Abstract
Violacein is an intensely purple pigment synthesized by various genera of bacteria that has been discovered to have a wide range of interesting biological activities which range from anticarcinogenic to antibacterial. One of the hindrances for its real-life application is that the first microorganisms found to produce the compound may act as opportunistic pathogens. Here, we report the isolation and characterization of violacein from a non-pathogenic Antarctic Iodobacter strain. Its anti-microbial properties were also tested. The method proposed here for the purification of violacein shows high yields, indicating that this Antarctic microorganism could be a valuable source for this important pigment. This is the first characterization of violacein from an Antarctic Iodobacter strain and here we also present a viable method to obtain this pigment for potential biotechnological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Violacein (Viol) is a natural indole derivative, intensely purple in color. It is biosynthesized by various bacterial genera from the condensation of two tryptophan molecules in response to quorum sensing signals (Gómez-Gómez et al. 2019). This pigment was first isolated from Chromobacterium violaceum, and its chemical structure was determined in early studies (Reilly and Pyne 1927).
Besides its role as a quorum-sensing metabolite, it has been found to possess a broad range of biological activities, including anti-tumoral (Bromberg et al. 2010), bacteriostatic and antibiotic potential (Nakamura et al. 2002; Shankar et al. 2014), as well as antifungal activity (Sasidharan et al. 2015). Viol not only works itself as an antibiotic but has also been demonstrated to potentiate the effects of other antibiotics acting synergistically.
The related pigment deoxyviolacein (dViol), which is a by-product of the biosynthesis of Viol, has also been found to have similar, but slightly differing antibacterial and anti-fungal properties. Studies showed that it acts as a less efficient anti-microbial agent, but it is a stronger antifungal (Wang et al. 2009). Structurally, dViol differs from Viol in a single oxygen atom located in the indole ring (Fig. 1). Compared to Viol, it is produced in considerably smaller amounts in wild-type strains. The relative difficulty in separating both pigments and producing them in sufficient quantities for laboratory analysis has remained a significant challenge.
Despite its medically interesting biological activities, their potential as drugs remains unexploited. To date, no human trials have been performed, although it has been shown to be harmless in animal models (Bromberg et al. 2010). Part of the hindrances in the development of these pigments as commercially available pharmaceuticals stems from the fact that the bacterial genus first reported to produce these pigments has also been found to act as an opportunistic pathogen (Batista and da Silva Neto 2017). Human infections with C. violaceum, while uncommon, show high mortality rates and life-threatening sepsis; the bacterium rapidly spreads to several organs, including lungs and liver (Durán and Menck 2001). This limits the real-life application of Viol and dViol obtained from such bacterium, because it could not apply for the FDA “Generally Regarded as Safe” approval.
An unexplored area of application for Viol is the functionalization of surfaces for the production of bioactive functionalized materials. Its relatively small size makes it a good candidate for this kind of application. One interesting example of this would be the production of metallic alloys functionalized with Viol to repel bacteria inhibiting the formation of biofilms on their surface. Biocorrosion, a bacteriogenic oxidation of metallic surfaces, is a major problem worldwide, and Viol holds promise as an interesting candidate for solving it.
With the increasing prominence of multidrug-resistant bacteria, a renewed effort has been put towards finding, isolating and characterizing novel bacterial strains capable of producing Viol. Various bacteria from diverse genera have been found to produce Viol, including Chromobacterium (Hoshino 2011), Collimonas (Hakvåg et al. 2009), Pseudoalteromonas (Timmermans et al. 2019), Janthinobacterium (Lu et al. 2009), Iodobacter (Doing et al. 2018) and Duganella (Wang et al. 2009).
This work focuses on the isolation and the development of a viable purification procedure for Viol from a non-pathogenic Iodobacter strain isolated recently from the Antarctic territory. We were able to successfully purify the compound, as determined by spectroscopic analysis. In contrast to pigments obtained from pathogenic bacteria, this finding could be relevant to the pharmaceutical industry for potential applications. It is also the first characterization of Viol production from an Antarctic Iodobacter.
