Abstract
Manganese peroxidases (MnPs) are a fascinating group of biocatalysts with various ecological and biotechnological implications. They are involved in the biodegradation of lignocellulose and lignin and participate in the bioconversion of other diverse recalcitrant compounds, like polycyclic aromatic hydrocarbons, chlorophenols, industrial effluents (mostly from the paper and pulp), and textile and petrochemical industries, and bioremediation of contaminated soils. This chapter presents an overview of the structural basis of the catalytic properties of MnPs and the enumeration of the molecular and protein homology characteristics of this enzyme. Multiple developments mainly pertaining to enzyme engineering for improved substrate specificity and stability of MnPs have also been highlighted. Inevitably, the progress in enzyme engineering research and the expression of MnPs have explored the vast genetic diversity of these enzymes with great interest being placed on exploiting these enzymes for a variety of industrial and scientific applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Manganese peroxidases
- Properties
- Heterologous production
- Molecular cloning
- Enzyme engineering research
- Crystal structure
- Industrial applications
Introduction
Manganese peroxidase (MnP) [EC 1.11.1.13, MnII:hydrogen-peroxide oxidoreductase] is an extracellular heme enzyme that utilizes hydrogen peroxide (H2O2) as an electron-accepting cosubstrate, for catalyzing the peroxide-dependent oxidation of MnII to MnIII. These enzymes have mainly been isolated from white-rot fungal species like Phanerochaete chrysosporium, Trametes versicolor, Heterobasidion annosum, and Irpex lacteus with the ability to degrade lignin (Eriksson et al. 1990; Cai and Tien 1993). A great majority of these enzymes contain a protoporphyrin IX (heme) prosthetic group. Lignin is a heterogeneous, optically inactive polymer, consisting of phenylpropanoid subunits. These phenylpropanoid subunits cannot be cleaved by hydrolytic enzymes unlike most other natural polymers, e.g., cellulose, starch, and proteins. Interestingly, some white-rot basidiomycetes that produce peroxidases have ability to degrade lignin (Sarkanen and Ludwig 1971). Peroxidases were first discovered in Phanerochaete chrysosporium (Kuwahara et al. 1984; Glenn and Gold 1985; Paszcynski et al. 1985, 1986). During the last decade, work has been done on the heterologous expression of peroxidases using X-ray crystallographic studies and active-site engineering to enhance substrate specificity and the thermal stability of MnP genes (Mino et al. 1988; Benner and Gerloff 1990; Petersen et al. 1993; Li et al. 2001; Sundaramoorthy et al. 2010). Attempts have also been made to determine the regulation of MnP gene at transcriptional and translational levels (Brown et al. 1991; Gettemy et al. 1998; Johansson et al. 2002). The nonspecific and non-stereoselective nature of MnP allows it to degrade a wide range of pollutants, such as polycyclic aromatic hydrocarbons (PAHs), chlorinated phenols, polychlorinated biphenyls, dioxins, pesticides, explosives, and dyes (Levin et al. 2004).
The main objective of this chapter has been to highlight the diversity of MnPs among basidiomycetes, their heterologous production, phylogenetic analysis, structural characteristics, and molecular features. This chapter also describes attempts made to engineer this enzyme for improved substrate specificity and stability and to quantify the utility of this enzyme.
Occurrence and Phylogenetic Analysis of MnPs
Production of extracellular MnP has been mainly observed from certain basidiomycetes, and thus far, no bacterium, yeast/mold, or mycorrhiza-forming basidiomycete have been reported to produce this enzyme (Cairney and Burke 1998; Hatakka 2001). Many ecophysiological groups of basidiomycetes have been found to secrete isoforms of MnP into their microenvironments (Hatakka 1994, 2001; Heinzkill et al. 1998; Steffen et al. 2000). C. subvermispora have been found to produce up to 11 different isoforms of MnP (Lobos et al. 1994; Urzua et al. 1995). Various white-rot fungi, which are well characterized for their ligninolytic ability, belong to phylogenetically older families such as Meruliaceae (P. radiata, P. sordida, P. chrysosporium, Merulius sp. M15), Coriolaceae (B. adusta, C. subvermispora, C. pruinosum, P. tephropora, A. biennis), and Polyporaceae (T. versicolor, T. gibbosa, T. trogii, T. hirsuta) as well as litter decomposers of euagaric families such as Strophariaceae and Tricholomataceae have been found to have notable expression of MnP (Table 6.1). In addition, some marine-derived fungal strains and strains dwelling on decaying sea grass (Raghukumar et al. 1999), cooling-tower wood (Schmidt et al. 1997), and brown coal (Willmann and Fakoussa 1997a, b) have MnP production ability. Sequences of different MnPs were retrieved from GenBank (available at: http://www.ncbi.nlm.nih.gov). For phylogenetic analysis of all MnPs, an alignment was created with ClustalW2 multiple sequence alignment tool (available at http://www.ebi.ac.uk/Tools/msa/clustalw2/). The evolutionary history was determined using the neighbor-joining method (Saitou and Nei 1987). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches (Felsenstein 1985). The evolutionary distances were computed using the Poisson correction method (Zuckerkand and Pauling 1965) and are in the units of the number of amino acid substitutions per site. The analysis involved 53 amino acid sequences. Evolutionary analyzes were conducted in MEGA5 (Tamura et al. 2011). It can be inferred from the phylogenetic tree that MnPs are divergent among different taxonomic groups. Among the 54 amino acid sequences analyzed, 44 basidiomycetes were grouped together at the same phenetic unit, whereas apart from main basidiomycete grouping, Coprinopsis cinerea, Arthromyces ramosus, Coprinellus disseminatus, and Ganoderma sp. arose as distinct genetic groups (Fig. 6.1). The MnP variant from Inonotus hispidus was the single gene variation ungrouped from the main cluster as aforementioned. Lactarius rufus along with Lactarius fulvissimus formed the third additional genetic group.
The maximum parsimony (MP) method was used to analyze the evolutionary history among different basidiomycete MnPs. The bootstrap consensus tree obtained from 1,000 replicates is taken to represent the evolutionary history of the taxa analyzed (Felsenstein 1985). Branches corresponding to partitions reproduced in less than 50 % of bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches (Felsenstein 1985). The MP tree was obtained using the close-neighbor-interchange algorithm (Nei and Kumar 2000) with search level 1 in which the initial trees were obtained with the random addition of sequences (ten replicates). Parsimony analysis revealed four distinct main evolutionary lineages among all basidiomycetes (Fig. 6.2). The MnP from Pleurotus ostreatus and Pleurotus sp. Florida evidenced two additional lineages as they were grouped separately from the main cluster. The MnPs from Pleurotus pulmonarius, Hygrophorus agathosmus, Laccaria bicolor S238N-H82, Agrocybe praecox, Phelli-nidium ferrugineofuscum, and Hymenochaete corrugata are evolutionarily closely related, and they arose as the sister group to the other remaining groups (Fig. 6.2).
