Abstract
A chitinolytic actinomycete Streptomyces vinaceusdrappus S5MW2 was isolated from water sample of Chilika lake, India and identified using 16S rRNA gene sequencing. It showed in vitro antifungal activity against the sclerotia producing pathogen Rhizoctonia solani in a dual culture assay and by chitinase enzyme production in a chitin supplemented minimal broth. Moreover, isolate S5MW2 was further characterized for biocontrol (BC) and plant growth promoting features in a greenhouse experiment with or without colloidal chitin (CC). Results of greenhouse experiment showed that CC supplementation with S5MW2 showed a significant growth of tomato plants and superior disease reduction as compared to untreated control and without CC treated plants. Moreover, higher accumulation of chitinase also recovered in the CC supplemented plants. Significant effect of CC also concurred with the Analysis of Variance of greenhouse parameters. These results show that the a marine antagonist S5MW2 has BC efficiency against R. solani and chitinase enzyme played important role in plant resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitin, a linear β-1, 4-linked homopolymer of N-acetylglucosamine, is one of the three most abundant polysaccharides in nature besides cellulose and starch. The antifungal activity and highly biocompatible quality make the chitin and its derivatives particularly useful for environmental and agricultural uses (Hoster et al. 2005). Although more than 2.3 million ton per year of chitin are produced annually in the aquatic biosphere alone, there is no substantial accumulation of chitin in ocean sediments (Jeuniaux and Voss-Foucart 1991). Chitin degradation is a bioconversion process that is naturally driven by chitinolytic marine bacteria. For example, chitin is an excellent carbon and nitrogen source for many chitinolytic Streptomyces strains (Robbins et al. 1998). The application of a BC agent along with chitin has great importance in plant disease management (Kishore et al. 2005). Recently, CC (1 %) supplemented Trichoderma/Hypocrea spp. formulations were reported to induce BC activity against Rhizoctonia solani in tomato (Solanki et al. 2011). These diseases are easily widespread, affecting a large number of agricultural plants all over the world (Szczech and Shoda 2006). Control of R. solani is difficult because it is soil-borne in nature, have a wide host range and survive in the soil for extended periods in the form of sclerotia. Chemical control of R. solani was not very effective and most of the chemicals caused hazardous effects on non-targeted organism and environment (Patil et al. 2011).
In modern agriculture, microbe-based BC agents, as a replacement or supplement for agrochemicals, have been addressed in many recent reports (Solanki et al. 2012). Among the microbes, actinomycetes could be attractive candidates for biological control agents against plant pathogens (Patil et al. 2011). Almost 80 % of world’s antibiotics are known to come from actinomycetes, especially Streptomyces and Micromonospora (Pandey et al. 2004). Actinomycetes are resistant to desiccation and nutrient stress, because of their ability to produce spores (Yandigeri et al. 2012). Interestingly, they have the capacity to synthesize bioactive secondary metabolites, hydrolytic enzymes, siderophores, phytostimulators and they solublise the minerals making them available for plant growth. Moreover, antibiotics and hydrolytic enzymes play important role in disease reduction (El-Tarabily and Sivasithamparamb 2006; Tsavkelova et al. 2006). Marine actinomycetes are the important source of novel antifungal metabolites (Pisano et al. 1992). Hence, the present study first deals with identification and characterization of antifungal potentiality of a marine actinomycetes from Chilika lake, India and to the best of our knowledge, there is little information concerning the influence of chitin on the efficacy of the a marine Streptomyces sp. in controlling root rot of tomato caused by R. solani. Therefore, second objective of this study was focused on the effect of chitin supplementation on PGP and BC efficacy of a marine Streptomyces sp.
