Abstract
Drought tolerant endophytic actinobacteria Streptomyces coelicolor DE07, S. olivaceus DE10 and Streptomyces geysiriensis DE27 were isolated from cultivated plants of arid and drought affected regions of Rajasthan, India. These isolates exhibited plant growth promotion traits and intrinsic water stress tolerance from −0.05 to −0.73 MPa. Maximum auxin production was observed in majority of actinobacterial cultures in the logarithmic to stationary phase of growth. Significant enhancement of wheat seedling vigour was recorded by the inoculation of these endophytic actinobacteria. S. olivaceus DE10 recorded maximum accumulation of indole 3-acetic acid (84.34 μg mg−1 protein). Culture and cell-free extract of the endophytes was applied on to wheat seeds to assess the effect on growth in water-stressed soil. Maximum yield was recorded with the inoculation of S. olivaceus DE10 culture (492.77 kg ha−1) and cell-free extract (262.31 kg ha−1). Co-inoculation of S. olivaceus DE10 + S. geysiriensis DE27 recorded highest yield of 550.09 kg ha−1 while their cell-free extract yielded 524.92 kg ha−1. Overall, wheat seeds treated with cultures showed better plant growth and yield in comparison to control. Direct coating of cultures on seeds yielded better performance than cell-free extract coated on seeds and co-inoculation of cultures or cell-free extract proved better than single culture inoculations. Production of phytohormones, plant growth promotion traits combined with water stress tolerance potential in these endophytic actinobacteria played a cumulative synergistic role that supported enhanced plant growth promotion of wheat in the stressed soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water deficit is among the most common environmental stresses to affect plant growth and influence crop quality and productivity in the arid and semiarid regions (Jones 2009). Plants growing under detrimental environmental conditions, such as those occurring in arid and semiarid soils, undergo water limitation and nutrient deficiencies. Rhizosphere microorganisms are adapted to adverse conditions and may compensate for such detrimental conditions (Ruiz-Lozano et al. 1996). Inoculation with native beneficial microorganisms may increase drought tolerance of plants growing in arid or semiarid areas (Marulanda et al. 2007). Endophytic microorganisms reside within living plant tissues at some phases of their life cycle without causing apparent damage to them (Petrini 1991). They can be extracted from inner plant parts or isolated from surface-disinfested plant tissues (Hallmann et al. 1997). Endophytic bacteria and filamentous fungi have been widely reported from seeds, roots, stems, leaves, needles, twigs and barks of various plant species and the role of endophytic microbial community in endophyte-plant associations has been intensively discussed (Seena and Sridhar 2004; Reinhold-Hurek and Hurek 2011). Root-colonizing non-pathogenic endophytic bacteria can increase plant resistance to biotic and abiotic stress factors (Dimkpa et al. 2009).
Plant-associated microorganisms are involved in symbiotic and associative microbial activities that help in plants to establish in their environment (Morrissey et al. 2004). These are economical and safer source of nutrition for increasing agricultural production and improving soil fertility. Suggested mechanisms for microbe associated plant growth include production of cytokinins, gibberellins, indole-3-acetic acid (IAA, an auxin), increased mineralization and nitrogen availability in the soil (Coombs and Franco 2003). Indole-3-acetic acid is quantitatively the most abundant phytohormone secreted by most plant-associated bacteria and play positive role in plant growth. IAA biosynthesis is related to environmental stress, including acidic pH, osmotic and matrix stress, and carbon limitation (Spaepen et al. 2007).
Microbial endophytes include both commensal microorganisms that have no direct effect on plants and beneficial microorganisms that could be used for the benefits of plants (Procopio et al. 2009; Shi et al. 2010). The role of endophytic microorganisms in the promotion of plant growth has received increasing attention in recent years as the introduction and/or manipulation of endophytic microbial population may provide a consistent and effective enhancement in the productivity of crops (Morrissey et al. 2004; Shi et al. 2010).