Mechanism of Catalysis
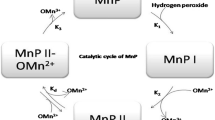
Manganese peroxidase (MnP) [EC 1.11.1.13, MnII:hydrogen-peroxide oxidoreductase, MnP] is an extracellular heme enzyme that utilizes hydrogen peroxide (H2O2) as electron-accepting cosubstrate for catalyzing the peroxide-dependent oxidation of MnII to MnIII. The MnIII formed is highly active, which in turn is stabilized by fungal chelators like oxalate and malonate. These chelators act as physiological regulators to the enzyme as they enhance the enzyme activity due to their ability to facilitate the dissociation of MnIII from the enzyme. The role of oxalate as an extracellular buffering agent has also been reported. It facilitates the ability of the fungus to control the pH of its environment (Timofeevski and Aust 1997; Zapanta and Tien 1997). Calcium sequestration by these chelators acts to increase the pore size of the plant cell wall and assist in the penetration of enzyme molecules. Oxidation of oxalic acid by MnIII produces a formate radical (HCO2 −) that reacts with dioxygen to form superoxide (O2 −) and subsequently H2O2 (Khindaria et al. 1994; Urzua et al. 1998). Chelated MnIII in turn acts as low molecular weight, diffusible redox mediator that attacks phenolic lignin structures and monomeric phenols, e.g., azo dyes, resulting in the formation of unstable free radicals that tend to disintegrate spontaneously. Characteristic features of the catalytic cycle of MnP resemble to those of other heme-containing peroxidases, such as horseradish peroxidase (HRP), lignin peroxidase, versatile peroxidase, and chloroperoxidases (Cai and Tien 1993; Magliozzo and Marcinkeviciene 1997; Longoria et al. 2008). However, MnP is unique in its ability to utilize MnII as a reducing substrate to oxidize it to MnIII (Kishi et al. 1994; Sundaramoorthy et al. 1997; Youngs et al. 2000; Deguchi et al. 2002). Spectroscopic studies have revealed that the heme iron of the native enzyme is in the ferric, high-spin, pentacoordinate state and is ligated to the proximal histidine (Mino et al. 1988; Wariishi et al. 1988; Gelpke et al. 2000). The reactions involved in the MnP catalytic cycle are (Wariishi et al. 1992; Gelpke et al. 1999)
Catalysis of Phenolic Substrates
During the oxidation of phenolic compounds, phenoxy radical intermediates are formed which undergo rearrangements, bond cleavages, and nonenzymatic degradation to yield various breakdown products (Fig. 6.3) (Tuor et al. 1992). MnIII generated by MnP is known to catalyze the oxidation of phenolic substrates, including simple phenols, amines, dyes, and also phenolic lignin substructure and dimers (Wariishi et al. 1989a; Urzua et al. 1995).
MnP-catalyzed oxidation of phenolic arylglycerol β-aryl ether lignin model compound (Modified according to Tuor et al. 1992)
Catalysis of Non-Phenolic Substrates
In contrast to LiP-catalyzed reactions, which involve electron abstraction from the aromatic ring, forming a radical cation, MnIII forms reactive radicals in the presence of a second mediator during the oxidation of non-phenolic substrates (Reddy et al. 2003). The presence of thiols, such as glutathione, mediates the oxidation of substituted benzyl alcohols and diarylpropane structures to their respective aldehydes by MnIII (Reddy et al. 2003). In these reactions, thiols are oxidized to thiyl radicals by MnIII, which subsequently removes hydrogen from the substrate to form a benzylic radical. The latter undergoes successive nonenzymatic reactions like addition of O2 at C1 position of benzylic radical followed by loss of • OOH, and homolytic C–O fission at C2 of benzylic radical expels a phenoxy radical that results in the formation of final products (Fig. 6.4) (Wariishi et al. 1989b, c).
MnP-catalyzed oxidation of non-phenolic β-O-4 lignin model compound (Modified according to Wong 2009)
Manganese peroxidase generated MnIII has also been coupled with peroxidation of lipids to catalyze Cα–Cβ cleavage and to β-aryl ether cleavage of non-phenolic diarylpropane and β-O-4 lignin structures, respectively (Fig. 6.4) (Bao et al. 1994; Daina et al. 2002; Reddy et al. 2003; Kapich et al. 2005). The steps involved in the mechanism are the following: firstly, hydrogen abstraction from the benzylic carbon via lipid peroxy radicals, and secondly, peroxy radicals are formed by addition of O2 and subsequent oxidative cleavage and nonenzymatic degradation. Absence of exogenous H2O2 directs the enzyme to oxidize nicotinamide adenine dinucleotide phosphate (NADPH) (reduced form), glutathione, dithiothreitol, and dihydroxymaleic acid to generate H2O2. One can infer (by observing the oxidase activity of MnP) that the H2O2 produced may become available for the enzyme to start the peroxidase cycle, thereby, assisting in the degradation of lignin by this fungal species (Paszczynski et al. 1986).
Molecular Cloning and Expression of MnP
In most fungi, MnP appears to be produced as a family of isoenzymes, which may be encoded by structurally related genes (Larrondo et al. 2001; Sakamoto et al. 2009). Commercial applications of MnP require significantly higher levels of extracellular enzyme production by fungal strains; however, the yield of MnP in its native hosts is too low (1.5–5 mgl−1) (Stewart et al. 1996; Li et al. 2001). Therefore, improvement in the yield and reduction in the production cost are the major goals to be focused for commercial exploitation of MnP.
The first extracellular fungal MnP that was characterized and homologously expressed was obtained from Phanerochaete chrysosporium (Glenn and Gold 1985). It was found that high concentrations of carbon and nitrogen in the medium significantly affected the transcription of the mnp l gene in P. chrysosporium (Mayfield et al. 1994). Sakamoto et al. (2009) studied the transcriptional and translational levels of different isoforms of MnP, i.e., lemnp1 and lemnp2, in Lentinula edodes using sawdust medium and reported lemnp2 as the major extracellular enzyme.
Heterologous expression of MnP from Phanerochaete chrysosporium has been reported in Aspergillus niger, Pichia pastoris, P. chrysosporium adel, Aspergillus oryzae, and Aspergillus nidulans (Pribnow et al. 1989; Mayfield et al. 1994; Stewart et al. 1996; Janse et al. 1998; Larrondo et al. 2001; Gu et al. 2003). MnPs from Trametes versicolor, P. eryngii, P. ostreatus, Dichomitus squalens, and Ceriporiopsis subvermispora have also been cloned and heterologously expressed in T. versicolor 9522-1, Coprinus cinereus, P. chrysosporium, and Aspergillus nidulans, respectively (Ogawa et al. 1998; Camarero et al. 2000; Li et al. 2001; Larrondo et al. 2001; Kim et al. 2005; Yeo et al. 2007). To date there have been no reports of successful expression of fungal MnPs in bacterial expression hosts.
Although the MnP production levels have often been improved significantly by expression in heterologous hosts, the reported levels are still rather low (100–400 mgl−1) for use in industrial applications (Conesa et al. 2000; Punt et al. 2002; Espinosa et al. 2012). The capability of the P. pastoris strain to perform various posttranslational modifications, such as heme insertion, glycosylation, folding, and protein secretion, had been reported for successful production of active MnP to a maximum yield of 120 UL−1 (Gu et al. 2003). As described by Yeo et al. (2007), increase in MnP activity of transformant TF6 was up to 45 % as compared to the recipient strain. A thermostable recombinant MnP from D. squalens has also been heterologously expressed in P. chrysosporium and purified. The recombinant protein appeared similar in kinetic and spectral characteristics to the wild-type MnP from D. squalens (Li et al. 2001), whereas the homologous expression of P. chrysosporium recombinant MnP resulted in 30 % of the level of MnP activity expressed under by wild-type strain (Mayfield et al. 1994). Data indicated that the addition of exogenous MnII, CdII, and ZnII conferred additional thermal stability to MnP from D. squalens and P. chrysosporium (Li et al. 2001). Aspergillus species have proven to be excellent hosts for the expression of heterologous proteins such as those of A. oryzae. The same has been shown to be effective in the expression of P. chrysosporium mnp1 with notable yields (Stewart et al. 1996).
Isozyme multiplicity of MnPs has been observed in various strains of C. subvermispora and P. chrysosporium. As an expression host, Aspergillus nidulans proved to be convenient system for MnPs. It has also been demonstrated that MnII is the key component that regulates the transcription of different recombinant MnP isoforms in carbon-limited cultures (Banci et al. 1992; Alic et al. 1997; Larrondo et al. 2001). Nutrient limitation such as Mn concentration, culture agitation, heat shock, H2O2 concentration, and other chemical stresses have also been reported to significantly regulate transcription of different isozymes of MnP (mnp1, mnp2, and mnp3) in P. chrysosporium (Janse et al. 1998). In contrast to manganese regulation, Tello et al. (2000) have proposed that the putative MREs (metal response elements) found in the upstream region of MnP genes in P. chrysosporium might have a role in the regulation of transcription of genes coding for MnP in filamentous fungi. Metallothionein genes are known to be regulated by MREs through various metals in animal cells, although these sites do not respond to manganese. Therefore, further work is required to decipher both the role of MREs in the upstream region of these genes and the mechanism of transcriptional regulation by manganese in basidiomycetes.