Materials and methods
Bacterial strain culture
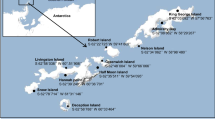
Iodobacter sp. 7MAnt was originally isolated from King George island near Eduardo Frei Montalva, Chilean Air Base (62°12′0″S58°57′51″W), Antarctic Peninsula.
This microorganism was grown in TSA media consisting of 15 g/L peptone, 5 g/L tryptone and 5 g/L NaCl supplemented with 0.03% tryptophan, adjusted to pH 7 and then inoculated at 10% with Iodobacter sp. 7MAnt. Inoculated media was incubated with agitation at 220 rpm for 16 h at 23 °C.
Identification of 7MAnt bacterial strain
Total DNA was isolated from Iodobacter sp. 7MAnt cultures, and the 16S rRNA gene was amplified using 515F, a specific primer for bacteria. We also used the universal primer 1492R. Reaction mix consisted of 0.25 μL of Taq DNA polymerase (500 U/mL, Sigma), 5 μL of each deoxynucleotide (2 mM) (dATP, dCTP, dGTP and dTTP), 5 μL of buffer, 4 μL of MgCl2 (0.75 mM) and 0.5 μL of each primer (100 mM). The following thermal conditions were applied: 95 °C for 45 s, 55 °C for 45 s and 72 °C for 45 s. Each cycle was repeated thirty times. Final elongation step was done at 72 °C for 10 min. Amplification reactions were carried on a Palm Gradient Cycler (Corbett).
Sequencing of the amplified DNA was performed by the Georgia Genomics Facility (University of Georgia, USA), using an Illumina MiSeq platform. Sequences obtained were then assembled, analyzed, and edited using Chromas Pro software (Technelysium Pty Ltd.). A nucleotide BLAST against the NCBI database was performed to identify the specie. For further insight, a Phylogenetic tree was constructed using Phylogeny.fr online server (Dereeper et al. 2008). 16S rRNA gene sequences from other Viol producing bacteria were used as input for the multiple sequence alignment. E. coli was used as an outgroup. Sequences were obtained from NCBI database.
Screening of enzyme activities
Activity for several enzymes was studied using a semi-quantitative method for detection of enzymatic activities. Metabolic profiling using an analytical profile index kits (API) was performed for further identification of the bacterial strain. API 20E and API 20Ne systems (BioMerieux). were used in parallel and results were in agreement with each other.
Violacein purification
Eight grams of Iodobacter sp. 7MAnt were harvested after centrifugation of bacterial culture at 36,389×g for 30 min in a Hitachi CP80WX preparative centrifuge. Cells were lysed by sonication using a VWR Branson 450 sonifier, and pigment was extracted using 99% ethanol. The lysate obtained was then centrifuged (36,389×g for 20 min) to remove cellular debris and the supernatant containing the pigment was collected. The pellet obtained from the centrifugation was resuspended in ethanol and the same centrifugation procedure previously described was repeated, until the pellet showed minimal color.
The total supernatant obtained was stored overnight at − 20 °C and then centrifuged (36,389×g × 20 min) to further precipitate impurities. The liquid phase was recovered and loaded into a PF-30C18AQ-F0-220 column (Interchim, France) using a FPLC system for chromatographic separation. The mobile phase used during the loading and chromatographic separation was 70% ethanol. Each fraction obtained from the chromatography was spectrophotometrically analyzed to find the fractions with the characteristic absorption wavelength for Viol. The UV–VIS spectrum for each fraction was obtained in the range between 700 nm and 300 nm using a Shimadzu spectrophotometer. All fractions with an absorbance peak between 574 and 577 nm were pooled and then solvent was removed in a rotatory evaporator (IKA RV10/HB10). Viol remaining in the aqueous phase was lyophilized (Liotop L101). The lyophilized fraction containing the partially purified Viol was dissolved in acetone and kept at − 20 °C overnight and then it was centrifuged at (36,389×g × 20 min) to further precipitate the remaining impurities. Acetone was then removed using a second cycle of rotatory evaporation and lyophilization. Purity of Viol was determined by Mass Spectroscopy using a triple quadrupole mass spectrometer (Agilent 6400) equipped with an Agilent LC 1200 series.
