chemically/physically hydrolysed biomasses were described (Table 6). Treatment of sugarcane bagasse hydrolysates with laccases from different sources was effective in removing most of phenolic compounds, resulting in improvement of yeast performances in subsequent ethanolic fermentation of hydrolysates (Martín et al. 2002; Chandel et al. 2007). Although laccase treatment did not eliminate other classes of inhibitors such as acetic acid, furfural, and 5-hydroxy-methyl-furfural, it brought about negligible loss in total sugars when compared with other chemical or physical detoxification methods (Chandel et al. 2007). As already described for delignification process (Sitarz et al. 2013; Giacobbe et al. 2018), laccases from different sources displayed also different performances in detoxification. For example, a newly isolated laccase from Coltricia perennis displayed a higher efficiency in removal of phenolic compounds in acid-pretreated rice straw, with respect to a commercial Novozyme laccase (Kalyani et al. 2012). On the other hand, the application of a bacterial laccase to detoxification of hydrolysed sugarcane bagasse did not increase following biohydrogen production, suggesting that detoxification provided by laccase action was not suitable for the thermophilic anaerobic bacteria used in the fermentation step (Hu et al. 2018).
3 Conclusions/Future Prospective
Laccase-based processes allow achieving ethanol yield, glucose conversion, and delignification and detoxification percentages similar or higher than conventional methodologies. Hence, in the current biorefinery concept, laccases constitute a powerful biotechnological tool towards the achievement of the EU Bioeconomy Strategy. The most important obstacle to laccases exploitation is the cost of both enzymes and redox mediators.
Now days, marked efforts are being made in order to achieve both cheap overproduction of laccases and their engineering aimed at gaining more robust and active enzymes (Piscitelli et al. 2011; Pezzella et al. 2017).
Moreover, the possibility to recover natural mediators and to produce lignocellulolytic enzymes from wastes themselves represents a step forward the process circularity.
References
Alvira P, Moreno AD, Ibarra D et al (2013) Improving the fermentation performance of Saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol Prog 29:74–82. https://doi.org/10.1002/btpr.1666
Asgher M, Ahmad Z, Iqbal HMN (2013) Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind Crops Prod 44:488–495. https://doi.org/10.1016/j.indcrop.2012.10.005
Avanthi A, Banerjee R (2016) A strategic laccase mediated lignin degradation of lignocellulosic feedstocks for ethanol production. Ind Crops Prod 92:174–185. https://doi.org/10.1016/j.indcrop.2016.08.009
Azadi P, Inderwildi OR, Farnood R, King DA (2013) Liquid fuels, hydrogen and chemicals from lignin: a critical review. Renew Sustain Energy Rev 21:506–523. https://doi.org/10.1016/J.RSER.2012.12.022
Banerjee R, Chintagunta AD, Ray S (2019) Laccase mediated delignification of pineapple leaf waste: an ecofriendly sustainable attempt towards valorization. BMC Chem 13. https://doi.org/10.1186/s13065-019-0576-9
Baruah J, Nath BK, Sharma R, et al (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6. https://doi.org/10.3389/fenrg.2018.00141
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98:1947–1950. https://doi.org/10.1016/j.biortech.2006.07.047
Chen Q, Marshall MN, Geib SM et al (2012) Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour Technol 117:186–192. https://doi.org/10.1016/j.biortech.2012.04.085
Chen CY, Hsieh ZS, Cheepudom J et al (2013) A 24.7-kDa copper-containing oxidase, secreted by Thermobifida fusca, significantly increasing the xylanase/cellulase-catalyzed hydrolysis of sugarcane bagasse. Appl Microbiol Biotechnol 97:8977–8986. https://doi.org/10.1007/s00253-013-4727-y