Characteristic Features of MnP Gene
MnP genes among different basidiomycetes (starting from the ATG codon) encompass a genomic region of 1.4–1.9 kbps. Lobos et al. (1998) had shown that MnP genes contain seven short intervening sequences with sizes ranging between 52 and 60 bp. The last intron restrained by mnp1 and mnp2 genes of P. chrysosporium segregates a codon for proline to give different isozymes (Fig. 6.5) (Godfrey et al. 1990; Mayfield et al. 1994). Sequences at the intron splicing junctions adhere to the GT–AG rule. In turn, three putative internal lariat formation sites match the consensus sequence CTRAY (Padgett et al. 1989). After structural comparison of mnp genes from the Cs-mnp1 gene of C. subvermispora and five mnp genes from different basidiomycetes, Lobos et al. (1994) revealed an almost perfect alignment between Cs-mnp1 and mnp2 of P. chrysosporium (Fig. 6.5), and both genes have an additional intron splitting exon 3 of mnp1 and mnp3 of P. chrysosporium at the codon for the distal histidine, H46 (Mayfield et al. 1994). The pattern of differential distribution of introns observed in the P. chrysosporium mnp genes might be because the mnp2 represents the ancestral gene structure and the mnp1and mnp3 genes arose with the loss of one intron each (Alic et al. 1997). In contrast, only one of the 15 introns (intron 12) in the P. ostreatus mnp gene aligns exactly with a C. subvermispora mnp1 intron (intron 6), and none of the 15 introns of the P. ostreatus mnp gene align precisely with introns of the P. chrysosporium mnp genes.
Intron/exon structure of MnP genes mnp1, mnp2, and mnp3 from P. chrysosporium, Cs-mnp1 from C. subvermispora, mnp from P. ostreatus, and mpg1 from T. versicolor. The exons are indicated by open boxes, whereas the solid black boxes correspond to the introns (Modified after Lobos et al. 1998)
The regulatory sequence of Cs-mnp2B contains a TATA box and an inverted CAAT (ATTG) element located 92 and 191 bp upstream of the ATG codon (Tello et al. 2000), respectively. Examination of the promoter regions of the mnp1 and mnp2 genes (Godfrey et al. 1990; Gold and Alic 1993; Mayfield et al. 1994) revealed the presence of putative MREs within 800 bp of the translation initiation codon. These sequences are identical to cis-acting MRE sequences responsible for heavy-metal induction of animal cell metallothionein genes (Gettemy et al. 1998). Interestingly, closer examination of the mnp1 promoter region also revealed the presence of putative HSEs within 400 bp upstream of the mnp1 translation initiation codon (Lobos et al. 1998). Mn2+ regulation of MnPs has been previously highlighted by different research groups (Bonnarme and Jeffries 1990). Godfrey et al. (1990) stated that 1,500 bp of sequence immediately upstream of the MnP translation start site is sufficient to regulate the ural reporter in a manner analogous to the regulation of the endogenous MnP genes with respect to Mn, nutrient nitrogen levels, and metabolic phase of growth.
The translocation of 48-bp fragment of promoter region of MnP isozyme 1 to a site 120 bp downstream of its original location has been shown to regulate Mn2+-dependent expression of downstream genes; this suggests the possibility of the presence of at least one Mn2+-responsive cis element in the fragment. However, deletion of a 48-bp fragment, located at 521 bp upstream of the translation start codon in the mnp1 promoter, or replacement of this fragment with an unrelated sequence resulted in egfp expression under nitrogen limitation, both in the absence and presence of exogenous Mn2+ (Ma et al. 2004).
Orth and coworkers (1994) have analyzed the organization of the MnP gene family of P. chrysosporium BKM1767 and concluded that the λMP-1 and λMP-2 genes hybridized to 3.6 and 3.8 Mb of DNA fragments located on separate chromosomes and in contrast to five LiP genes that are localized to a dimorphic chromosome of about 3.7 and 3.5 Mb (Gaskell et al. 1991).
Crystallographic Analysis of MnP
MnP from different basidiomycetes has been crystallized and subsequently analyzed (Poulos et al. 1993; Sundaramoorthy et al. 1994a, b, 1995; Duenas et al. 1999). MnP is a glycoprotein with the molecular weight of 46 kDa and contains one heme group (Sundaramoorthy et al. 1994a, b). The structural features of manganese peroxidase (pdb 1mnp) from P. chrysosporium are displayed/shown in Fig. 6.6, with a resolution of 2.06 Å (retrieved from http://www.rcsb.org/pdb). The substrate-bound MnP (Mn–MnP) consists of 357 amino acids, three sugar residues, a heme prosthetic group, two structural calcium ions, substrate MnII ion, and 478 solvent molecules, including two glycerol molecules (Sundaramoorthy et al. 2010). The sequence of the heme distal helix Glu35–Ala48 is highly conserved in the key residues (Selvaggini et al. 1995). The active site consists of a proximal His ligand H-bonded to an Asp residue, and a distal side peroxide-binding pocket consisting of a catalytic His and Arg is the same among all peroxidases (Fig. 6.7). MnP differs with respect to having five rather than four disulfide bonds. The additional disulfide bond is located near the C-terminus of the polypeptide chain. The ligands constituting the Mn2+ binding site include Asp179, Glu35, Glu39, a heme propionate, and two water molecules. The overall structure is similar to that of two other fungal peroxidases, i.e., lignin peroxidase from Phanerochaete chrysosporium and Arthromyces ramosus peroxidase. Like the other fungal peroxidases, MnP also has two structural calcium ions and N-acetylglucosamine residues N-linked to Asn131 (Sundaramoorthy et al. 1994a, b).
The overall structure of P. chrysosporium MnP (pdb 1mnp) as analyzed by UCSF chimera 1.4.1. The cyan spheres are structural CaII ions conserved in extracellular heme peroxidases. The location of the substrate, Mn+2, near the heme, is indicated. The purple color shows active-site structure of MnP. This architecture is highly conserved in heme peroxidase
Multiple alignments of amino acid sequences of fungal peroxidases. MnP, LiP, VP, CcP TP, and other peroxidases from P. chrysosporium (Pc) and T. versicolor (Tv), P. ostreatus (Po), P. brasiliensis Pb01 (Pb), P. eryngii (Pe), Brassica rapa turnip (turnip), and A. ramosus (ArP) are compared. The alignment was generated and conserved residues were identified by using Clustal X 2.0.11. The sequences highlighted in red denote heme binding sites; calcium binding sites are highlighted in blue. Green shows conserved residues in the substrate binding site. Blank boxes encompass the common residues in heme and substrate binding sites. The following marks are used in the consensus line: Mn2+ binding site (▼); distal and proximal histidine (■). Accession numbers of the amino acid sequences are as given below: TvLiP, AAA34049.1; TvMnP, CAA83148.1; PcLiP, AAA03748.1; PeVP, AAD54310.1; PoMnP, AAA84397.1; ArP, P28313.3; PcMnP, AAA33743.1; Turnip, P00434.3; PbCcP, EEH41729.1
Proximal pocket of MnP active site contains His173 that coordinates to heme atom and the side chain of Asp242 interacts with His173 through H bond. The Phe190 residue is positioned just below the heme ring, near the His173 residue. There is a second opening between the heme propionate residues; this region is polar and characterized by residues Arg177, Asp179, Glu35, and Glu39 (Selvaggini et al. 1995). Its ability to oxidize Mn2+ with high substrate affinity is related to the presence of a Mn binding site (involving Glu36, Glu40, and Asp179) which enables the oxidation of this cation by the internal heme propionate (Sundaramoorthy et al. 1997).