Determination of the pigment concentration
Pigment concentration was determined with two independent methods, a colorimetric method measuring absorbance at 575 nm and using HPLC profile and the area of peaks obtained at a given retention time in comparison with the HPLC profile of the standard solution prepared with commercial Viol (Sigma V9389). Both methods gave similar results. For colorimetric determination, a calibration curve using commercial Viol was prepared.
Identification of the pigment
The chemical structure of the purified pigment was determined by 1H NMR. Pigment was dissolved in deuterated dimethyl sulphoxide (DMSO) and deuterated ethanol and then analyzed (600 MHz, Varian Innova-600). For corroboration of the identity of the compound a Viol standard solution (Sigma V9389) was also used.
Antibacterial assay
Solid culture plates were grown using bacterial lawns of a pathogenic strain of Pseudomonas aeruginosa. For this test, TSA media supplemented with 1% agar was used. 34 µg, 54 µg and 72 µg of purified Viol were dropped before incubation of the bacterial strain for 20 h at 37 °C. The inhibition of bacterial growth was visually monitored.
Results
Characterization of the bacterial strain
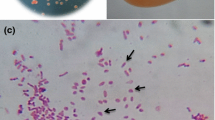
A sample of meltwater stream sediments taken during Chilean Antarctic expedition ECA 46 from a small lagoon located in King George Island, located at South Shetland, Antarctica. (coordinates of sampling site: 62°12′02.5″S and 58°58′18.9″W) was used to inoculate TSA media. Using serial dilution, a gram-negative, rod-shaped, motile, psychrotolerant bacterium was isolated from the inoculated enriched media (Fig. 2). This bacterium was found to grow at pH 7 in the temperature range between 8 °C and 25 °C.
Bacterial colonies formed in solid culture were observed to develop a strong purple color after 24 h of incubation (Fig. 3). In liquid media, the purple pigmentation reached its maximum after 16 h (Fig. 4). When culture media was supplemented with 0.03% tryptophan, the color became more intense, suggesting the indolic nature of the compound.
Total DNA was extracted from bacterial cultures and the 16S rRNA gene was amplified for sequencing. The sequence obtained (Fig. 5) was used to identify the species in a BLAST analysis, showing that the microorganism found and cultured is closely related to the Iodobacter genus. To better establish the identity of the isolate, a phylogenetic tree was constructed using 16S rRNA sequences from other Viol producing bacteria (Fig. 6). This once again placed the sequence in close proximity with other Iodobacter species and it is most closely related to Iodobacter arcticus. The isolate was, therefore, classified as Iodobacter sp 7MAnt.
A broad metabolic characterization of the bacteria was performed using API kits (Table 1). Results obtained indicate that this bacterium is a nitrate reducing microorganism capable of assimilating various carbon sources.
Purification of the pigment
After incubating Iodobacter sp. 7MAnt for 16 h, the strongly violet pigment reached maximum on culture media and in cells. The cells were harvested by centrifugation and then lysed by sonication to purify the pigment using a combination of organic solvent extraction and liquid chromatography. Rotatory evaporation was used to remove the ethanol, followed by a lyophilization step. After this step, insoluble contaminants were still present upon resuspension, so an acetone wash was applied to the lyophilizate.
To remove acetone, three independent methods were assayed: room temperature evaporation, overnight incubation at 40 °C, and rotatory evaporation followed by a second lyophilization step. Based on HPLC/MS analysis, the best method used was rotary evaporation and lyophilization, as the purity obtained for the three methods was 87, 91 and 97%, respectively.
Following this procedure, 8 grams of bacteria yielded 1.089 mg of pigment, giving a yield of 0.01% of pure pigment. The scalability of this procedure needs to be developed.