Davidi L, Moraïs S, Artzi L et al (2016) Toward combined delignification and saccharification of wheat straw by a laccase-containing designer cellulosome. Proc Natl Acad Sci 113:10854–10859. https://doi.org/10.1073/pnas.1608012113
De La Torre M, Martín-Sampedro R, Fillat Ú et al (2017) Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J Ind Microbiol Biotechnol 44:1561–1573. https://doi.org/10.1007/s10295-017-1977-1
Fang Z, Liu X, Chen L et al (2015) Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production David Wilson. Biotechnol Biofuels 8. https://doi.org/10.1186/s13068-015-0235-x
Faraco V, Pezzella C, Giardina P et al (2009) Decolourization of textile dyes by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus. J Chem Technol Biotechnol 84. https://doi.org/10.1002/jctb.2055
Fillat Ú, Ibarra D, Eugenio M et al (2017) Laccases as a potential tool for the efficient conversion of lignocellulosic biomass: a review. Fermentation 3:17. https://doi.org/10.3390/fermentation3020017
Galbe M, Zacchi G (2002) A review of the production of ethanol from softwood. Appl Microbiol Biotechnol 59:618–628. https://doi.org/10.1007/s00253-002-1058-9
Giacobbe S, Pezzella C, Lettera V et al (2018) Laccase pretreatment for agrofood wastes valorization. Bioresour Technol 265:59–65. https://doi.org/10.1016/j.biortech.2018.05.108
Giacobbe S, Piscitelli A, Raganati F et al (2019) Butanol production from laccase-pretreated brewer’s spent grain. Biotechnol Biofuels 12. https://doi.org/10.1186/s13068-019-1383-1
Gutiérrez A, Rencoret J, Cadena EM et al (2012) Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour Technol 119:114–122. https://doi.org/10.1016/j.biortech.2012.05.112
Heap L, Green A, Brown D et al (2014) Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catal Sci Technol 4:2251–2259. https://doi.org/10.1039/c4cy00046c
Hu BB, Li MY, Wang YT, Zhu MJ (2018) Enhanced biohydrogen production from dilute acid pretreated sugarcane bagasse by detoxification and fermentation strategy. Int J Hydrogen Energy 43:19366–19374. https://doi.org/10.1016/j.ijhydene.2018.08.164
Hyeon JE, You SK, Kang DH et al (2014) Enzymatic degradation of lignocellulosic biomass by continuous process using laccase and cellulases with the aid of scaffoldin for ethanol production. Process Biochem 49:1266–1273. https://doi.org/10.1016/j.procbio.2014.05.004
Ji L, Yang J, Fan H et al (2014) Synergy of crude enzyme cocktail from cold-adapted Cladosporium cladosporioides Ch2-2 with commercial xylanase achieving high sugars yield at low cost. Biotechnol Biofuels 7. https://doi.org/10.1186/s13068-014-0130-x
Jönsson LJ, Alriksson B, Nilvebrant N-O (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels 6:16. https://doi.org/10.1186/1754-6834-6-16
Jurado M, Prieto A, Martínez-Alcalá Á et al (2009) Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour Technol 100:6378–6384. https://doi.org/10.1016/j.biortech.2009.07.049
Kalyani D, Dhiman SS, Kim H et al (2012) Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochem 47:671–678. https://doi.org/10.1016/j.procbio.2012.01.013
Kapoor RK, Rajan K, Carrier DJ (2015) Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresour Technol 189:99–106. https://doi.org/10.1016/j.biortech.2015.03.100
Kolb M, Sieber V, Amann M et al (2012) Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour Technol 104:298–304. https://doi.org/10.1016/j.biortech.2011.11.080
Kudanga T, Prasetyo EN, Sipilä J et al (2010) Reactivity of long chain alkylamines to lignin moieties: implications on hydrophobicity of lignocellulose materials. J Biotechnol 149:81–87. https://doi.org/10.1016/j.jbiotec.2010.06.020
Kuila A, Mukhopadhyay M, Tuli DK, Banerjee R (2011a) Accessibility of enzymatically delignified Bambusa bambos for efficient hydrolysis at minimum cellulase loading: an optimization study. Enzyme Res 2011:1–8. https://doi.org/10.4061/2011/805795