Multiple sequence alignment of amino acid sequences (retrieved from www.ncbi.nlm.nih.gov/protein/) conducted on several characteristic peroxidases (P. chrysosporium MnP (378 amino acids) and LiP (371 amino acids), T. versicolor MnP (365 amino acids) and LiP (372 amino acids), P. ostreatus MnP (361 amino acids), P. brasiliensis Pb01 cytochrome c peroxidase (374 amino acids), and P. eryngii versatile peroxidase (370 amino acids), Brassica rapa TP7 (296 amino acids), and Arthromyces ramosus peroxidase (364 amino acids)) indicated significant conserved structural residues (Fig. 6.7). All the enzymes have the conserved proximal histidine, the distal histidine and the distal arginine. The distal arginine residue has been proposed to participate in the formation of the peroxide-binding pocket together with distal histidine, while Asn131 has been revealed to be the only carbohydrate binding site in MnP1 (Sundaramoorthy et al. 1994a, b, 2005). The iron coordination and the residues involved in the active site are the most conserved region among all the aforementioned fungal peroxidases, as well as cytochrome c peroxidase. The two glutamic acid and the aspartic acid residues present in the manganese binding site are also conserved. It could be inferred from Fig. 6.7 that heme binding sites are conserved among all the characteristic peroxidases with some variations found in the case of the turnip (Brassica rapa) and cytochrome c peroxidases. The distal Ca2+-binding residues are conserved in all fungal peroxidases (coordination being completed by two water molecules), whereas some differences exist in the residues binding Ca2+ at the proximal side.
Comparative Analysis of MnP, LiP, and VP
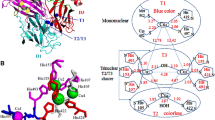
Comparative studies were performed between P. chrysosporium MnP (pdb 1mnp), T. cervina LiP (pdb 3q3u), and P. eryngii VP (pdb 3fmu) on the basis of tertiary structure alignment using UCSF chimera 1.4.1 software (Fig. 6.8). Structural alignment depicted approximately 50 % similarity of the residues among these peroxidases. Several functionally relevant structurally important features were also observed: (a) the very close structural similarity between MnP, LiP, and VP active sites, suggesting a similar mode of hydrogen-peroxide activation. The heme prosthetic group is found embedded between the N-terminal and the C-terminal domains, along with the surrounding conserved residues H46 (MnP)/H47 (LiP)/H47(VP), H173 (MnP)/H175 (LiP)/H169(VP), and R42 (MnP)/R43 (LiP)/R43(VP); (b) the substitution of polar residues for the hydrophobic amino acids exposed at the edge of the channel involved in substrate recognition in lignin peroxidase, suggesting that manganese peroxidase does not directly bind aromatic substrates; (c) the location of residues potentially able to bind Mn2+, spatially positioned on the side of the 3-CH3 heme edge. The close sequence similarity of all the peroxidases strongly suggests a very similar three-dimensional fold. Lignin peroxidase was characterized by two calcium binding sites involving the side-chain residues, i.e., Asp194, Ser177, Thr196 (proximal site) and Asp65, Asp86 (distal site). The corresponding residues of aligned manganese peroxidase were Asp198, Thr199, Thr219 and Asp64, Asp85 and of versatile peroxidase were Ser195, Asp194, Lys215 and Asp65, Asp86; (d) the high degree of conservation of the aspartic acid residue at N + 1 site position after the distal histidine suggests that the calcium distal site is involved in maintaining the integrity of the active site is in accordance with Henrisatt et al. (1990) and Welinder (1992); (e) the sequence corresponding to the solvent-exposed a helix Ala12–Gln25, connected with other portions of the protein by disulfide bridges involving Cys3–Cysl5 and Cysl4–Cys289, is closely similar to the Ala12–Gln25 segment of LiP and VP with some closely related mutations. The coil region located at residues Ala50–Gly65 in the MnP sequence was not completely conserved in the lignin peroxidase and versatile peroxidase. The presence of glycine and proline in this region strongly suggests a coil arrangement (Benner 1989; Benner and Gerloff 1990; Branden and Tooze 1991). The region Pro163–Val175 (heme proximal helix) is highly similar to the aligned segments of lignin peroxidase and versatile peroxidase sequences except for some minor changes in this region.
Tertiary structural comparison of MnP, LiP, and VP using UCSF chimera 1.4.1 software. (a) Whole structures of P. chrysosporium MnP (pdb 1mnp), T. cervina LiP (pdb 3q3u), and P. eryngii VP (pdb 3fmu) are shown respectively from left to right. (b) Structural alignments of MnP, LiP, and VP are shown in pink, cyan, and green, respectively. (c) Structural alignment of the region surrounding heme and the MnP, LiP, and VP residue numbers are shown
Superimposition of the MnP, LiP, and VP structures clearly depicts the presence of five disulfide bonds in MnP in contrast to four disulfide bonds present in the LiP and VP. The initial four disulfide bonds in MnP, viz., Cys3–Cys15, Cys33–Cys117, Cys14–Cys289, and Cys253–Cys319, are the same as observed in Lip, VP, and A. ramosus peroxidase and also had perfect alignment as portrayed by superimposed structures in Fig. 6.8b. The additional disulfide bond found in MnP (Cys341–Cys348 located near the C-terminus of the polypeptide chain) aids in the formation of the MnII binding site and is responsible for pushing the C-terminus segment away from the main body of the protein (Sundaramoorthy et al. 1994a, b). LiP, VP, and MnP all have a Phe position 190, but the orientation differs. In LiP the Phe ring is nearly parallel to the proximal His imidazole ring, whereas in MnP the Phe ring is almost perpendicular to the plane of the proximal His. Owing to the differences in orientation of the outer propionate in MnP, the distal Arg42 cannot form a hydrogen bond with the propionate, whereas in LiP, the distal arginine directly interacts with the propionate. The second (inner) propionate interacts with Mn2+ ion and water molecules, and the peptide NH group of a propionate together with a main chain peptide nitrogen is also found in LiP.
The residues forming the helices found to be structurally aligned in MnP, LiP, and VP models are summarized in Table 6.2.
MnP and Active-Site Engineering
Major challenges remain in understanding the role of functional domains and their structural/functional relationships. The desired features required for the commercial exploitation of these enzymes are stability, notable yields, and enhanced or superannuated activities. Site-directed mutagenesis (SDM) or active-site engineering is an invaluable tool to alter bases at precise positions in the gene. Engineered enzymes are then subjected to analysis for the above-mentioned favorable characteristics using various molecular tools. Site-directed mutagenesis is a major approach that provides opportunities to study unique structural/functional relationships in MnPs and allows the detailed characterization of the MnII binding site.
Kishi et al. (1994) developed a series of mutants (E35Q, E39Q, and E35Q-D179N) from the gene encoding manganese peroxidase isozyme 1 (mnp1) from Phanerochaete chrysosporium, using site-directed mutagenesis. The mutations demonstrated that changing any of the acidic amino acid MnII ligands, Asp179, Glu35, or Glu39, significantly affects the oxidation of MnII, most probably by decreasing the affinity of the enzyme for MnII. Asp179, Glu35, and Glu39 residues at the catalytic site are essentially required for MnII oxidation, since the double mutation, i.e., E35Q–D179N, had almost completely resulted into the loss of MnII oxidation (Sundaramoorthy et al. 1994a, b). The coordination of MnII at this site is octahedral, which is typical of MnII coordination complexes (Demmer et al. 1980). It has been postulated that Glu39 is a Mn ligand and its precise geometry within the Mn binding site of MnP is essential for the efficient binding, oxidation, and release of Mn by this enzyme and that mutation of this ligand decreases both the Mn binding and the rate of Mn oxidation (Martınez 2002; Li et al. 2001).
Miyazaki and Takahashi (2001) found that the site-directed mutagenesis of oxidizable Met273 located near the H2O2-binding pocket to a non-oxidizable Leu had resulted into improved stability of IMnP, as it retained more than 60 % of its initial activity in presence of 1 mM H2O2 and more than 30 % at a concentration of 3 mM H2O2 as compared to wild type that was completely inactivated by 1 mM H2O2. Stability in the presence of hydrogen peroxide may be attributed to the above-mentioned mutation that makes it resistant to oxidation by the conformational stabilization around the H2O2-binding pocket. Manganese peroxidase (MnP) is susceptible to thermal inactivation due to the loss of calcium. Engineering of a disulfide bond near the distal calcium binding site of MnP by double mutation A48C and A63C showed the improvement in thermal stability as well as pH stability in comparison to native MnP. The disulfide bond adjacent to the distal calcium ligands Asp47 and Asp64 stabilizes the recombinantly expressed MnP against the loss of calcium (Reading and Aust 2000).