Identification of violacein
The pigment was identified on the basis of its absorption spectrum and its retention time on HPLC when compared to a standard (Fig. 7). The characteristic absorption peak of Viol was obtained at 575 nm. A small peak was also evident at a slightly longer retention time in the chromatogram.
HPLC Chromatograms and 3-D graphs. a Shows the chromatogram of the purified pigment, while b corresponds to the chromatogram of the violacein standard. c Shows a 3-D graph relating absorption spectra of the Viol standard solution in relation to its retention time. d Shows the absorption spectra and retention time of the purified violacein
The structure of the molecule was confirmed by analyzing the purified pigment by 1H-NMR (Fig. 8). NMR spectra were obtained in DMSO and deuterated ethanol. Signals obtained were consistent with those obtained with a Viol standard solution. Chemical shifts obtained were also in agreement with the ones reported in a work by Aruldass et al. (2015). Although the resolution was low in the spectrum obtained due to the concentration of the sample, all the relevant peaks were visible and identifiable. The spectrum obtained using deuterated ethanol showed no peaks in the area between 10 and 12 ppm, in contrast the one obtained when DMSO was the solvent for the sample. A combined analysis of spectra under both experimental conditions mentioned above, allowed us to confirm the structure of the molecule.
Antibacterial activity assays
The bioactivity of the purified pigment towards biofilm-forming bacteria was tested in agar plates containing a culture lawn of the opportunistic pathogen Pseudomonas aeruginosa. Three different quantities of Viol were cast dropped on the agar plate and the effect was observed visually as an inhibition halo in the bacterial lawn (Fig. 9). 54 µg of the pigment was sufficient to inhibit bacterial growth. The antibacterial effect appears to achieve saturation when the amount of Viol was 72 µg. The inhibition halo shows no apparent difference between the two highest amounts of pigment used during the test.
Anti-bacterial activity assay. A bacterial lawn of P. aeruginosa was grown, and three different amounts of Viol were applied to investigate its antibacterial properties. a Is a control, with no Viol added. b–d Show the effect of adding 54 ug, 72 ug and 108 ug, respectively, to the plate. The dotted line circles were added to help visualizing the inhibition halos
Discussion
Iodobacter sp. 7MAnt showed to be a producer of violet pigment when media was supplemented with tryptophan. The highest absorption peak of the pigment was obtained at 575 nm, which is consistent with what is reported for Viol in the literature (Jiang et al. 2012; Kothari et al. 2017). NMR spectrum of the purified pigment coincides with the spectrum obtained for a commercial standard Viol, confirming the identity of the pigment purified from Iodobacter sp. 7MAnt. NMR spectra were obtained using deuterated DMSO and ethanol as solvents for the spectroscopic measurements. The spectrum obtained using deuterated ethanol did not show the peaks that are characteristic of the indole ring. Thus, DMSO was preferred in our analysis for the identification of the molecule.
The yield of Viol biosynthesis is approximately 0.01%, obtaining 1.1 mg of Viol per 1 L of bacterial cells. Although with this yield Iodobacter sp. 7MAnt is not as proficient in producing the pigment as other bacteria, like Duganella violaceinigra which is reported to produce 18.6 mg per culture liter, or Chromobacter piscinae, with approximately 15 mg per liter (Choi et al. 2015a), this is nevertheless an important discovery because it is the only report of an Antarctic Iodobacter producing Viol. Improvement on the procedure for induction of Viol produce by the microorganism is required.
To the best of our knowledge, the only studies on Viol production using Antarctic bacteria corresponds to a microorganism of the Janthinobacterium genera (Mojib et al. 2011; Smith et al. 2016). This is the first characterization of Viol production from an Antarctic Iodobacter. As opposed to Janthinobacterium, Iodobacter is described as a non-pathogenic genus, opening the way for potential biotechnological application of its pigment. The fact that this microorganism is also psychrotolerant, gives better opportunities to manipulate the microorganism in an industrial setting, for their cultivation at large scale avoids the additional cost of incubation at higher temperatures. The use of psychrophilic bacteria is also desirable because their adaptation to cold environments diminishes the possibility that they could act as human pathogens. For the eventual pharmaceutical production of violacein this is a key point.