Actinobacteria are constantly found in the phytosphere (Shirokikh et al. 2006; Norovsuren et al. 2007; Merzaeva and Shirokikh 2006). They are the exciting novel sources of bioactive compounds and have been reported from several hosts such as tomato, banana, wheat, and maize with promising anti-microbial activity against pathogenic strains (Coombs and Franco 2003; Cao et al. 2004; Castillo et al. 2007) and root endophytic actinobacteria play an important role in plant development (Verma et al. 2009). Their involvement in the phytohormonal regulation of plant growth (Khamna et al. 2010; Shi et al. 2010) has received little attention in relation to crop plants under environmental stresses. The relevant literature contains just sparse references to the ability of the representatives of the genera Streptomyces, Micromonospora, Corynebacterium, Frankia, Mycobacterium (Khamna et al. 2010), and Rhodococcus (Tsavkelova et al. 2005) in plant growth promotion. Their presence as endophytic symbiont with plant and ability to help plants in coping with the detrimental soil conditions like drought has not gained much attention although actinobacterial existence with the plants is an established phenomenon (Khamna et al. 2010). This versatility of endophytic actinobacteria encouraged us to explore these organisms from arid and drought affected regions for their utilization in alleviating drought stress in wheat (Triticum aestivum) crop.
Materials and methods
Site description and sample collection
The plant samples were collected from arid and drought-affected fields of Bikaner and Jaisalmer, Rajasthan, India. The roots from five plant species Bui (Aerva tomentosa, Amaranthaceae), Keeker (Acacia nilotica, Mimosaceae), Kheep (Leptadenia pyrotechnica, Asclepiadaceae), Phog (Calligonum polygonides, Polygonaceae) and Bajra (Pennisetum glaucum, Poaceae) were collected from Beechwal (28°04′N,73°20′E), Arjanser (28°56′N,73°52′E), Napaser (27°57′N,73°33′E) in Bikaner and Sam (26°49′N,70°30′E) village in Jaisalmer, India. The samples were kept in sterile polythene bags and transported to laboratory in Styrofoam boxes maintained at 4 °C.
Isolation and characterization of endophytic actinobacteria
Plants roots were thoroughly washed by a sonication step to dislodge soil and organic matter, dipped in Tween 20 for 5 min and sterilized by sequential immersion in 70 % ethanol for 5 min and in a solution of sodium hypochlorite (0.9 % available chlorine) for 20 min (Coombs and Franco 2003). Samples were washed (3×) in sterile water and soaked in 10 % NaHCO3 solution for 10 min to disrupt plant tissues and inhibit the growth of endophytic fungi (Baker 1990). After washing for three times for 15 min in sterile distilled water, roots were homogenized with necessary dilutions (10−2), plated on petriplates containing three different media (Actinomycetes isolation agar, Starch casein agar, Humic acid-Vitamin agar) with 50 μg ml−1 each of cycloheximide and nalidixic acid and incubated at 32 °C for 7–15 days. Actinobacterial colonies appearing on isolation plates were picked on the basis of colony morphology and diffusible pigments and characterized for plant growth promoting (PGP) attributes like ammonia (Cappuccino and Sherman 1992), siderophore (Schwyn and Neilands 1987) and IAA production (Brick et al. 1991). In addition to PGP attributes, lysozyme sensitivity, urea and casein hydrolysis, gelatin degradation and hydrogen sulphide production were also tested according to Cappuccino and Sherman (1992). To study the drought tolerance ability of actinomycetes at various water stress levels, Actinomycetes isolation agar (AIA) plates with different water potentials (−0.05, −0.15, −0.30, −0.49, and −0.73 MPa) were prepared by adding filter sterilized 5–25 % polyethylene glycol (PEG6000) (Michel and Kaufmann 1973) and was allowed to dry overnight in laminar airflow chamber. The plates were inoculated with actinobacterial cultures and incubated at 32 °C for 1–2 weeks under continuous observation. Growth of isolates at various stress levels was recorded. All experiments were repeated three times and pooled data was analyzed.