Timofeevski et al. (1999) have described that a single mutation (S168W) in rMnP added veratryl alcohol oxidase activity to the enzyme without significantly affecting Mn2+ oxidase activity. This surface tryptophan residue, present in various LiP isoenzymes but absent in MnP, may be the site of VA binding and oxidation by LiP. Other research conducted observed how the hydrophobicity of the heme pocket could affect the reactivity of compound I (formed during MnP catalysis). Leu169 and Ser172 were mutated and converted Phe and Ala, respectively. Steady-state kinetics characterization indicated that the Leu169Phe mutation had little effect on activity, whereas the Ser172Ala mutation decreased kcat to 45 s −1 as compared to wild type (449 s−1) and also the specificity constant (kcat/Km) of Ser172Ala mutant decreased from 1.1 × 107 M−1s−1 to 5.3 × 105 M−1s−1 for Mn2+, but not H2O2. It has been shown/demonstrated that compound II is the most sensitive to changes in the heme environment when compared to compound I (Balay et al. 2000).
The role of the axial ligand hydrogen-bonding network on heme reactivity was analyzed by Whitwam et al. (1999); D242 is hydrogen bonded to the proximal His of MnP, in other peroxidases, and this conserved Asp, in turn, is hydrogen bonded to a Trp. In MnP and other fungal peroxidases, the Trp is replaced by a Phe (F190). Both residues are thought to have a direct influence on the catalytic center of the enzyme. Mutagenesis of D242 and F190 has shown that these residues affect the reactivity of the heme active site. The changes in the axial ligand H-bonding network largely influence the reactivity of compound II (Fe4+) and have little influence on the reactivity of compound I (porphyrin cation radical).
Zhang et al. (2009) described the role of Arg42 and Asn131 in the oxidation of 2,6-DMP after performing site-directed mutagenesis with in vitro synthesis. As previously described, R177A and R177K mutants of P. chrysosporium had specifically altered binding of Mn, whereas the rate of electron transfer from Mn2+ to the oxidized heme was apparently not affected (Gelpke et al. 1999). Whitwam et al. (1997) had suggested that Arg177 may anchor to the carboxylate of Glu35 in the Mn2+-occupied closed configuration of the protein. Shortening the side chain of this residue by one methylene in the E35D mutant probably does not affect the salt bridge to Arg177, but it may restrain the carboxylate of this ligand from making a strong bond with the MnII atom. This results in a disruption of the ligation for MnII and hence in the electron-transfer rate as observed by Gelpke et al. (1999).
MnPs and Industrial/Commercial Applications
Industrial applications for manganese peroxidases that have been proposed include bleaching of unbleached kraft pulp in pulp and paper industries, treatment of textile industry effluents, in generating natural aromatic flavors in foods, degradation of environmental pollutants like polyaromatic hydrocarbons (PAHs), azo dyes, TNT, and DTT. Manganese peroxidase is versatile and energy saving, and its ability to bioremediate displays the capacity of this enzyme to be a significant tool for a number of eco-friendly commercial applications.
Ecotoxic organic chemicals generated from textile, pulp, and paper industry effluents are major contributors for the environmental pollution and are the major health hazards for a number of vertebrates and to human population. Detoxification of these compounds poses an immense technical challenge (Evans et al. 2004; Brar et al. 2006). Conventional methods for treatment of various industrial effluents include physical (adsorption, membrane filtration, ion exchange, irradiation, etc.) and chemical (oxidation, coagulation, electrochemical, etc.) processes; these methods have earlier been reviewed extensively (Hao et al. 2000; Forgacs et al. 2004; Joshi et al. 2004; Kuhad et al. 2004). The major drawbacks of physicochemical approa-ches are that these are prohibitively expensive, less efficient, not versatile, and have interference by other wastewater constituents. Biological methods consisting of biosorption, biodegradation, and enzymatic processes are eco-friendly, simpler, and cost-effective and are receiving greater attention for treatment of industrial effluents (Kuhad et al. 2004; Kaushik and Malik 2009).
Manganese peroxidases are the part of the extracellular oxidative system which evolved in white-rot fungi for lignin degradation (Kirk and Cullen 1991; Hatakka 1994; Vares and Hatakka 1996). Lignin is a heterogeneous, optically inactive polymer consisting of phenylpropanoid interunits, which are linked by several covalent bonds (e.g., aryl-ether, aryl–aryl, carbon–carbon bonds) (Hofrichter 2002). The structure of the lignin polymer implies that lignolytic enzymes possess the ability to oxidize substrates of high redox potential in a nonspecific manner. Paper and pulp industries employ a combination of chlorine-based chemicals and alkaline extraction multistage procedures for bleaching of the kraft pulp. However, chemical bleaching procedures end up with chlorinated organic substances as by-products which contain toxic, mutagenic, and carcinogenic polychlorinated dioxins, dibenzofurans, and phenols. Discharge of these organic compounds into the effluent generates serious environmental concern. Partially purified manganese peroxidase in the presence of oxalate preparations is known to be effective in decolorizing kraft effluents and oxidizing a broad range of xenobiotic compounds (Harazono et al. 1996; Sasaki et al. 2001). In vitro depolymerization studies using LiP and MnP showed that the enzymes were able to degrade to a variety of aromatic substrates (Conesa et al. 2002).
MnP also demonstrated the ability to decolorize a range of azo and anthraquinone dyes, as well as textile industry effluents in aqueous cultures and in packed bed bioreactors (Robinson et al. 2001; Mielgo et al. 2001; Kasinath et al. 2003; Shin 2004; Yang et al. 2004; Snajdar and Baldrian 2007; Asgher et al. 2008; Sedighi et al. 2009). Susla et al. (2008) have evaluated the contribution of MnP from D. squalens and laccase in degradation of azo, anthraquinone, phthalocyanine, and oligocyclic aromatic dyes. MnP has been observed to be capable of decolorizing the mixture of azo dyes at a concentration range of 10–200 mgl−1 each (Singh and Pakshirajan 2010), and also MnP from P. chrysosporium sp. HSD has been reported to rapidly decolorize a higher concentration (up to 600 mgl−1) of azo dyes (Hailei et al. 2009).
MnP has been shown to have the mineralization ability for many environmental contaminants. Besides having ability to degrade azo, heterocyclic, reactive, and polymeric dyes (Champagne and Ramsay 2005), it can degrade 1.1.1-trichloro-2.2-bis-(4-chlorophenyl) ethane (DDT), 2.4.6-trinitrotoluene (TNT), and polycyclic aromatic hydrocarbons (PAHs) too (Maciel et al. 2010).
Manganese peroxidase (MnP) from two metabolically distinct fungi Phanerochaete chrysosporium BKM-F-1767 (ATCC 24725) and Bjerkandera sp. BOS55 (ATCC90940) has the ability to degrade (98 %) anthracene to generate anthraquinone in organic solvent mixtures after 6 h of operation under optimal conditions (Eibes et al. 2005). Utilization of MnP (from the basidiomycete Bjerkandera adusta) for acrylamide polymerization has also been reported (Iwahara et al. 2000). MnPs from Phanerochaete chrysosporium have also been employed in styrene degradation, an important industrial polymer used as a raw material for wrapping and transporting goods. Its disposal poses serious environmental concerns (Soto et al. 1991; Lee et al. 2006).
MnP a redox enzyme has the potential of directly transferring the electrons to the electrodes. This enables the use of this enzyme for various applications in the development of biosensors, designing effective biofuel cells, and for selective bioorganic synthesis (Maciel et al. 2010).