Materials and methods
Isolation and characterization
Chilika lake (19°28′–19°54′N and 85°06′–86°36′E) is a largest brackish water lake situated on the east coast of India. Streptomycetes strain S5MW2 was isolated from Ramsar site water sample (pH 8.34 ± 0.22) of Chilika lake on starch casein agar (SCA) medium containing (gL−1): Starch, 10.0; Casein, 0.3; K2HPO4, 2.0; NaCl, 2.0; KNO3, 2.0; MgSO4·7H2O, 0.2; FeSO4·7H2O, 0.01; CaCO3, 0.02; agar, 18; (pH 8.5) supplemented with nalidixic acid and cycloheximide (10 mg L−1 each) to inhibit Gram negative bacteria, some Gram-positive bacteria, and fungi, respectively. Identification of Streptomycetes was carried out by using standard methods of morphological and physiological traits (Shirling and Gottlieb 1966). PGP attributes like nitrate reduction, siderophore (You et al. 2005), indole 3-acetic acid (IAA) production (Patten and Glick 1996), nitrate reduction (Glass et al. 1997) and cyanogenesis were carried out according to Schippers et al. (1990) and BIOLOG sugar utilization pattern was performed according to Tripathi et al. (2011). BC activity of Streptomyces isolate against R. solani (NAIMCC-F-01970) were also evaluated according to Khamna et al. (2009). R. solani isolate was maintained on Potato dextrose agar (Himedia, India) at 26 ± 2 °C and 8 mm fungal disc (5 days old) was placed on the mixed agar (1:1, PDA and Chitin agar, pH 6.8). After that, Streptomyces strain (3 days grown on SCA) was streaked on the plate 3 cm away from the fungal disc. All plates were incubated on 26 ± 2 °C for 7 days and percent inhibition were calculated and interaction zone was also analyzed by scanning electron microscope (SEM) (Yuan and Crawford 1995; Malviya et al. 2011).
Streptomyces strain genomic DNA was extracted using modified method (Boudjella et al. 2006) and 16S rRNA gene was amplified using primers fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and Rp2 (5′-AAGGAGGTGATCCAGCCGCA-3′) with PCR amplification kit of Promega. The final volume of reaction mixture (50 µL) contained 1 × PCR buffer (10 mM Tris–HCl, 50 mM KCl, pH 9.0 at 25 °C), 1.5 mM MgCl2, 200 mM of each dNTP, 1 mM of each primer, 0.25U of Taq polymerase and 500 ng of template DNA. The amplification was performed on BioRad thermal cycler (initial denaturation step at 98 °C for 3 min, after which Taq polymerase was added, followed by 30 amplification cycles of 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 2 min). PCR products (approx. 1500 bp) were purified by using QiaexII purification kit (Qiagen) and cloned into the pCR®-XL-TOPO® vector (Invitrogen). Plasmid DNAs were extracted and purified with a QIAprep Spin Miniprep kit (Qiagen) and sequenced by using BigDye terminator chemistry with an automated capillary sequencer (Applied Biosystems). Inserts were sequenced twice on each strand by using vector-specific primers and a suite of 16S rDNA-specific primers to generate an overlapping set of sequences that were assembled into one contiguous sequence using online CAP3 Sequence Assembly Program (Huang and Madan 1999). The contiguous sequences were used for homology search of the 16S rDNA sequences using the BlastN with the sequences deposited in public databases. The identification were based on percentage similarity (>97 % compared with public database sequences, NCBI), by BLAST homology.
Preparation of CC and chitinase assay
Five grams of crab shell chitin (HiMedia, India) was slowly added into 100 mL of 0.25 M HCl with vigorous stirring and kept overnight at 4 °C. The mixture was filtered through glasswool into 200 mL of ice-cold ethanol at 4 °C with rapid stirring. The resultant chitin suspension was centrifuged at 12,000×g for 20 min and the chitin pellets were washed repeatedly with distilled water until the pH became neutral and prepared aqueous CC was lyophilized and 1 % lyophilized powder was used in this study as supplement for plants.
For chitinase assay, fresh culture of S5MW2 isolate (~106 CFU mL−1) was grown in the minimal medium (MM) (containing gL−1: MgSO4·7H2O, 0.2; K2HPO4, 0.9; KCl, 0.2; NH4NO3, 1.0; FeSO4·7H2O, 0.002; MnSO4, 0.002; ZnSO4, 0.002; pH 6.8) supplemented with CC powdered (1 % w:v) and incubated for 15 days at 28 ± 2 °C in flasks. Six individual flasks were used for different time intervals and this experiment was performed in three individual replicates. Minimal broth samples were taken at every 3 days intervals (3, 6, 9, 12 and 15) for the colorimetric estimation of chitinase by using the method of Boller and Mauch (1988) after centrifuging the culture broth in 30 mL Oakridge tubes at 8000×g for 10 min. 1 mL of CC (1 % v:v) in citrate phosphate buffer (0.1 M, pH 6.5) was incubated with 1.0 mL of culture filtrate at 37 °C for 2 h. The resulting chitin oligomers were treated with 2 mL of 4-dimethylaminobenzaldehyde at 37 °C for 20 min and absorbance was measured at 585 nm. N-acetylglucosamine (GlcNAc) served as the standard. One unit of enzyme activity is defined as the amount of enzyme required to produce 0.5 μM mL−1 of N-acetylglucosamine per hour. Specific activity is expressed as Units mL−1.