Scanning electron microscopy
Scanning electron microscopy was carried out to study the mycelial morphology and root colonization. Germinated seedlings with mycelia were taken (after 10 days of sowing) and washed in 0.1 M sodium cacodylate buffer (pH 7.4). Mycelia were fixed in 2.5 % glutaraldehyde in 0.1 M sodium cacodylate buffer for 4 h at 4 °C followed by post-fixation with 1 % OsO4 in 0.1 M sodium cacodylate buffer (pH 7.4) and dried in a critical point dryer (EMITECH model K850, Hitachi). The preparations were mounted onto aluminium holders, sputter-coated with 10 nm Au and observed by Scanning electron microscope (Hitachi model S3400 at 15–30 kV, 2–5.00 μm).
Identification of endophytic actinobacteria
Genomic DNA of actinobacterial isolates was extracted by the modified protocol of Boudjella et al. (2006). 16S rRNA gene was PCR amplified using two universal primer pairs fD1 (5′-GAGTTTGATCCTGGCTCA-3′) and Rp2 (5′-CGGCTACCTTGTTACGACTT-3′) according to the method of Malviya et al. (2011). The forward and reverse rDNA sequences were joined into contiguous sequence using CAP3 Sequence Assembly Program (http://pbil.univ-lyon1.fr/cap3.php). The 16S rRNA sequences were identified by comparing the partial 16S rRNA sequences of the isolated strains with those available in the GenBank database using BLASTn program. A total of 3 sequences of 16S rRNA gene were deposited in public databases (GenBank, NCBI) under the accession numbers JN204723, JN204724 and JN204725, and the cultures were deposited under the accession number B-1006, B-1007 and B-1008 at the National Agriculturally Important Microbial Culture Collection, National Bureau of Agriculturally Important Microorganisms, Maunath Bhanjan, Uttar Pradesh, India.
Effect of water stress on growth kinetics
Effect of water stress on the growth kinetics and IAA production by endophytic actinobacteria was investigated using Bioscreen-C Micro Biology Reader (Labsystems). Actively growing culture (~106 CFU ml−1) was inoculated in −0.73 MPa concentration of polyethylene glycol (PEG6000) in liquid media according to manufacturer’s instructions and subjected to growth kinetics analysis (absorbance 600 nm) at 24 h interval. The liquid media without PEG6000 served as control for each culture. The experiment was repeated thrice and pooled data was used for analysis.
Extraction of IAA
Cultures were tested for IAA production with same inoculum size using the procedure of Brick et al. (1991). The content of IAA produced from each isolate at different phase of growth was determined using high performance liquid chromatography (HPLC). Seven selected isolates were grown in 10 ml of GYMA broth (gl−1: 4.0 g glucose; 4.0 g yeast extract; 10.0 g malt extract) in 3 replicates and allowed to grow in incubator shaker at 32 °C for 15 days. Supernatant was collected by centrifugation at 10,000×g for 10 min at every 24 h from each replicate, added with equal volume of ethyl acetate in screw cap tubes, rigorously shaken for 2 h on a gyratory shaker (50 rpm) and the content was allowed to settle for 1 h at 37 °C. The ethyl acetate extract was finally air dried and re-dissolved in methanol (HPLC grade) prior to HPLC analysis.
HPLC analysis
HPLC analysis was performed on HPLC system (Waters, USA) equipped with binary 515 reciprocating pumps, variable photodiode array (PDA) detector (Waters 2996), and system controller equipped with Waters® Empower™ software for data integration and analysis. Reverse phase analysis was carried out in isocratic conditions on C-18 column (250 × 4.6 mm i.d., 5 μm particle size) at 25 ± 1 °C and detection at 254 and 280 nm. IAA was analyzed at a flow rate of 1 ml min−1 of methanol: 1 % aqueous acetic acid (60:40, v/v). Samples were filtered through a 0.45 μm membrane filter prior to injection in the sample loop (injection volume 10 μl). HPLC grade solvents and chemicals (EMerck and HiMedia, India) were used throughout the analysis. Qualitative characterization of the compounds in the sample was done by comparing retention time (Rt) and co-injection while quantitative analysis was performed by comparing peak areas of the standard compounds obtained from HiMedia, India.