Future Perspectives
This review highlights the various developments related to the molecular features, cloning, heterologous production, crystal structure refinements of MnPs, and its possible industrial and biotechnological applications. MnPs are promising enzymes and an eco-friendly alternative to the conventional physicochemical processes as presently employed for various such as the pulp and paper, textile, pharmaceutical, and for food industries. However, a major challenge in the commercialization of the MnPs is due to its lower thermal stability. Despite recent progress, our understanding of the process is still limited due to its substrate complexity and because of multiplicity of the peroxidases. Manganese peroxidases currently generated are so far not promising enough for their commercial scale exploitation. Although efforts have been made for improving the thermal stability of MnPs, further efforts are required for the development of designer enzymes with desired levels of thermal stability for its industrial applications. Therefore, the tailor-made enzymes can be designed using a combination of molecular approaches like site-directed mutagenesis, saturation mutagenesis, and directed evolution enabling the industrial exploitation of the unique catalytic abilities of these biocatalysts. The various developments in enzyme engineering research open a wide spectrum of possible applications in the near future.
References
Alic M, Akileswaran L, Gold MH (1997) Characterization of the gene encoding manganese peroxidase isozyme 3 from Phanerochaete chrysosporium. Biochim Biophys Acta 1338:1–7
Asgher M, Bhatti HN, Ashraf M, Legge RL (2008) Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19:771–783
Balay KA, Dougherty M, Tien M (2000) Reactivity of manganese peroxidase: site-directed mutagenesis of residues in proximity to the porphyrin ring. Arch Biochem Biophys 382:89–94
Banci L, Bertini I, Pease EA, Tien M, Turano P (1992) 1H NMR investigation of manganese peroxidase from Phanerochaete chrysosporium. A comparison with other peroxidases. Biochemistry 31(41):10009–10017
Bao W, Fukushima Y, Jensen KA, Moen MA, Hammel KE (1994) Oxidative degradation of non-phenolic lignin during lipid peroxidation by fungal manganese peroxidase. FEBS Lett 354:297–300
Benner SA (1989) Patterns of divergence in homologous proteins as indicators of tertiary and quaternary structure. Adv Enzyme Regul 28:219–236
Benner SA, Gerloff D (1990) Patterns of divergence in homologous proteins as indicators of secondary and tertiary structure: a prediction of the structure of the catalytic domain of protein kinases. Adv Enzyme Regul 31:121–181
Bonnarme P, Jeffries TW (1990) MnII regulation of lignin peroxidases and manganese dependent peroxidases from lignin-degrading white rot fungi. Appl Environ Microbiol 56:210–217
Branden C, Tooze J (1991) Introduction to protein structure. Garland Publishing, New York/London
Brar SK, Verma M, Surampalli RY, Misra K, Tyagi RD, Meunier N, Blais JF (2006) Bioremediation of hazardous wastes: a review. Pract Period Hazard, Toxic Radioact Waste Manag 10:59–72
Brown JA, Alic M, Gold MH (1991) Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J Bacteriol 173:4101–4106
Cai D, Tien M (1993) Lignin-degrading peroxidases of Phanerochaete chrysosporium. J Biotechnol 30:79–90
Cairney JWG, Burke RM (1998) Do ecto- and ericoid mycorrhizal fungi produce peroxidase activity. Mycorrhiza 8:61–65
Camarero S, Ruiz-Duen FJ, Sarkar S, Martinez MJ, Martinez AT (2000) The cloning of a new peroxidase found in lignocellulose cultures of Pleurotus eryngii and sequence comparison with other fungal peroxidases. FEMS Microbiol Lett 191:37–43
Champagne PP, Ramsay JA (2005) Contribution of manganese peroxidase and laccase to dye decoloration by Trametes versicolor. Appl Microbiol Biotechnol 69:276–285
Conesa A, van Den Hondel CA, Punt PJ (2000) Studies on the production of fungal peroxidases in Aspergillus niger. Appl Environ Microbiol 66:3016–3023
Conesa A, Punt PJ, vanden Hondel CAMJJ (2002) Fungal peroxidases: molecular aspects and applications. J Biotechnol 93:143–158
Daina S, Orlandi M, Bestetti G, Wiik C, Elegir G (2002) Degradation of β-5 lignin model dimers by Ceriporiopsis subvermispora. Enzyme Microb Technol 30:499–505
Deguchi T, Matsubara M, Nishida T (2002) NADH oxidation by manganese peroxidase with or without alpha-hydroxy acid. Biosci Biotechnol Biochem 66(4):717–721
Demmer H, Hinz I, Keller-Rudex H, Koeber K, Kottelwesch H, Schneider D (1980) In: Schleitzer-Rust E (ed) Coordination compounds of manganese, vol 56, 8th edn. Springer, New York, pp 1–185
Duenas FJR, Martnez MJ, Martnez A (1999) Molecular characterization of a novel peroxidase from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol 31:223–235
Eibes G, Lú-Chau T, Feijoo G, Moreira MT, Lema JM (2005) Complete degradation of anthracene by Manganese Peroxidase in organic solvent mixtures. Enzyme Microb Technol 37:365–372
Eriksson KEL, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. Springer, Berlin/Heidelberg/New York
Espinosa DVC, Absalón AE, Sanchez N, Loera O, Vazquez RR, Fernández FJ (2012) Heterologous expression of manganese peroxidase in Aspergillus niger and its effect on phenanthrene removal from soil. J Mol Microbiol Biotechnol 21:120–129
Evans FF, Rosado S, Sebastian GV, Casella R, Machado PLOA, Holmstrom C, Kjelleberg S, Van Elsas JD, Seldin L (2004) Impact of oil contamination and biostimulation on the diversity of indigenous bacterial communities in soil microcosms. FEMS Microbiol Ecol 49:295–305
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Gaskell J, Dieperink E, Cullen D (1991) Genomic organization of lignin peroxides genes of Phanerochaete chrysosporium. Nucleic Acids Res 19:599–603
Gelpke SMD, Loccoz PM, Gold MH (1999) Arginine 177 is involved in MnII binding by manganese peroxidase. Biochemistry 38:11482–11489
Gelpke MD, Youngs HL, Gold MH (2000) Role of arginine 177 in the MnII binding site of manganese peroxidase. Studies with R177D, R177E, R177N, and R177Q mutants. Eur J Biochem 267(24):7038–7045
Gettemy JM, Ma B, Alic M, Gold MH (1998) Reverse transcription-PCR analysis of the regulation of the manganese peroxidase gene family. Appl Environ Microbiol 64:569–574
Glenn JK, Gold MH (1985) Purification and characterization of an extracellular MnII-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys 242:329–341
Godfrey BJ, Mayfield MB, Brown JA, Gold MH (1990) Characterization of a gene encoding a manganese peroxidase from Phanerochaete chrysosporium. Gene 93:119–124
Gold MH, Alic M (1993) Molecular biology of the lignin degrading basidiomycetes Phanerochaete chrysosporium. Microbiol Rev 57:605–622
Gu L, Lajoie C, Kelly C (2003) Expression of a Phanerochaete chrysosporium manganese peroxidase gene in the yeast Pichia pastoris. Biotechnol Prog 19:1403–1409
Hailei W, Ping L, Min P, Zhijun Z, Guangli Y, Guosheng L, Jianming Y (2009) Rapid decolourization of azo dyes by a new isolated higher manganese peroxidase producer: Phanerochaete sp. Hsd Biochem Eng J 46:327–333
Hao OJ, Kim H, Chiang PC (2000) Decolourization of wastewater. Crit Rev Environ Sci Technol 30:449–505
Harazono K, Kondo R, Sakai K (1996) Bleaching of hardwood kraft pulp with manganese peroxidase from Phanerochaete sordida YK-624 without addition of MnSO(inf4). Appl Environ Microbiol 62(3):913–917
Hatakka A (1994) Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev 13:125–135
Hatakka A (2001) Biodegradation of lignin. In: Hofrichter M, Steinbüchel A (eds) Biopolymers. Lignin, humic substances and coal. Wiley-VCH, Weinheim, pp 129–180
Heinzkill M, Bech L, Halkier T, Schneider P, Anke T (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl Environ Microbiol 64:1601–1606