One of the difficulties in the purification of Viol is the separation from the structurally related compound dViol. Retention time between both compounds is similar enough that most protocols reported end up with a mixture of both compounds. As dViol is a common byproduct in the biosynthesis of Viol (Rodrigues et al. 2012), it is likely that it would be found as an impurity in the isolated pigment. We observed a small, additional peak in the chromatogram of our purified compound, which is speculated on the basis of its absorption peak at 560 nm, to be dViol. To completely confirm the identity of the compound causing this additional peak, it would be necessary to purify it and analyze it using NMR spectroscopy.
Nonetheless, the method described here for the purification of Viol allowed us to obtain the pigment at remarkably high purity. Using mass spectroscopy, the purity of the pigment was estimated to be at 97%, which is higher than the commercially available Viol Sigma V9389, which has 85% purity according to the distributor.
The pigment purified performed as an inhibitor of bacterial growth on solid media as demonstrated with the experiment using Pseudomonas aeruginosa. The presence of an antibiotic compound from an Antarctic microorganism hints at the necessity to eliminate any possible competitors in an environment with such harsh conditions for life as is the Antarctic soil. As violacein is a wide-spectrum abiotic, effective against bacteria and fungi, both of which can be found in Antarctic soil, it is an efficient resource for survival in a competitive and extreme environment.
References
Aruldass CA, Rubiyatno R, Venil CK, Ahmad WA (2015) Violet pigment production from liquid pineapple waste by Chromobacterium violaceum UTM5 and evaluation of its bioactivity. RSC Adv 5(64):51524–51536. https://doi.org/10.1039/c5ra05765e
Batista JH, da Silva Neto JF (2017) Chromobacterium violaceum Pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front Microbiol 8:2213. https://doi.org/10.3389/fmicb.2017.02213
Bromberg N, Dreyfuss JL, Regatieri CV, Palladino MV, Durán N, Nader HB, Haun M, Justo GZ (2010) Growth inhibition and pro-apoptotic activity of violacein in Ehrlich ascites tumor. Chem Biol Interact 186(1):43–52. https://doi.org/10.1016/j.cbi.2010.04.016
Choi SY, Kim S, Lyuck S, Kim SB, Mitchell RJ (2015) High-level production of violacein by the newly isolated Duganella violaceinigra str. NI28 and its impact on Staphylococcus aureus. Sci Rep 5:15598. https://doi.org/10.1038/srep15598
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36(Web Server issue):W465–W469. https://doi.org/10.1093/nar/gkn180
Doing G, Perron GG, Jude BA (2018) Draft genome sequence of a violacein-producing Iodobacter sp. from the Hudson valley watershed. Genome Announc 6(1):e01428-17. https://doi.org/10.1128/genomea.01428-17
Durán N, Menck CF (2001) Chromobacterium violaceum: a review of pharmacological and industrial perspectives. Crit Rev Microbiol 27:201–222. https://doi.org/10.1080/20014091096747
Gómez-Gómez B, Arregui L, Serrano S, Santos A, Pérez-Corona T, Madrid Y (2019) Selenium and tellurium-based nanoparticles as interfering factors in quorum sensing-regulated processes: violacein production and bacterial biofilm formation. Metallomics. https://doi.org/10.1039/c9mt00044e(Epub ahead of print)
Hakvåg ES, Fjærvik E, Klinkenberg G, Borgos SE, Josefsen KD, Ellingsen TE, Zotchev SB (2009) Violacein-producing Collimonas sp from the sea surface microlayer of costal waters in Trøndelag, Norway. Mar Drugs 7(4):576–588. https://doi.org/10.3390/md7040576(Published online Nov 12)