Plant growth under drought stress
Seedling vigour assay
Wheat seeds (cultivar WR-544) of late variety sensitive to drought stress obtained from Directorate of Seed Research, Maunath Bhanjan, Uttar Pradesh, India were used for field experiments including seedling vigour assays. Seeds were surface sterilized with a mixture of 3 % hydrogen peroxide and 96 % ethanol (1:1), coated with actinobacterial culture (~108 cells ml−1) and cell-free extract using 1 % carboxymethyl cellulose (CMC) as binder. Coated seeds were dried overnight and sown in a plastic pot filled with sterilized soil in a greenhouse. The plants were uprooted at 24 h intervals after 2 days of germination for measurement of root and shoot length. The seedling vigour indices were calculated after 10 days using the formula: Seedling vigour index = (mean root length + mean shoot length) × germination (%). The experiment was repeated three times and the data was pooled before statistical analysis.
Field experiments
Field experiment was conducted in drought affected field of Directorate of Seed Research, Maunath Bhanjan, Uttar Pradesh, India. The air-dried and sieved (<2 mm) field soil possessed following physico-chemical parameters: sand 71 %, silt 3 %, and clay 26 % with bulk density 1.60 mg m−3, total porosity 38.9 %, and water holding capacity 36.9 %; pH 8.8, EC 4.6 dS m−1, organic C 0.41 %, total N 0.14 %, and Olsen P 101 mg per 100 g soil. Surface sterilized, actinobacteria and cell-free extract coated wheat seeds were sown in the field in randomized complete block design (RCBD). Surface-sterilized non-inoculated seeds served as control. Two independent RCBD experimental fields were maintained for culture and cell-free extract coated seeds. Fertilizer application and other package of practices were carried out till harvest of the crop. Experimental field with wheat crop received irrigation only at the germination stage to maintain the stress level. After harvesting, shoot and root length, fresh shoot and root weight of the plants along with their dry weight, number of tillers and panicles and yield were recorded. The field experiment was repeated three times and pooled data was used for analysis.
Statistical analysis
One-way ANOVA was applied to determine the significance between different treatments using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Critical difference (P ≤ 0.05) and standard error of means (SEM) were tabulated. Mean separations were calculated based on the mean rankings at P ≤ 0.05 using Duncan’s Multiple Range Test.
Results
Isolation and screening of drought tolerant endophytic actinobacteria
Fourty six morphotypes of endophytic actinobacteria were isolated from drought tolerant plant roots. Most of the endophytes possessed dry colonies with varying colour of aerial and substrate mycelium. Only 19 morphotypes were able to show growth under −0.73 MPa (25 % PEG) water stress condition. Growth kinetics of water stress tolerant isolates indicated normal growth curve with typical life cycle of 6–8 days. All isolated actinobacteria showed different biochemical attributes like lysozyme sensitivity, urea and casein hydrolysis, gelatin degradation and hydrogen sulphide production. Among the endophytes 7 isolates DE07, DE10, DE20, DE27, DE39, DE46 and DE52 that showed significant growth and IAA production at −0.73 MPa of PEG6000 were chosen for seedling vigour assay and colonization studies (Supplementary table).
Seedling vigour assay and colonization
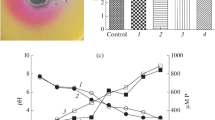
Among the seven actinobacterial isolates, DE07, DE10 and DE27 showed higher seedling vigour with cell treatment as compared to cell-free extracts (Fig. 1). Maximum seedling vigour was recorded with isolate DE10, followed by DE07 and DE27 and showed significant vigour over the control. Out of seven actinobacterial cell-free extracts, only DE10 cell-free extracts showed significant vigour over control. Isolate DE20 showed decrease in seedling vigour in comparison to culture extract. Over all the cultures DE07, DE10 and DE27 increased the seedling vigour (P ≤ 0.05) significantly in comparison to other isolates with cell culture. Scanning electron microscope studies also showed actinobacterial colonization on the root surface by the isolate DE07, DE10 and DE27, where actinobacteria were connected together by an extracellular polymeric matrix colony formation under water stress conditions (Fig. 2).