Kuila A, Mukhopadhyay M, Tuli DK, Banerjee R (2011b) Production of ethanol from lignocellulosics: an enzymatic venture. EXCLI J 10:85–96
Kumar S, Gujjala LKS, Banerjee R (2017) Simultaneous pretreatment and saccharification of bamboo for biobutanol production. Ind Crops Prod 101:21–28. https://doi.org/10.1016/j.indcrop.2017.02.028
Kumar V, Patel SKS, Gupta RK et al (2019) Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J 1800468. https://doi.org/10.1002/biot.201800468
Larsson S, Reimann A, Nilvebrant N-O, Jönsson LJ (2003) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77:91–104. https://doi.org/10.1385/abab:77:1-3:91
Li J, Sun F, Li X et al (2012) Enhanced saccharification of corn straw pretreated by alkali combining crude ligninolytic enzymes. J Chem Technol Biotechnol 87:1687–1693. https://doi.org/10.1002/jctb.3818
Liu ZH, Xie S, Lin F, et al (2018) Combinatorial pretreatment and fermentation optimization enabled a record yield on lignin bioconversion. Biotechnol Biofuels 11. https://doi.org/10.1186/s13068-018-1021-3
Lu C, Wang H, Luo Y, Guo L (2010) An efficient system for pre-delignification of gramineous biofuel feedstock in vitro: application of a laccase from Pycnoporus sanguineus H275. Process Biochem 45:1141–1147. https://doi.org/10.1016/j.procbio.2010.04.010
Ludwig D, Amann M, Hirth T et al (2013) Development and optimization of single and combined detoxification processes to improve the fermentability of lignocellulose hydrolyzates. Bioresour Technol 133:455–461. https://doi.org/10.1016/j.biortech.2013.01.053
Martín C, Galbe M, Wahlbom CF et al (2002) Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae
Martin-Sampedro R, Capanema EA, Hoeger I et al (2011) Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips. J Agric Food Chem 59:8761–8769. https://doi.org/10.1021/jf201605f
Martín-Sampedro R, Eugenio ME, García JC et al (2012) Steam explosion and enzymatic pre-treatments as an approach to improve the enzymatic hydrolysis of Eucalyptus globulus. Biomass Bioenergy 42:97–106. https://doi.org/10.1016/j.biombioe.2012.03.032
Mirabella N, Castellani V, Sala S (2014) Current options for the valorization of food manufacturing waste: a review. J Clean Prod 65:28–41
Moilanen U, Kellock M, Galkin S, Viikari L (2011) The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzyme Microb Technol 49:492–498. https://doi.org/10.1016/j.enzmictec.2011.09.012
Moilanen U, Kellock M, Várnai A et al (2014) Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol Biofuels 7:1–13. https://doi.org/10.1186/s13068-014-0177-8
Moreno AD, Ibarra D, Fernández JL, Ballesteros M (2012) Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour Technol 106:101–109. https://doi.org/10.1016/j.biortech.2011.11.108
Moreno AD, Ibarra D, Ballesteros I et al (2013a) Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour Technol 135:239–245. https://doi.org/10.1016/j.biortech.2012.11.095
Moreno AD, Ibarra D, Ballesteros I et al (2013b) Ethanol from laccase-detoxified lignocellulose by the thermotolerant yeast Kluyveromyces-Effects of steam pretreatment conditions, process configurations and substrate loadings. Biochem Eng J 79:94–103. https://doi.org/10.1016/j.bej.2013.07.006
Moreno AD, Tomás-Pejó E, Ibarra D et al (2013c) In situ laccase treatment enhances the fermentability of steam-exploded wheat straw in SSCF processes at high dry matter consistencies. Bioresour Technol 143:337–343. https://doi.org/10.1016/j.biortech.2013.06.011
Moreno A, Ibarra D, Mialon A, Ballesteros M (2016a) A bacterial laccase for enhancing saccharification and ethanol fermentation of steam-pretreated biomass. Fermentation 2:11. https://doi.org/10.3390/fermentation2020011
Moreno AD, Ibarra D, Alvira P et al (2016b) Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J Chem Technol Biotechnol 91:1816–1825. https://doi.org/10.1002/jctb.4774
Nakanishi A, Bae JG, Fukai K et al (2012) Effect of pretreatment of hydrothermally processed rice straw with laccase-displaying yeast on ethanol fermentation. Appl Microbiol Biotechnol 94:939–948. https://doi.org/10.1007/s00253-012-3876-8