Hoshino T (2011) Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: biosynthetic mechanism and pathway for construction of violacein core. Appl Microbiol Biotech 91:1463–1475. https://doi.org/10.1007/s00253-011-3468-z
Jiang P, Wang H, Xiao S et al (2012) Pathway redesign for deoxyviolacein biosynthesis in Citrobacter freundii and characterization of this pigment. Appl Microbiol Biotechnol 94:1521. https://doi.org/10.1007/s00253-012-3960-0
Kothari V, Sharma S, Padia D (2017) Recent research advances on Chromobacterium violaceum, Asian Pac. J Trop Med 10:744–752. https://doi.org/10.1016/j.apjtm.2017.07.022
Lu Y et al (2009) Production of violet pigment by a newly isolated psychrotrophic bacterium from a glacier in Xinjiang, China. Biochem Eng J 43:135–141. https://doi.org/10.1016/j.bej.2008.09.009
Mojib N, Nasti TH, Andersen DT, Attigada VR, Hoover RB, Yusuf N, Bej AK (2011) The antiproliferative function of violacein-like purple violet pigment (PVP) from an Antarctic Janthinobacterium sp. Ant5-2 in UV-induced 2237 fibrosarcoma. Int J Dermatol 50:1223–1233. https://doi.org/10.1111/j.1365-4632.2010.04825.x
Nakamura Y, Sawada T, Morita Y, Tamiya E (2002) Isolation of a psychrotrophic bacterium from the organic residue of a water tank keeping rainbow trout and antibacterial effect of violet pigment produced from the strain. Biochem Eng J 12:79–86. https://doi.org/10.1016/S1369-703X(02)00079-7
Reilly J, Pyne G (1927) On the pigment produced by Chromobacterium violaceum. Biochem J 21:1059–1064
Rodrigues AL, Göcke Y, Bolten C, Brock NL, Dickschat JS, Wittmann C (2012) Microbial production of the drugs violacein and deoxyviolacein: analytical development and strain comparison. Biotechnol Lett 34:717–720. https://doi.org/10.1007/s10529-011-0827-x
Sasidharan A, Sasidharan NK, Amma DB, Vasu RK, Nataraja AV, Bhaskaran K (2015) Antifungal activity of violacein purified from a novel strain of Chromobacterium sp. NIIST (MTCC 5522). J Microbiol 10:694–701. https://doi.org/10.1007/s12275-015-5173-6
Shankar S, Venkatraman R, Aravind S (2014) Synergistic antimicrobial profiling of violacein with commercial antibiotics against pathogenic microorganisms. Pharm Biol 52(1):86–90. https://doi.org/10.3109/13880209.2013.815634
Smith HJ, Foreman CM, Akiyama T, Franklin MJ, Devitt NP, Ramaraj T (2016) Genome sequence of Janthinobacterium sp. CG23_2, a violacein-producing isolate from an Antarctic supraglacial stream. Genome Announc 4:e01468-15. https://doi.org/10.1128/genomeA.01468-15
Timmermans ML, Picott KJ, Ucciferri L, Ross AC (2019) Culturing marine bacteria from the genus Pseudoalteromonas on a cotton scaffold alters secondary metabolite production. Microbiologyopen 8(5):e00724. https://doi.org/10.1002/mbo3.724
Wang HS et al (2009) Optimization of culture conditions for violacein production by a new strain of Duganella sp B2. Biochem Eng J 44:119–124. https://doi.org/10.1016/j.bej.2008.11.008
Acknowledgements
We would like to thank Chilean Antarctic Institute (INACH) and project Conicyt, PIA ACT1412.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests to declare.
Additional information
Communicated by M. Moracci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript is part of a special issue of Extremophiles journal for the 12th International Congress of Extremophiles (Extremophiles 2018) that was held on 16–20 September 2018 in Ischia, Naples, Italy.
Rights and permissions
About this article
Cite this article
Atalah, J., Blamey, L., Muñoz-Ibacache, S. et al. Isolation and characterization of violacein from an Antarctic Iodobacter: a non-pathogenic psychrotolerant microorganism. Extremophiles 24, 43–52 (2020). https://doi.org/10.1007/s00792-019-01111-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-019-01111-w