Seedling vigour indices of culture and cell free extracts of actinobacteria treated wheat seeds at 10 days after inoculation. Control: seedlings without treatment of culture or cell free extracts of actinobacteria, DE07: Streptomyces coelicolor DE07, DE10: S. olivaceus DE10, DE27: S. geysiriensis DE27, DE20: Streptomyces sp. DE20, DE39: Streptomyces sp. DE39, DE46: Streptomyces sp. DE46, DE52: Streptomyces sp. DE52. Experiment was repeated three times and mean with different letters on the top of error bars indicate statistically different values at P ≤ 0.05 using Duncan’s Multiple Range Test (DMRT). Seedling vigour index = (mean root length + mean shoot length) × germination (%)
Scanning electron microscopy (SEM) of actinobacteria isolates, showing variations in spore chain morphology of a Streptomyces coelicolor DE07, b S. olivaceus DE10, c S. geysiriensis DE27 and wheat root colonization with d Streptomyces coelicolor DE07, e S. olivaceus DE10 and f S. geysiriensis after 10 days of inoculation. Thin arrows represent the wheat roots and thick arrows represent endophytic actinobacterial colonization on roots
Identification of endophytic actinobacteria
BLASTn homology search of NCBI for 16S rRNA gene sequences of all the three strains were confirmed as Streptomyces sp. The strain DE10 had 100 % similarity with Streptomyces olivaceus whereas strains DE07 and DE27 showed 99 % similarity with Streptomyces coelicolor and S. geysiriensis. The gene sequences of DE07, DE10 and DE27 were submitted to GenBank (NCBI) with accession numbers JN204723, JN204724 and JN204725, respectively (Table 1). The molecular identification was further confirmed on the basis of morphological characteristics using scanning electron microscopy. The scanning electron microscopic observations ascertained that spores of all the three actinobacteria were spirals and loops forms (Fig. 2) with characteristic aerial mycelium, type I cell wall (LL-DAP and without characteristics sugars) indicating that they belonged to the Streptomyces genus.
Actinobacterial IAA synthesis in water stress condition
Quantitative IAA estimation at different time intervals resulted through growth kinetics and IAA synthesis in liquid culture demonstrated that actinobacterial isolates grew well in normal conditions and synthesized IAA significantly as compared to water stress conditions (Fig. 3). Isolate DE07 and DE10 synthesized and accumulated IAA in the logarithmic phase whereas isolate DE27 produced IAA after stationary phase of growth till to death phase under normal conditions. However, isolate DE07 and DE27 produced highest IAA under water stress condition till end of logarithmic phase of growth, whereas DE10 produced highest IAA in the midst of logarithmic phase. In general cultures showed increased IAA production under normal conditions, and decreased IAA production under water stress conditions after peak growth phase. DE27 showed minimum accumulation in normal and water stress condition in comparison to other isolates.
Plant growth under drought stress
All the treatments with actinobacterial inoculation (single and combined) were significant (P ≤ 0.05) in comparison to control for all the studied growth and yield parameters. Among all the treatments, S. geysiriensis DE10 culture treated plants recorded maximum shoot length, number of tillers and panicles, fresh shoot and root weight, dry shoot and root weight, and yield. In combined inoculations, S. coelicolor DE07 + S. geysiriensis DE27 resulted highest plant growth through root length, number of tillers and panicles, fresh shoot and root weight and dry shoot weight. Highest yield was recorded with combined inoculation of S. olivaceus DE10 + S. geysiriensis DE27 cultures and lowest yield with S. coelicolor DE07 + S. geysiriensis DE27 in comparison to control (242 kg ha−1). Combined inoculations were better in yield as compared to single inoculations and cell culture treatments were better than cell-free extract treated seeds (Table 2).
Discussion
Beneficial plant–microbe interactions, impact of microbial inoculation on plant growth and differential mechanisms underlying growth promotion under stress conditions are documented (Yang et al. 2008; Meena et al. 2010; Jha et al. 2011). Bacteria isolated from different stressed habitats possess stress tolerance along with the plant growth-promoting traits and therefore are potential candidates for seed treatment (Tiwari et al. 2011). Impact assessment of drought tolerant actinobacteria on plant growth promotion and underlying biochemical mechanisms is scarcely documented, and therefore, need for special attention because of the potential intrinsic properties of actinobacteria isolated from drought soil ecosystems.