Inoculum preparation of Streptomycetes
For Streptomycetes inoculum preparation, a loopful pure culture was inoculated in starch casein broth (250 mL) and incubated for 10 days, at 28 ± 2 °C at 150 rpm. The cell suspension was centrifuged at 8000×g and the pellet was washed with liquid suspension containing 2.0 % polyvinylpyrrolidone (PVP), 1.5 % polyethylene glycol (PEG6000) and 2.5 % glycerol and finally liquid suspension (~108 cells mL−1) was used for in vivo study.
Greenhouse experiment
Effect of chitin supplementation on efficiency of S5MW2
The effect of chitin on efficiency of isolate was evaluated under greenhouse condition with or without pathogen. Tomato (var. Navratan) seeds were sown in earthen pots (15 cm dia., 4 seeds/pot) containing sterile saline soil. This experiment was conducted during the winter season, and natural saline soil obtained from the salt affected field of National Bureau of Agriculturally Important Microorganisms, Kusmaur, Mau, Uttar Pradesh, India. Saline soil texture was sandy loam with a pH of 7.6, ECe 6.9 dS m−1, sodium absorption ratio of 76, Organic C 0.63 %, available N 164.7 kg ha−1, available PO4 7.1 kg ha−1 and available K2O 45.3 kg ha−1. After 25 days of germination, thinning was done to maintain two seedlings per pot and twenty pots per treatment. Pots were kept in ambient greenhouse conditions with natural temperature (25 ± 2 °C), 12 h light/dark photoperiod and relative humidity of 85–90 %. For inoculation of R. solani, the inoculum was prepared by following this method: 2 agar discs (1 cm diameter) from an actively growing fungal culture were mixed with 100 g of sterile wheat bran and incubated for 10 days at 24 °C. Wheat bran containing pathogen was mixed at a rate of 1.5 % (w/w) with the same soil with which the pots were filled and then 10 g of the same soil (containing pathogen inoculum) was spread over the surface of the pots having 25 days old plants. After pathogen acclimatization for 3–4 days, the plants were inoculated with actinomycete suspension (5 mL pot−1) alone and with colloidal chitin (10 mL pot−1) with the use of sterilized measuring cylinder. Aqueous solutions of CC (1 % w/v) was prepared in deionized water and added after 24 h of actinomycetes inoculation. Two type of experiments conducted with actinomycete in the greenhouse with or without R. solani and all treatments were as follows: (1) isolate S5MW2, (2) isolate S5MW2 + R. solani, (3) isolate S5MW2 with CC, (4) isolate S5MW2 + R. solani with CC, (5) CC alone, (6) R. solani with CC (7) Untreated control (only water) and (8) R. solani alone.
Disease assessment, physiological parameter and chitinase assay
Forty-five days after inoculation with the isolate S5MW2, the all plants (40 seedlings) were uprooted and washed with distilled water and used for root rot disease assessment. Root rot symptoms were scored by using 0–4 scale (0 = Healthy roots, 1 = 1/3 secondary roots do not exist, 2 = 3/4 secondary roots do not exist, 3 = Primary roots exist, but secondary roots do not exist and 4 = Primary roots do not exist) and disease index (%) was calculated as followed: \(DI\left( \% \right) = \left[ { \sum\nolimits_{di = 4}^{0} {\left( {n \times di} \right)/\left( {N \times 4} \right)} } \right] \times 100\) where: di is the disease scale, n is the number of diseased plants in the index and N is the total number of plants investigated. After disease assessment, all uprooted plants were used for measurement of plant length (cm) and fresh weight (g) (all lateral and primary root were used in measurement). All uprooted tomato plants were gently washed with sterilized distilled water. Fresh leaf samples from washed plants were homogenized in liquid nitrogen, powdered sample (1 g) was used for the estimation of chitinase (Boller and Mauch 1988) and expressed in µg GlcNAc min−1 g−1 fresh weight.
