Abstract
Opuntia ficus-indica Mill. (forage cactus) is farmed with relative success in the semi-arid region of the Brazilian northeast for commercial purposes, particularly as forage and food. Endophytic microorganisms are those that can be isolated inside plant tissues and can be a new source to production of enzymes with different potentialities. The objective of this study was to describe the richness of endophytic fungi from O. ficus-indica and to detect the capacity of these species to produce extracellular hydrolytic enzymes. Forty-four endophytic fungi species were isolated. Among them, the most commonly found were Cladosporium cladosporioides (20.43%) and C. sphaerospermum (15.99%). Acremonium terricola, Monodictys castaneae, Penicillium glandicola, Phoma tropica and Tetraploa aristata are being reported for the first time as endophytic fungi for Brazil. The majority of isolated fungi exhibited enzymatic potential. Aspergillus japonicus and P. glandicola presented pectinolytic activity. Xylaria sp. was the most important among the other 14 species with positive cellulase activity. All 24 isolates analysed were xylanase-positive. Protease was best produced by isolate PF103. The results indicate that there is a significant richness of endophytic fungi in O. ficus-indica, and that these isolates indicate promising potential for deployment in biotechnological processes involving production of pectinases, cellulases, xylanases and proteases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endophytic microorganisms are those that spend all or part of their life cycle colonizing the interior of tissues in host plants without causing them any apparent harm (Tan and Zou 2001). These microorganisms have been described as protectors against the attack of other microorganisms, insects, and herbivore animals, in addition to producing phyto-hormones, enzymes and other chemical compounds, providing advantages for the host plant (Azevedo et al. 2000). Endophytic fungi can also become a new and important resource for the degradation of polycyclic aromatic hydrocarbons (PAH), a toxic class of environmental pollutants (Dai et al. 2010). When colonization results in protection for plant tissues to biotic or abiotic stress, these fungi are called mutualistic (Latch 1993), as both benefit from this interaction (Wang and Dai 2011).
Studies on endophytic fungi of plants are necessary to provide fundamental information for the assessment of global fungal diversity and distribution, as well as for the discovery of new species (Stone et al. 2004; Siqueira et al. 2008). Currently, nearly all host plants studied have had endophytic microorganisms isolated (Wang and Dai 2011).
Endophytic microorganisms produce hydrolytic extracellular enzymes as part of their mechanism of resistance in overcoming the host’s defenses against microbial invasion and/or to obtain nutrients from the soil (Tan and Zou 2001). These enzymes include pectinases, esterases, cellulases, lipases, proteases and xylanases (Petrini et al. 1992; Silva et al. 2006; Suto et al. 2002). As a way of establishing the functional role of endophytic fungi, among other factors, there is a need for a detection of extracellular enzymes (Carroll and Petrini 1983).
There are few studies in the literature regarding the endophytic mycobiota of Cactaceae (Bills 1996), except for a preliminary study of endophytic fungi associated with Opuntia stricta in the semi-arid regions of Australia (Fischer et al. 1994) and of species of cacti in Arizona (Suryanarayanan et al. 2005). Opuntia ficus-indica Mill., a forage cactus, is known for its broad usage in agriculture as a producer of edible fruit and cladodes, which can be used as food (fodder) for animal, including man (Scheinvar 1995). It has medicinal properties and is used as an antioxidant (Lee et al 2002), anti-inflammatory agent (Park et al. 2000) and in the prevention of ulcers (Galati et al. 2001). It is grown with relative success in the semi-arid region of the Brazilian northeast since the beginning of the twentieth century, similar to what happens in the arid and semi-arid regions of the United States, Mexico, South Africa, Australia, for displaying morphological and physiological features that render them appropriate for these regions, thereby becoming an important food base for cattle (Teixeira et al. 1999).
Due to the importance of the Caatinga, a biome unique to Brazil, and the scarcity of studies about the diversity of fungi in this environment, the present paper targeted investigating the richness of endophytic fungi in O. ficus-indica from the state of Pernambuco, northeast of Brazil, and their capacity to produce pectinases, cellulases, xylanases and proteases.