Water stress tolerant morphotypes of endophytic actinobacteria isolated from roots of five plant species produced dry powdery colonies on plates and possessed morphological characteristics of Streptomyces genus that has been the predominant genera of endophytes in the majority of hosts (Sardi et al. 1992; Coombs and Franco 2003). Among 46 morphotypes, nineteen were capable of producing IAA in the growth medium (Supplementary table). Majority of actinobacteria and coryneform bacteria associated with plants have been reported to produce auxins (Merzaeva and Shirokikh 2006). Seven endophytes producing relatively high levels of IAA enhanced root and shoot growth in wheat seedlings and out of seven three actinobacteria (DE07, DE10 and DE27) enhanced maximum seedling vigour and produced maximum auxin. Molecular characterization of these potent isolates confirmed their identification as S. coelicolor DE07, S. olivaceus DE10 and S. geysiriensis DE27 with 99, 100 and 99 % similarity (Table 1), which was further validated by scanning electron microscopic studies (Fig. 2). Cell-free extracts showed relatively more enhanced seedling vigour over culture as in case of DE20 (Fig. 1). Scanning electron microscopy of isolate DE07, DE10 and DE27 revealed successful actinobacterial colonization on the root surface by an extracellular polymeric matrix colony formation under water stress conditions (Fig. 2). Possible reasons for decreased vigour of DE20 culture coated seedlings over the extracts might be attributed to relatively slow colonization of actinobacteria on the roots. Similarly, root exudates have definite selective and promoting effects on specific microbial population (Hartmann et al. 2009) and actinobacteria, being a slow grower in the soil, may colonize roots successfully but slowly. Since, Streptomyces species isolated from unexplored and underexplored habitats are reported to be a rich source of bioactive compounds (Bull et al. 2005; Fiedler et al. 2005), the coating of cell-free extracts of actinobacteria to plant seeds may serve the purpose of potent actinobacterial inoculation in a more pronounced manner.
Comparison of biomass and IAA concentration in liquid culture revealed that maximum auxin accumulation observed in the logarithmic phase to stationary growth phase (Fig. 3), which corresponded well with other reports on the dependence of auxin synthesis on the growth phase of cultures (Muronets et al. 1997; Tsavkelova et al. 2005).
Microbial population as natural bio-inoculants is advantageous, not only from the economical but also from the ecological point of view (Diby et al. 2005). Some microbes live inside plants for at least a part of their life cycle as endophytes conferring on the host benefit such as stress reduction, increased root growth and nutrient availability (Hardoim et al. 2008). Microbial inoculations significantly alter the rhizospheric changes particularly in the small molecules like sugars, amino acids, fatty acids, flavonoids and strigolactones, as well as various classes of proteins leading to interesting modification in the plant (Badri and Vivanco 2009). In the rhizosphere, plant–microbe interactions are interdependent as plants exude a wide array of organic and inorganic compounds (Lugtenberg and Kamilova 2009). In turn, microbes inhabiting the rhizosphere release phytohormones, small molecules, or volatile compounds which regulate plant growth and root morphogenesis (Castro-Sowinski et al. 2007). Although growth and development of plants is reduced in stress conditions due to damaged biochemical and physiological mechanisms, such stresses may be relieved to some extent by the application of microbial inoculants which evoke various natural mechanisms to help plants sustain their growth under stress conditions (Yang et al. 2008). In the present study, single as well as combined inoculation of actinobacteria performed better plant growth promotion in comparison to control; combined inoculation of microbes has always been proved to be more effective than single inoculation in plant growth promotion as is also reported in this study (Meena et al. 2010; Jha et al. 2011). It is interesting to note that wheat seeds coated with cell-free extracts yielded reduced plant growth parameters in comparison to culture inoculation (Table 2) except in case of DE20. Long-term effect of actinobacterial live cultures colonized on roots served as a continuous source of phytohormones throughout different plant growth stages rather than one time initial application of cell-free extracts (Shakirova et al. 2003). Endophytic actinobacteria S. geysiriensis DE27, S. coelicolor DE07 and S. olivaceus DE10 showed increase in shoot and root length, number of tillers and panicles, biomass, and yield of wheat crop under field conditions (Table 2). These observations are in accordance with the role of endophytic actinobacteria to produce auxins and enhance drought tolerance in plants (Hasegawa et al. 2004; Meguro et al. 2006). Our results support the hypothesis that microbial colonization induce different physical and biochemical changes in plants with enhanced plant growth under stressed soil conditions. Endophytic actinobacteria have been implied in the production of metabolites that affect plant’s life directly by affecting physiology of the host plants (Hasegawa et al. 2004; Meguro et al. 2006) which is correlated with the results of current investigation. The plant–microbe and microbe–microbe interactions are complex and dependent on multiple traits. The strong promotion of growth and yield of wheat plants due to combined inoculation may serve as a new finding to improve crop productivity, particularly for dry soils, using stress-tolerant actinobacteria.