Nie S (2019) Laccase pretreatment for enhancing microwave-assisted alkaline extraction of hemicellulose from bagasse. National Natural Science Foundation of China 31760192. The degradation of hexenuronic acid and its effect on AOX formation during xylanase aided chlorine dioxide bleaching of pulp. View project Guangxi Natural Science Foundation of China (2018GXNSFDA281050). Fundamental study on conversion of lignocellulosic biomass into high-value materials. View project
Oliva-Taravilla A, Moreno AD, Demuez M et al (2015a) Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour Technol 175:209–215. https://doi.org/10.1016/j.biortech.2014.10.086
Oliva-Taravilla A, Tomás-Pejó E, Demuez M et al (2015b) Inhibition of cellulose enzymatic hydrolysis by laccase-derived compounds from phenols. Biotechnol Prog 31:700–706. https://doi.org/10.1002/btpr.2068
Oliva-Taravilla A, Tomás-Pejó E, Demuez M et al (2016) Phenols and lignin: key players in reducing enzymatic hydrolysis yields of steam-pretreated biomass in presence of laccase. J Biotechnol 218:94–101. https://doi.org/10.1016/j.jbiotec.2015.11.004
Palonen H, Viikari L (2004) Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol Bioeng 86:550–557. https://doi.org/10.1002/bit.20135
Palonen H, Saloheimo M, Viikari L, Kruus K (2003) Purification, characterization and sequence analysis of a laccase from the ascomycete Mauginiella sp. Enzyme Microb Technol 33:854–862. https://doi.org/10.1016/S0141-0229(03)00247-3
Parfitt J, Barthel M, MacNaughton S (2010) Food waste within food supply chains: quantification and potential for change to 2050. Philos Trans R Soc B Biol Sci 365:3065–3081
Parmar I, Rupasinghe HPV (2012) Optimization of dilute acid-based pretreatment and application of laccase on apple pomace. Bioresour Technol 124:433–439. https://doi.org/10.1016/j.biortech.2012.07.030
Pezzella C, Giacobelli VG, Lettera V et al (2017) A step forward in laccase exploitation: recombinant production and evaluation of techno-economic feasibility of the process. J Biotechnol 259. https://doi.org/10.1016/j.jbiotec.2017.07.022
Piscitelli A, Pezzella C, Giardina P et al (2010) Heterologous laccase production. 1:252–262
Piscitelli A, Del Vecchio C, Faraco V et al (2011) Fungal laccases: versatile tools for lignocellulose transformation. Comptes Rendus – Biol 334:789–794. https://doi.org/10.1016/j.crvi.2011.06.007
Plácido J, Capareda S (2014) Analysis of alkali ultrasonication pretreatment in bioethanol production from cotton gin trash using FT-IR spectroscopy and principal component analysis. Bioresour Bioprocess 1. https://doi.org/10.1186/s40643-014-0023-7
Plácido J, Imam T, Capareda S (2013) Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour Technol 139:203–208. https://doi.org/10.1016/j.biortech.2013.04.012
Pleissner D, Qi Q, Gao C et al (2016) Valorization of organic residues for the production of added value chemicals: a contribution to the bio-based economy. Biochem Eng J 116:3–16
Qiu W, Chen H (2012) Enhanced the enzymatic hydrolysis efficiency of wheat straw after combined steam explosion and laccase pretreatment. Bioresour Technol 118:8–12. https://doi.org/10.1016/j.biortech.2012.05.033
Rashid Shah S, Chinonso Ishmael U, Vejayan Palliah J et al (2016) Optimization of the enzymatic saccharification process of empty fruit bunch pretreated with laccase enzyme. BioResources 11
Ravindran R, Jaiswal AK (2016) A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102
Rencoret J, Pereira A, del Río JC et al (2016) Laccase-mediator pretreatment of wheat straw degrades lignin and improves saccharification. Bioenergy Res 9:917–930. https://doi.org/10.1007/s12155-016-9745-z
Rencoret J, Pereira A, Del Río JC et al (2017) Delignification and saccharification enhancement of sugarcane byproducts by a laccase-based pretreatment. ACS Sustain Chem Eng 5:7145–7154. https://doi.org/10.1021/acssuschemeng.7b01332