Henrisatt B, Saloheimo M, Lavaitte S, Knowles JKC (1990) Structural homology among the peroxidase enzyme family revealed by hydrophobic cluster analysis. Protein Struct Funct Genet 8:251–257
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microb Technol 30:454–466
Iwahara K, Hirata M, Honda Y, Watanabe T, Kuwahara M (2000) Free-radical polymerization of acrylamide by manganese peroxidase produced by the white-rot basidiomycete Bjerkandera adusta. Biotechnol Lett 22:1355–1361
Janse BJH, Gaskell J, Akhtar M, Cullen D (1998) Expression of Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl Environ Microbiol 64:3536–3538
Johansson T, Nyman PO, Cullen D (2002) Differential regulation of mnp2, a new manganese peroxidase-encoding gene from the ligninolytic fungus Trametes versicolor PRL 572. Appl Environ Microbiol 68:2077–2080
Joshi M, Bansal R, Purwar R (2004) Colour removal from textile effluents. Indian J Fibre Text Res 29:239–259
Kapich AN, Steffen KT, Hofrichter M, Hatakka A (2005) Involvement of lipid peroxidation in the degradation of a non-phenolic lignin model compound by manganese peroxidase of the litter-decomposing fungus Stropharia coronilla. Biochem Biophys Res Commun 330:371–377
Kasinath A, Novotny C, Svobodova K, Patel KC, Saseck V (2003) Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed-bed bioreactor. Enzyme Microb Technol 32:167–173
Kaushik P, Malik A (2009) Fungal dye decolourization: recent advances and future potential. Environ Int 35:127–141
Khindaria A, Grover TA, Aust SD (1994) Oxalate-dependent reductive activity of manganese peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys 314:301–306
Kim Y, Yeo S, Kum J, Song HG, Choi HT (2005) Cloning of a manganese peroxidase cDNA gene repressed by manganese in Trametes versicolor. J Microbiol 43:569–571
Kirk TK, Cullen D (1991) Enzymology and molecular genetics of wood degradation by white-rot fungi. Wiley, New York
Kishi K, Wariishi H, Marquez L, Dunford BH, Gold MH (1994) Mechanism of manganese peroxidase II reduction. Effect of organic acid and chelators and pH. Biochemistry 34:8694–8701
Kuhad RC, Sood N, Tripathi KK, Singh A, Ward OP (2004) Developments in microbial Methods for the treatment of dye effluents. Adv Appl Microbiol 56:185–213
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Larrondo LF, Lobos S, Stewart P, Cullen D, Vicuña R (2001) Isoenzyme multiplicity and characterization of recombinant manganese peroxidases from Ceriporiopsis subvermispora and Phanerochaete chrysosporium. Appl Environ Microbiol 67:2070–2075
Lee JW, Lee SM, Hong EJ, Jeung EB, Kang HY, Kim MK, Choi IG (2006) Estrogenic reduction of styrene monomer degraded by Phanerochaete chrysosporium KFRI 20742. J Microbiol 44:177–184
Levin L, Papinutti L, Forchiassin F (2004) Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresour Technol 94:169–176
Li D, Youngs HLA, Gold MH (2001) Heterologous expression of a thermostable manganese peroxidases from Dichomitus squalens in Phanerochaete chrysosporium. Arch Biochem Biophys 385:348–356
Lobos S, Larram J, Salas L, Cullen D, Vicuña R (1994) Isoenzymes of manganese dependent peroxidase and laccase produced by the lignin degrading basidiomycete Ceriporiopsis subvermispora. Microbiology 14:2691–2698
Lobos S, Larrondo L, Salas L, Karahanian E, Vicun R (1998) Cloning and molecular analysis of a cDNA and theCs-mnp1 gene encoding a manganese peroxidase isoenzyme from the lignin-degrading basidiomycete Ceriporiopsis subvermispora. Gene 206:185–193
Longoria A, Tinoco R, Duhalt VR (2008) Chloroperoxidase-mediated transformation of highly halogenated monoaromatic compounds. Chemosphere 72:485–490
Ma B, Mayfield MB, Godfrey BJ, Gold MH (2004) Novel promoter sequence required for manganese regulation of manganese peroxidase isozyme 1 gene expression in Phanerochaete chrysosporium. Eukaryot Cell 3:579–588
Maciel MJ, Silva AC, Ribeiro HCT (2010) Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Elect J Biotechnol 13(6). (ISSN: 0717-3458) doi:org/10.2225/, full-text2
Magliozzo RS, Marcinkeviciene JA (1997) The role of Mn(II)-peroxidase activity of mycobacterial catalase-peroxidase in activation of the antibiotic isoniazid. J Biol Chem 272(14):8867–8870
Martınez AT (2002) Molecular biology and structure-function of lignin-degrading heme peroxidases. Enzyme Microb Technol 30:425–444
Mayfield MB, Godfrey BJ, Gold MH (1994) Characterization of the mnp2 gene encoding manganese peroxidase isozyme 2 from the basidiomycete Phanerochaete chrysosporium. Gene 142:231–235
Mielgo I, Moreira MT, Feijoo G, Lema JM (2001) A packed-bed fungal bioreactor for the continuous decolourisation of azo-dyes (Orange II). J Biotechnol 89:99–106
Mino Y, Wariishi H, Blackburn NJ, Loehrldx TM, Gold MH (1988) Spectral characterization of manganese peroxidase, an extracellular heme enzyme from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. J Biol Chem 263:7029–7036
Miyazaki C, Takahashi H (2001) Engineering of the H2O2-binding pocket region of a recombinant manganese peroxidase to be resistant to H2O2. FEBS Lett 509(1):111–114
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
Ogawa K, Yamazaki T, Hasebe T, Kajiwara S, Watanabe A, Asada Y, Shishido K (1998) Molecular breeding of the basidiomycete Coprinus cinereus strains with high lignindecolorization and β-degradation activities using novel heterologous protein expression vectors. Appl Microbiol Biotechnol 49:285–289
Orth AB, Rzhetskaya M, Cullen D, Tien M (1994) Characterization of a cDNA encoding a manganese peroxidase from Phanerochaete chrysosporium: genomic organization of lignin and manganese peroxidase-encoding genes. Gene 148:161–165
Padgett R, Konarska M, Grabowski P, Hardy S, Sharp P (1989) Lariat RNAs as intermediates and products in the splicing of messenger RNA products. Science 225:898–903
Paszcynski A, Huynh V-B, Crawford R (1985) Enzymatic activities of an extracellular, manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiol Lett 29:37–41
Paszcynski A, Huynh V-B, Crawford R (1986) Comparison of ligninase-1 and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys 244:750–765
Petersen JF, Tams JW, Vind J, Svensson A, Dalbøge H, Welinder KG, Larsen S (1993) Crystallization and X-ray diffraction analysis of recombinant Coprinus cinereus peroxidase. J Mol Biol 232:989–991
Poulos TL, Edwards SL, Wariishi H, Gold MH (1993) Crystallographic refinement of lignin peroxidase at 2 Å. J Biol Chem 268:4429–4440
Pribnow D, Mayfield MB, Nipper VJ, Brown JA, Gold MH (1989) Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem 264:5036–5040
Punt PJ, Biezen NV, Conesa A et al (2002) Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol 20:200–206
Raghukumar C, D’Souza TM, Thorn RG, Reddy CA (1999) Lignin-modifying enzymes of Flavodon flavus, a basidiomycete isolated from a coastal marine environment. Appl Environ Microbiol 65:2103–2111
Reading NS, Aust SD (2000) Engineering a disulfide bond in recombinant manganese peroxidase results in increased thermostability. Biotechnol Prog 16:326–333
Reddy GVB, Sridhar M, Gold MH (2003) Cleavage of nonphenolic β-1 diarylpropane lignin model dimers by manganese peroxidase from Phanerochaete chrysosporium. Eur J Biochem 270:284–292
Robinson T, Chandran B, Nigam P (2001) Studies on the production of enzymes by white-rot fungi for the decolourisation of textile dyes. Enzyme Microb Technol 29:575–579
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakamoto Y, Nakade K, Nagai M, Uchimiya H, Sato T (2009) Cloning of Lentinula edodes lemnp2, a manganese peroxidase that is secreted abundantly in sawdust medium. Mycoscience 50:116–122
Sarkanen KV, Ludwig CH (1971) Lignins: occurrence, formation and structure. Wiley–Interscience, New York