References
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Baker D (1990) Methods for the isolation, culture and characterization of the Frankiaceae: soil actinomycetes and symbionts of actinorhizal plants. In: Labeda DP (ed) Isolation of biotechnological organisms from nature. McGraw-Hill Publishing Company, New York, pp 213–236
Boudjella H, Baute K, Zitoune A, Mathieu F, Lebsehi A, Sabaou N (2006) Taxonomy and chemical characterization of antibiotics of Streptosporangium Sg10 isolated from a Saharan soil. Microbiol Res 161:288–298
Brick JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Bull AT, Stach JEM, Ward AC, Goodfellow M (2005) Marine actinobacteria: perspectives, challenges and future directions. Antonie Van Leeuwenhoek 87:65–79
Cao LX, Qiu ZQ, You JL, Tan HM, Zhou S (2004) Isolation and characterization of endophytic Streptomyces antagonists of Fusarium wilt pathogen from surface sterilized banana roots. FEMS Microbiol Lett 247:147–152
Cappuccino JG, Sherman N (eds) (1992) Microbiology: a laboratory manual, 3rd edn. Rockland Community College, Suffern, New York
Castillo UF, Browne L, Strobel G, Hess WM, Ezra S, Pacheco G, Ezra D (2007) Biologically active endophytic streptomycetes from Nothofagus spp. and other plants in Patagonia. Microb Ecol 53:12–19
Castro-Sowinski S, Herschkovitz Y, Okon Y, Jurkevitch E (2007) Effects of inoculation with plant growth-promoting rhizobacteria on resident rhizosphere microorganisms. FEMS Microbiol Lett 276:1–11
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol 69:5603–5608
Diby P, Sarma YR, Srinivasan V, Anandraj M (2005) Pseudomonas fluorescens mediated vigour in black pepper (Piper nigrum L.) under greenhouse cultivation. Ann Microbiol 55:171–174
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32:1682–1694
Fiedler HP, Bruntner C, Bull AT, Goodfellow M, Potterat O, Puder C, Mihm G (2005) Marine actinomycetes as a source of new secondary metabolites. Antonie Van Leeuwenhoek 87:37–42
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hardoim PR, Overbeek V, Leo S, Elsas DJV (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Hasegawa S, Meguro A, Nishimura T, Kunoh H (2004) Drought tolerance of tissue-cultured seedlings of mountain laurel (Kalmia latifolia L.) induced by an endophytic actinomycete. I. Enhancement of osmotic pressure in leaf cells. Actinomycetologica 18:43–47
Jha A, Sharma D, Saxena J (2011) Effect of single and dual phosphate-solubilizing bacterial strain inoculations on overall growth of mung bean plants. Arch Agron Soil Sci 1–15
Jones MG (2009) Using resources from the model plant Arabidopsis thaliana to understand effects of abiotic stress. Salin Water Stress 44:129–132
Khamna S, Yokota A, Peberdy JF, Lumyong S (2010) Indole-3-acetic acid production by Streptomyces isolated from some Thai medicinal plant rhizosphere soils. EurAsian J Biosci 4:23–32
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Malviya N, Yadav AK, Yandigeri MS, Arora DK (2011) Diversity of culturable Streptomycetes from wheat cropping system of fertile regions of Indo-Gangetic Plains, India. World J Microbiol Biotechnol 27:1593–1602
Marulanda A, Porcel R, Barea JM, Azcon R (2007) Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb Ecol 54:543–552
Meena KK, Mesapogu S, Kumar M, Yandigeri MS, Singh G, Saxena AK (2010) Co-inoculation of the endophytic fungus Piriformospora indica with the P-solubilizing bacteria Pseudomonas striata affects population dynamics and plant growth in chickpea. Biol Fertil Soils 46:169–174