Rhizospheric colonization
To monitor the rhizospheric population of the introduced isolate S5MW2, the soil samples were collected from the actinomycetes inoculated pots (3 cm depth and 2 cm away from tomato stems) at different time interval 5, 15, 30 and 45 days after inoculation. For enumeration of S5MW2, rhizosphere soil (1 g each) of the treatment were serially diluted up to 10−4 in the sterile saline water and 100 µL aliquot was spread on SCA medium supplemented with an antifungal antibiotics nystatin (25 µg mL−1) and actidione (50 µg mL−1). The morphological characteristic of S5MW2 was confirmed based on grey aerial mycelia colour on SCA medium. Enumeration was done with three replicates and all the data collected were pooled for statistical analysis.
Statistical analysis
One-way and two-way ANOVA was applied to determine the significance between different treatments using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Critical difference (P ≤ 0.05) and degree of freedom, F value, p value, critical variance and standard error of means (SEm±) were tabulated.
Results
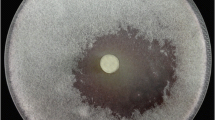
Actinomycetes strain S5MW2 was isolated using starch casein agar medium from water sample of Chilika lake, India and was characterized using cultural, micromorphological and carbon utilization characteristics (Table 1). Based on phenotypic characteristics, actinomycetes strain S5MW2 was identified as Streptomyces species (Figs. 1, 2). Moreover, isolate S5MW2 showed inhibition of R. solani growth up to 65 % in the dual culture plate assay (Table 1; Fig. 1a, b) and mycoparasitic activity of S5MW2 was confirmed by the SEM observation at the interaction zone of S5MW2 and R. solani (Fig. 2b, c). Complete destruction of R. solani mycelia showed coiling and spore proliferation of S5MW2 on the mycelia in the microscopic observations (Fig. 2b, c). Further, this potent strain was identified as Streptomyces vinaceusdrappus on the basis of 99 % similarity with S. vinaceusdrappus strain W8A-43 (HQ889270) by 16S rRNA gene sequencing and sequence was submitted to GenBank, NCBI under the accession number JN400103.
Role of chitinase in the antagonism was evaluated based on chitinase assay. Isolate S5MW2 produced significant amount of chitinase enzyme in minimal broth supplemented with chitin and maximum chitinase production (14.5 ± 0.4 Units mL−1) was recorded after 9 days of inoculation in minimal media showing pH 7.4 (Fig. 3).
After in vitro study, this marine isolate was evaluated in the greenhouse with chitin on tomato plants. Chitin supplementation with S5MW2 significantly (P ≤ 0.05) enhanced PGP parameters, viz., shoot and root length, plant fresh weight, etc. Appreciable level of plant root lengths were recorded from the seedlings treated with Chitin + S5MW2 (with and without pathogen) as compared to the alone and untreated control. Overall, tomato seedlings treated with Chitin + S5MW2 recorded significant (P ≤ 0.05) enhancement in PGP attributes compared with untreated control (Table 2). Similarly, in the in vivo greenhouse experiment the Chitin + S5MW2 treated plants showed vital BC potential against R. solani through higher chitinase accumulation in tomato leaves as compared to infected control. Quantitative estimation of chitinase showed significant enhancement of chitinase activity in healthy tomato plants with chitin supplementation as compared to untreated control (Tables 2, 3). Similarly, maximum control of disease index percent was observed in tomato seedlings that received Chitin + S5MW2, and followed by S5MW2 (71.07 %). Control treatments had 75 % disease index with the majority of plants left completely stunted or dead (Fig. 4). Population of S5MW2 increased in rhizosphere in both conditions with or without pathogen in the presence of CC, however, it reduced with pathogen in the absence of CC (Fig. 5). Maximum population of S5MW2 was observed in the presence of CC only (without pathogen) and followed by CC + S5MW2 + Rs (Fig. 5).