Rico A, Rencoret J, del Río JC et al (2014) Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock
Rico A, Rencoret J, del Río JC et al (2015) In-depth 2D NMR study of lignin modification during pretreatment of eucalyptus wood with laccase and mediators. Bioenergy Res 8:211–230. https://doi.org/10.1007/s12155-014-9505-x
Rizal NFAA, Ibrahim MF, Zakaria MR et al (2018) Combination of superheated steam with laccase pretreatment together with size reduction to enhance enzymatic hydrolysis of oil palm biomass. Molecules 23. https://doi.org/10.3390/molecules23040811
Rocha-Martín J, Martínez-Bernal C, Zamorano LS et al (2018) Inhibition of enzymatic hydrolysis of pretreated corn stover and sugar cane straw by laccases. Process Biochem 67:88–91. https://doi.org/10.1016/j.procbio.2018.01.021
Roth S, Spiess AC (2015) Laccases for biorefinery applications: a critical review on challenges and perspectives. Bioprocess Biosyst Eng 38:2285–2313
Sánchez-Ramírez J, Martínez-Hernández JL, López-Campos RG et al (2018) Laccase validation as pretreatment of agave waste prior to saccharification: free and immobilized in superparamagnetic nanoparticles enzyme preparations. Waste Biomass Valor 9:223–234. https://doi.org/10.1007/s12649-016-9774-z
Schroyen M, Vervaeren H, Van Hulle SWH, Raes K (2014) Impact of enzymatic pretreatment on corn stover degradation and biogas production. Bioresour Technol 173:59–66. https://doi.org/10.1016/j.biortech.2014.09.030
Schroyen M, Vervaeren H, Vandepitte H et al (2015) Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour Technol 192:696–702. https://doi.org/10.1016/j.biortech.2015.06.051
Sherpa KC, Ghangrekar MM, Banerjee R (2018) A green and sustainable approach on statistical optimization of laccase mediated delignification of sugarcane tops for enhanced saccharification. J Environ Manage 217:700–709. https://doi.org/10.1016/j.jenvman.2018.04.008
Singh R, Hu J, Regner MR et al (2017) Enhanced delignification of steam-pretreated poplar by a bacterial laccase. Sci Rep 7. https://doi.org/10.1038/srep42121
Sitarz AK, Mikkelsen JD, Højrup P, Meyer AS (2013) Identification of a laccase from Ganoderma lucidum CBS 229.93 having potential for enhancing cellulase catalyzed lignocellulose degradation. Enzyme Microb Technol 53:378–385. https://doi.org/10.1016/j.enzmictec.2013.08.003
Thangavelu K, Desikan R, Taran OP, Uthandi S (2018) Delignification of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: process optimization by response surface methodology. Biotechnol Biofuels 11. https://doi.org/10.1186/s13068-018-1204-y
Wang FQ, Xie H, Chen W et al (2013) Biological pretreatment of corn stover with ligninolytic enzyme for high efficient enzymatic hydrolysis. Bioresour Technol 144:572–578. https://doi.org/10.1016/j.biortech.2013.07.012
Wang Y, Shao Y, Zou X et al (2017) Synergistic action between extracellular products from white-rot fungus and cellulase significantly improves enzymatic hydrolysis. Bioengineered:1–8. https://doi.org/10.1080/21655979.2017.1308991
Zhang J, Qu Y, Xiao P et al (2012) Improved biomass saccharification by Trichoderma reesei through heterologous expression of lacA gene from Trametes sp. AH28-2. J Biosci Bioeng 113:697–703. https://doi.org/10.1016/j.jbiosc.2012.01.016
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Giacobbe, S., Pezzella, C., Sannia, G., Piscitelli, A. (2020). Old Enzymes at the Forefront of Lignocellulosic Waste Valorization. In: Schlosser, D. (eds) Laccases in Bioremediation and Waste Valorisation. Microbiology Monographs, vol 33. Springer, Cham. https://doi.org/10.1007/978-3-030-47906-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-47906-0_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47905-3
Online ISBN: 978-3-030-47906-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)