Meguro A, Ohmura Y, Hasegawa S, Shimizu M, Nishimura T, Kunoh H (2006) An endophytic actinomycete, Streptomyces sp. MBR-52, that accelerates emergence and elongation of plant adventitious roots. Actinomycetologica 20:1–9
Merzaeva OV, Shirokikh IG (2006) Colonization of plant rhizosphere by actinomycetes of different genera. Microbiology 75:226–230
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Physiol 51:914–916
Morrissey JP, Dow JM, Mark GL, O’Gara F (2004) Are microbes at the root of a solution to world food production? Rational exploitation of interactions between microbes and plants can help to transform agriculture. EMBO Rep 5:922–926
Muronets EM, Belavina NV, Mitronova TN, Kameneva SV (1997) Synthesis of indole-3-acetic acid by the saprophytic plant-associated bacterium Agrobacterium radiobacter. Microbiology 66:423–428
Norovsuren Zh, Zenova GM, Mosina LV (2007) Actinomycetes in the rhizosphere of semidesert soils of Mongolia. Eurasian Soil Sci 40:415–418
Petrini O (1991) Fungal endophytes in tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Springer, New York, pp 179–197
Procopio REL, Araujo WL, Maccheroni W Jr, Azevedo JL (2009) Characterization of an endophytic bacterial community associated with Eucalyptus spp. Genet Mol Res 8:1408–1422
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443
Ruiz-Lozano JM, Azcon R, Palma JM (1996) Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. New Phytol 134:327–333
Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S (1992) Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl Environ Microbiol 58:2691–2693
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Seena S, Sridhar KR (2004) Endophytic fungal diversity of 2 sand dune wild legumes from the southwest coast of India. Can J Microbiol 50:1015–1021
Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR (2003) Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci 164:317–322
Shi Y, Lou K, Li C (2010) Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynth Res 105:5–13
Shirokikh IG, Zenova GM, Merzaeva OV, Lapygina EV, Lysak LV (2006) Number and structure of actinomycetes complexes in the rhizosphere winter rye, oat and red clover. Izv Akad Nauk Ser Biol 4:496–501
Spaepen S, Versees W, Gocke D, Pohl M, Steyaert J, Vanderleyden J (2007) Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J Bacteriol 189:7626–7633
Tiwari S, Singh P, Tiwari R, Meena KK, Yandigeri M, Singh DP, Arora DK (2011) Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol Fertil Soils 47:907–916
Tsavkelova EA, Cherdyntseva TA, Netrusov AI (2005) Auxin production by bacteria associated with orchid roots. Mikrobiologiia 74:55–62
Verma VC, Gond SK, Kumar A, Mishra A, Kharwar RN, Gange AC (2009) Endophytic actinomycetes from Azadirachta indica A. Juss.:isolation, diversity, and anti-microbial activity. Microb Ecol 57:749–756
Yang J, Kloepper JW, Ryu CM (2008) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Acknowledgments
Authors are thankful to the Indian Council of Agricultural Research (ICAR), New Delhi, India for financial assistance under the Network project ‘Application of Microorganisms in Agriculture and Allied Sectors’ (AMAAS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yandigeri, M.S., Meena, K.K., Singh, D. et al. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul 68, 411–420 (2012). https://doi.org/10.1007/s10725-012-9730-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9730-2