Discussion
There has been increasing interest in the discovery of agriculturally important microbes from rare and uncommon actinomycetes for their exploitation in BC and PGP (Malviya et al. 2011; Patil et al. 2011; Yandigeri et al. 2012). Conventionally, soil-derived actinomycetes have been most frequently screened for PGP and BC (Khamna et al. 2009). But, most of actinomycetes present in marine, desert and forest ecosystems are still unexplored or under explored for their PGP and BC potential (Vijayakumar et al. 2012). Though some reports are available on antibiotic and enzyme production by marine actinomycetes, but marine environment is still a potential source for new actinomycetes, which can yield novel bioactive compounds and industrially important enzymes (Ramesh and Mathivanan 2009). In recent years, plant pathogenic microorganisms are acquiring resistance to chemical fungicides; hence there is an urgent need for search of novel, safe and effective microbe-based biocontrol agents. As a part of our search, we isolated and characterized Streptomycetes strain (S5MW2) from a less explored marine system of Chilika lake, India. Functional characterization of strain S5MW2 for PGP traits demonstrated IAA, HCN production and nitrate reduction. PGP attributes like IAA and HCN were also reported from different rhizospheric actinomycetes (Khamna et al. 2009; Patil et al. 2011). Moreover, antagonism of S5MW2 against R. solani and chitinase enzyme production ability in the presence of CC provided a base for evaluating this biocontrol agent. Some of the previous results on rhizospheric Streptomycetes also correlated with current findings (El-Tarabily and Sivasithamparam 2006; Patil et al. 2011). Antagonistic behavior of actinomycetes against fungi through several mechanisms have been explained using scanning electron microscopic studies (Patil et al. 2011).
Importance of chitinolytic microorganisms in biocontrol of disease causing organisms has also been recognized. Besides many factors, enzymatic action on fungal cell wall seems to play an active role in antagonism (Hoster et al. 2005). Marine isolate S5MW2 was thus evaluated in the greenhouse with CC and resulted in higher growth of plants with S5MW2 treated plants over untreated control. These results showed the PGP ability of this isolate and indole acetic acid production could play an important role in the enhancement of plant growth. These results are in concurrence with ability of Streptomyces from many crop rhizosphere soils to produce IAA and promote plant growth (El-Tarabily and Sivasithamparam 2006; Tsavkelova et al. 2006; Gopalakrishnan et al. 2011). Maruyama et al. (1989) reported previously that marine microbes isolated from sediments produced IAA in presence of tryptophan. In the rhizosphere, root exudates are the natural source of tryptophan for microorganisms, which enhances auxin biosynthesis in the rhizosphere. Furthermore, biocontrol activity of S5MW2 against R. solani also confirmed that the marine isolate could be utilized as biocontrol agent and CC supplementation showed higher PGP parameters as compared to the single treatment and treatment with infected control. Improved control of Rhizoctonia root rot by S5MW2 + CC corresponded with the enhanced survival of the isolates in the soil, though the microclimate changed from marine to terrestrial for the antagonist. S5MW2 colonised easily in the tomato rhizosphere with CC, but with R. solani the colonization is less than other treatment, may be R. solani also produced some inhibitory compounds against the S5MW2 and population was enhanced by CC which could possibly attributed due to lytic enzymes produced by antagonist. Enhanced population of S5MW2 might have created an unfavorable environment for R. solani in soil. Disease control activity of marine actinomycete also correlated with the accumulation of chitinase and its improvement with chitin played the significant role in the leaves of tomato plants. Chitinase enzyme has ability of catalyzing the hydrolysis of chitin to its oligomers (chitooligosaccharides) and/or monomers (N-acetylglucosamine) by terrestrial and marine actinomycetes (Pisano et al. 1992; El-Dein et al. 2010). Chitinolytic enzymes have been widely used in various processes including the agricultural, biological and environmental fields. Several chitinolytic enzymes have been identified in various Streptomyces sp., including, Streptomyces plicatus, S. lividans, S. virdificans, S. halstedii