Materials and methods
Plant material and isolation of endophytic fungi
The samples of forage cactus were collected in the municipality of Itaíba, Pernambuco, Brazil (09°08.895S, 037°12.069 W) and processed within a maximum of 24 h. For the purpose of isolating endophytic fungi, 45 fragments of about 1 cm2 were used. During processing, the cactus fragments were immersed in the following solutions: ethanol 75% for 20 s, sodium hypochlorite 4% for 90 s, ethanol 75% for 10 s, and rinsed three times in distilled and sterilized water (Fischer et al. 1994; Araújo et al. 2002; Suryanarayanan et al. 2005), and then transferred aseptically to the surface of the potato-dextrose-agar gel culture medium (PDA) supplemented with chloramphenicol (100 mg l−1), to suppress bacterial growth in the Petri dishes. The dishes were then incubated at a temperature of 28 ± 2°C for up to 30 days. Dishes were inspected daily and any fungal colony found was isolated, purified and maintained in PDA for later identification. In order to check the efficacy of the surface sterilization, water samples (1 ml) from the last rinse were inoculated into Petri dishes containing the same medium, using the same incubation conditions.
Identification of endophytic fungi
For identification of endophytic fungi, micro-cultivations were performed and the macro and micro morphological aspects of the somatic and reproductive structures were observed, using specific methodology and literature (Ellis 1971; Sutton 1980; Samson and Frisvad 2004; Domsch et al. 2007).
Preliminary selection of endophytic fungi with screening for enzyme production
Fragments (5 mm) of the endophytic fungal cultures grown in PDA for 7 days were then transferred to the center of the Petri dishes containing the solid medium with specific substrates to each enzyme: citric pectin for pectinases (Uenojo and Pastore 2006), carboxymethylcellulose for cellulases (Neirotti and Azevedo 1988), xylan for xylanases (Sarath et al. 1989) and milk casein for proteases (Lacaz et al. 2002). The cultures were incubated at 28°C for 7 days. The zone of activity (ZA) was expressed by the relationship between the average diameter of the colony growth (cm) and the average diameter of the colony growth (cm) + average diameter of the degradation halo (cm) (Serda and Yucel 2002). The scores for the production of each enzyme were based on the following criteria: ZA between 0.9 and 1: very weak; ZA between 0.89 and 0.80: weak; ZA between 0.79 and 0.70: strong, and ZA smaller than 0.69: very strong. According to this system a lower ZA ratio corresponds with a higher enzyme activity.
Frequency of endophytic fungi
The absolute and relative frequency of endophytic fungi isolated was calculated. The absolute frequency was calculated as the total number of endophytic isolates and for the relative frequency, the number of isolates of each species was divided by the total of isolates (Larran et al. 2002).
Results and discussion
Endophytic fungi from forage cactus (Cactaceae)
There is a dearth of papers studying endophytic fungi in plants from arid to semi-arid regions (Bills 1996). Brazil has an exclusive natural region, the Caatinga biome, which features a seasonally dry forest, being home to endemic species of plants, birds, mammals, fish and other species that ensure its importance. Little attention has been given to preserving the outstanding and varied landscape of the Caatinga, and the contribution made by its biota to the high level of biodiversity found in Brazil has been severely underestimated (Silva et al. 2004; Leal et al. 2005).
A total of 45 fragments of the cactus O. ficus-indica were analysed and 44 endophytic fungi were isolated, belonging to 12 genera and 13 species. Table 1 shows the frequency of endophytic fungi found in O. ficus-indica. Many of the genera identified in this study were found by Suryanarayanan et al. (2005), who isolated endophytic fungi associated with Arizona cacti, including Cladosporium, the most frequent species in this study, with a frequency of 36.42%. Colletotrichum and Phyllosticta have being commonly reported as endophytic fungi (Larran et al. 2002; Baayen et al. 2002; Glienke-Blanco et al. 2002; Hata et al. 2002; Santamaría and Bayman 2005; Chareprasert et al. 2006; Wang et al. 2007; Rakotoniriana et al. 2008; Xing et al. 2010; Juan Chen et al. 2011), but we did not isolate any species from these genera from O. ficus-indica. This was also observed by Fischer et al. (1994) who studied endophytic fungi from Opuntia stricta in the semi-arid regions of Australia and by Suryanarayanan et al. (2005) who analysed the endophytic fungi from cacti in Arizona.