Sasaki T, Kajino T, Li B, Sugiyama H, Takahashi H (2001) New pulp biobleaching system involving manganese peroxidase immobilized in a silica support with controlled pore sizes. Appl Environ Microbiol 67(5):2208–2212
Schmidt O, Schmitt U, Moreth U, Potsch T (1997) Wood decay by the white-rotting basidiomycete Physisporinus vitreus from a cooling tower. Holzforschung 51:193–200
Sedighi M, Karimi A, Vahabzadeh F (2009) Involvement of ligninolytic enzymes of Phanerochaete chrysosporium in treating the textile effluent containing Astrazon Red FBL in a packed-bed bioreactor. J Hazard Mater 169(1–3):88–93
Selvaggini C, Salmona M, Gioia LD (1995) Manganese peroxidase from Phanerochaete chrysosporium. A homology-based molecular model. Eur J Biochem 228:955–961
Shin KS (2004) The role of enzymes produced by white-rot fungus Irpex lacteus in the decolorization of the textile industry effluent. J Microbiol 42:37–41
Singh S, Pakshirajan K (2010) Enzyme activities and decolourization of single and mixed azo dyes by the white-rot fungus Phanerochaete chrysosporium. Int biodeterior Biodegrad 64:1–5
Snajdar J, Baldrain P (2007) Temperature effect the production, activity and stability of ligninolytic enzymes in Pleurotus ostreatus and Trametes versicolor. Folia Microbiol 52(5):498–502
Soto AM, Justicia H, Wray JW, Sonnens Chein C (1991) p-nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspect 92:167–173
Steffen K, Hofrichter M, Hatakka A (2000) Mineralization of 14C-labelled lignin and production of ligninolytic enzymes by selected litter decomposing basidiomycetes. Appl Microbiol Biotechnol 54:819–825
Stewart P, Whitwam RE, Kersten PJ, Cullen D, Tien M (1996) Efficient expression of a Phanerochaete chrysosporium manganese peroxidase gene in Aspergillus oryzae. Appl Microbiol Biotechnol 62:860–864
Sundaramoorthy M, Kishi K, Gold MH, Poulos TL (1994a) Preliminary crystallographic analysis of manganese peroxidase from Phanerochaete chrysosporium. J Mol Biol 238:845–848
Sundaramoorthy M, Kishi K, Gold MH, Poulos TL (1994b) The crystal structure of manganese peroxidase from Phanerochaete chrysosporium at 2.06-A resolution. J Biol Chem 269:32759–32767
Sundaramoorthy M, Terner J, Poulos TL (1995) The crystal structure of chloroperoxidase: a heme peroxidase-cytochrome P450 functional hybrid. Structure 3:1367–1377
Sundaramoorthy M, Kishi K, Gold MH, Poulos TL (1997) Crystal structures of substrate binding site mutants of manganese peroxidase. J Biol Chem 272:17574–17580
Sundaramoorthy M, Youngs HL, Gold MH, Poulos TL (2005) High-resolution crystal structure of manganese peroxidase: substrate and inhibitor complexes. Biochemistry 44:6463–6470
Sundaramoorthy M, Gold MH, Poulos TL (2010) Ultrahigh (0.93 Å) resolution structure of manganese peroxidase from Phanerochaete chrysosporium: implications for the catalytic mechanism. J Inorg Biochem 104:683–690
Susla M, Novotny C, Erbanova P, Svobodova K (2008) Implication of Dichomitus squalens manganese-dependent peroxidase in dye decolorization and cooperation of the enzyme with laccase. Folia Microbiol 53:479–485
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tello M, Corsini G, Larrondo L, Salas L, Lobos S, Vicun R (2000) Characterization of three new manganese peroxidase genes from the ligninolytic basidiomycete Ceriporiopsis subvermispora. Biochim Biophys Acta 1490:137–144
Timofeevski SL, Aust SD (1997) Effects of Mn2+ and oxalate on the catalytic activity of manganese peroxidase. Biochem Biophys Res Commun 239:645–649
Timofeevski SL, Nie G, Reading NS, Aust SD (1999) Addition of veratryl alcohol oxidase activity to manganese peroxidase by site-directed mutagenesis. Biochem Biophys Res Commun 256:500–504
Tuor U, Wariishi H, Schoemaker HE, Gold MH (1992) Oxidation of phenolic aryglycerol β- aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: oxidative cleavage of an α-carbonyl model compound. Biochemistry 31:4986–4995
Urzua U, Larrondo LF, Lobos S, Larraın J, Vicuna R (1995) Oxidation reactions catalyzed by manganese peroxidase isoenzymes from Ceriporiopsis subvermispora. FEBS Lett 371:132–136
Urzua U, Kersten PJ, Vicuna R (1998) Manganese peroxidase-dependent oxidation of glyoxylic and oxalic acids synthesized by Ceriporiopsis subvermispora produces extracellular hydrogen peroxide. Appl Environ Microbiol 64:68–73
Vares T, Hatakka A (1996) Lignin-degrading activity and ligninolytic enzymes of different white-rot fungi: effects of manganese and malonate. Can J Bot 75:61–71
Wariishi H, Akaleswaran L, Gold MH (1988) Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of oxidized states and the catalytic cycle. Biochemistry 27:5365–5370
Wariishi H, Dunford HB, MacDonald ID, Gold MH (1989a) Manganese peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. Transient state kinetics and reaction mechanism. J Biol Chem 264:3335–3340
Wariishi H, Valli K, Renganathan V, Gold MH (1989b) Oxidative cleavage of a phenolic diarylpropane lignin model dimer by manganese peroxidase from Phanerochaete chrysosporium. Biochemistry 28:6017–6023
Wariishi H, Valli K, Renganathan V, Gold MH (1989c) Thiol-mediated oxidation of nonphenolic lignin model compounds by manganese peroxidase of Phanerochaete chrysosporium. J Biol Chem 264:14185–14191
Wariishi H, Valli K, Gold MH (1992) Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. J Biol Chem 267:23688–23695
Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol 2:388–393
Whitwam RE, Brown KR, Musick M, Natan MJ, Tien M (1997) Mutagenesis of the Mn2+ binding site of manganese peroxidase affects oxidation of Mn2+ by both compound I and compound II. Biochemistry 36:9766–9773
Whitwam RE, Koduri RS, Natan M, Tien M (1999) Role of axial ligands in the reactivity of Mn peroxidase from Phanerochaete chrysosporium. Biochemistry 38:9608–9616
Willmann G, Fakoussa RM (1997a) Biological bleaching of water-soluble coal macromolecules by a basidiomycete strain. Appl Microbiol Biotechnol 47:95–101
Willmann G, Fakoussa RM (1997b) Extracellular oxidative enzymes of coal attacking fungi. Fuel Process Technol 52:27–41
Wong DWS (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209
Yang HS, Yoon JS, Kim MN (2004) Effects of storage of a mature compost on its potential for biodegradation of plastics. Polym Degrad Stab 84:411–417
Yeo PSN, Song HG, Choi HT (2007) Generation of a transformant showing higher manganese peroxidase (Mnp) activity by overexpression of Mnp Gene in Trametes versicolor. J Microbiol 45:213–218
Youngs HL, Gelpke SMD, Li D, Sundaramoorthy M, Gold MH (2000) The role of Glu39 in MnII binding and oxidation by manganese peroxidase from Phanerochaete chrysoporium. Biochemistry 40:2243–2250
Zapanta LS, Tien M (1997) The roles of veratryl alcohol and oxalate in fungal lignin degradation. J Biotechnol 53:93–102
Zhang X, Wang Y, Wang L, Chen G, Liu W, Gao P (2009) Site-directed mutagenesis of manganese peroxidase from Phanerochaete chrysosporium in an in vitro expression system. J Biotechnol 139:176–178
Zuckerkand E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Evolving genes and proteins. Academic, New York
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer India
About this chapter
Cite this chapter
Saroj, S., Agarwal, P., Dubey, S., Singh, R.P. (2013). Manganese Peroxidases: Molecular Diversity, Heterologous Expression, and Applications. In: Shukla, P., Pletschke, B. (eds) Advances in Enzyme Biotechnology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1094-8_6
Download citation
DOI: https://doi.org/10.1007/978-81-322-1094-8_6
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1093-1
Online ISBN: 978-81-322-1094-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)