and S. champvatii (El-Dein et al. 2010). In this study, chitinase production was observed in the presence of chitin and it could be possible that oligomers and monomers of chitin might have enhanced the chitinase activity and thus the suppression of R. solani. For instance, the obvious antifungal activity of chitinase from the novel marine Streptomyces strain was proven against Aspergillus nidulans and phytopathogens such as Botrytis cinerea, Fusarium culmorum, Guignardia bidwellii, and Sclerotia sclerotiorum (Hoster et al. 2005). The effect of chitin in inducing resistance either alone or in combination with the biocontrol agents, has been reported against R. solani (Solanki et al. 2011). The observations of Anitha and Rabeeth (2009) also confirm our findings on supplementation of chitin in the antagonistic formulations of Trichoderma spp. But, most of the biocontrol agents and plant growth promoters show good performance in the controlled condition, whereas in field, their performance is affected by soil factors like low organic matter, soil moisture status, soil salinity, soil pH and temperature. This marine habitat isolate S5MW2 possessed potential for salinity tolerance, utilized more than 21 different carbon sources, produced HCN, IAA and chitinolytic activity. These vital characters correlated with its biocontrol potential and survival of the isolate under extreme habitats, and it could be utilized as inoculants to explore the potential applications of microbes in the terrestrial ecosystem. In conclusion, our results showed that the antagonistic activity of a marine S. vinaceusdrappus was directly enhanced by CC and thus had potential in reducing the tomato root rot disease. Kishore et al. (2005) also reported that supplementation of Bacillus circulans GRS 243 and Serratia marcescens GPS5 with 1 % colloidal chitin in the greenhouse reduced lesion frequency of late leaf spot of groundnut caused by Phaeoisariopsis personata by 60 %, when compared with application of bacterial cells alone. Yu et al. (2008) also discussed similar kind of effect by amendment of colloidal chitin with Cryptococcus laurentii against Penicillium expansum in pear fruit. The chitin supplementation with marine actinomycetes offered a effective biocontrol of R. solani under greenhouse. This study also showed the importance of chitinolytic marine actinomycete and its potential. A comparative study on the efficacy of actinomycetes from different ecological origin (rhizospheric soil, aquatic system and arid ecosystem) against many plant pathogens may be explored for possible new applications of less explored microbes.
References
Anitha A, Rabeeth M (2009) Control of Fusarium wilt of tomato by bioformulation of Streptomyces griseus in greenhouse condition. Afr J Basic Appl Sci 1:9–14
Boller T, Mauch F (1988) Colourimetric assay for chitinase. Method Enzymol 161:430–435
Boudjella H, Baute K, Zitoune A, Mathieu F, Lebsehi A, Sabaou N (2006) Taxonomy and chemical characterization of antibiotics of Streptosporangium sg 10 isolated from a Saharan soil. Microbiol Res 161:288–298
El-Dein A, Hosny MS, El-Shayeb NA, Abood A, Abdel-Fattah AM (2010) A potent chitinolytic activity of marine actinomycete sp. and enzymatic production of chitooligosaccharides. Aust J Basic Appl Sci 4:615–623
El-Tarabily KA, Sivasithamparam K (2006) Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem 38:1505–1520
Glass C, Silverstein JA, Denton L (1997) Bacterial populations in activated sludge denitrifying high nitrate waste reflect pH differences. In: Proceedings, second international conference on microorganisms in activated sludge and biofilm processes, Berkely, pp 377–380
Gopalakrishnan S, Pande S, Sharma M, Humayun P, Kiran BK, Sandeep D, Vidya MS, Deepthi K, Rupela O (2011) Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot 30:1070–1078
Hoster F, Schmitz JE, Daniel R (2005) Enrichment of chitinolytic microorganisms: isolation and characterization of a chitinase exhibiting antifungal activity against phytopathogenic fungi from a novel Streptomyces strain. Appl Microbiol Biotechnol 66:434–442
Huang X, Madan A (1999) CAP3: a DNA sequence assembly program. Genome Res 9:868–877
Jeuniaux C, Voss-Foucart MF (1991) Chitin biomass and production in the marine environment. Biochem Syst Ecol 19:347–356
Khamna S, Yokota A, Lumyong S (2009) Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J Microbiol Biotechnol 25:649–655
Kishore GK, Pande S, Podile AR (2005) Chitin-supplemented foliar application of Serratia marcescens GPS 5 improves control of late leaf spot disease of groundnut by activating defense-related enzymes. J Phytopathol 153:169–173
Malviya N, Yadav AK, Yandigeri MS, Arora DK (2011) Diversity of culturable Streptomycetes from wheat cropping system of fertile regions of Indo-Gangetic Plains, India. World J Microbiol Biotechnol 27:1593–1602
Maruyama A, Maeda M, Simidu U (1989) Microbial production of auxin indole-3-acetic acid in marine sediments. Mar Ecol Prog Ser 58:69–75
Pandey B, Ghimire P, Agrawal VP (2004) Studies on antibacterial activity of soil from Khumbu region of mount everest In: International conference on the great himalayas: climate, health, ecology, management and conservation, Kathmandu University, Nepal and the Aquatic Ecosystem Health and Management Society, Canada, p 53
Patil HJ, Srivastava AK, Singh DP, Chaudhari BL, Arora DK (2011) Actinomycetes mediated biochemical responses in tomato (Solanum lycopersicum) enhances bioprotection against Rhizoctonia solani. Crop Prot 30:1269–1273
Patten C, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Pisano MA, Sommer MJ, Taras L (1992) Bioactivity of chitinolytic actinomycetes of marine origin. Appl Microbiol Biotechnol 36:553–555
Ramesh S, Mathivanan N (2009) Screening of marine actinomycetes isolated from the Bay of Bengal, India for antimicrobial activity and industrial enzymes. World J Microbiol Biotechnol 25:2103–2111
Robbins PW, Albright C, Benfield B (1998) Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem 263:443–447
Schippers B, Bakker AW, Bakker PAHM, Peer R (1990) Beneficialand deleterious effects of HCN-producing pseudomonads on rhizosphere interactions. Plant Soil 129:75–83
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Solanki MK, Singh N, Singh RK, Singh P, Srivastava AK, Kumar S, Kashyap PL, Arora DK (2011) Plant defense activation and management of tomato root rot by a chitin-fortified Trichoderma/Hypocrea formulation. Phytoparasitica 39:471–481
Solanki MK, Kumar S, Panday AK, Srivastava S, Singh RK, Kashyap PL, Srivastava AK, Arora DK (2012) Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol Sci Technol 22:203–217
Szczech M, Shoda M (2006) The effect of mode of application of Bacillus subtilis RB14C on its efficacy as a biocontrol agent against Rhizoctonia solani. J Phytopathol 154:370–377
Tripathi BM, Kaushik R, Kumari P, Saxena AK, Arora DK (2011) Genetic and metabolic diversity of streptomycetes in pulp and paper mill effluent treated crop fields. World J Microbiol Biotechnol 27:1603–1613
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI (2006) Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol 42:117–126
Vijayakumar R, Gopika G, Dhanasekaran D, Saravanamuthu R (2012) Isolation, characterisation and antifungal activity of marine actinobacteria from Goa and Kerala, the west coast of India. Arch Phytopathol Plant Prot 45:1010–1025
Yandigeri MS, Meena KK, Singh D, Malviya N, Singh DP, Solanki MK, Yadav AK, Arora DK (2012) Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul 68:411–420
You JL, Cao LX, Liu GF, Zhou SN, Tan HM, Lin YC (2005) Isolation and characterization of actinomycetes antagonistic to pathogenic Vibrio spp. from nearshore marine sediments. World J Microbiol Biotechnol 21:679–682
Yu T, Wang L, Yin Y, Wang Y, Zheng X (2008) Effect of chitin on the antagonistic activity of Cryptococcus aurentii against Penicillium expansum in pear fruit. Int J Food Microbiol 122:44–48
Yuan WM, Crawford DL (1995) Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl Environ Microbiol 61:3119–3128
Acknowledgments
This work was funded by the Indian Council of Agriculture Research (ICAR) by a network project ‘Application of Microorganisms in Agriculture and Allied Sectors (AMAAS)’. The help of the culture collection unit of NBAIM is highly appreciated for providing cultures for this study. All the co-authors are acknowledged for help in execution of experiments, data collection, analysis and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yandigeri, M.S., Malviya, N., Solanki, M.K. et al. Chitinolytic Streptomyces vinaceusdrappus S5MW2 isolated from Chilika lake, India enhances plant growth and biocontrol efficacy through chitin supplementation against Rhizoctonia solani . World J Microbiol Biotechnol 31, 1217–1225 (2015). https://doi.org/10.1007/s11274-015-1870-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1870-x