First reports of endophytic fungi for Brazil
Most species found in this study are commonly reported as endophytic to tropical, subtropical or temperate plants (Mariano et al. 1997; Pereira et al. 1999; Araújo et al. 2001; Photita et al. 2001; Kumar and Hyde 2004). However, Acremonium terricola (J.H. Mill., Giddens & A.A. Foster) W. Gams, Monodictys castaneae (Wallr.) S. Hughes, P. glandicola (Oudem.) Seifert & Samson, Phoma tropica R. Schneid. & Boerema and Tetraploa aristata Berk. & Broome are being listed for the first time for Brazil as endophytic species.
Acremonium terricola was reported by Gams (1971) in forest soil, and this species was isolated in leaves and litter of the sub-antarctic phanerogam Poa flabellata (Poaceae) (Hurst et al. 1983), from soil impacted by copper mining in Brazil (Costa et al. 2006) and in the rhizosphere of sugarcane (Braz et al. 2009); Monodictys castaneae was isolated in dead stem and rotting wood (Ellis 1971), in leaf surface of Labiatae, Solanaceae and Umbelliferae in Egypt (El Kady et al. 1997), home dust (El Bokhary 1999), as saprobe in Proteaceae and Restionaceae in a study in South Africa (Lee et al. 2004) and in soil of the Caatinga (Cavalcanti et al. 2006).
Penicilium glandicola was reported in a wide range of substrate types such as stored apples (Ates 1991), in soil of farm areas (Haliki and Dizbay 1997), foods (Topal 1998), forest soil (Paterson and Russell 2004; Kara and Bolat 2007), hospital air (Okten 2008), bat dung, mammal feces and earthworms (Nováková 2009) and in cereal products (Doolotkeldieva 2010); Phoma tropica was isolated from Heliconia sp. (Heliconiaceae) and from Cordia colococca (Boraginaceae) (Sutton 1980), isolated in water environment of the Atlantic Rain Forest (Schoenlein-Crusius and Milanez 1998), brown algae Fucus spiralis (Fucaceae) (Osterhage et al. 2002), necrotic spots in leaves of Heliconia psittacorum (Heliconiaceae) (Costa 2007) and in soil (Schoenlein et al. 2008), and as a phytopathogen in leaves of Lablab purpureus (Papilionoideae) in India (Patil et al. 2010);
Tetraploa aristata, usually found on the basis of leaves and stems just above soil surface (Ellis 1971), was registered as a saprophyte from Syzygium (Myrtaceae), Cenchrus (Poaceae) and in dead branches in South African provinces (Sinclair 1990); in dead stalks of Elegia capensis (Restionaceae) Lee et al. (2004), in organic matter collected in the Santiago River in Argentina (Liberto and Saparrat 2005), decomposing leaf litter of Caesalpinia echinata (Fabaceae) (Grandi and Silva 2006), fruit of Psidium guajava (Myrtaceae) in decomposition (Pérez et al. 2003) and was also isolated in the dead trunk of Alpinia formosa (Zingiberaceae) in work in Japan (Tanaka et al. 2009).
Preliminary selection for production of enzymes
Fungi are important producers of enzymes, relatively easy to grow in controlled environments and highly sensitive to genetic alterations, enabling enhanced strains to be obtained in terms of production and quality of enzymes (Santos 2007).
Endophytic fungi have high capability for production of extracellular enzymes, such as pectinases, cellulases, proteases, phenol-oxidases, proteases and other catabolic enzymes (Oses et al. 2006; Tan and Zou 2001; Bischoff et al. 2009). Different studies have shown the efficacy of these enzymes in the degradation of residual plant matter (Wang and Dai 2011). Jordaan et al. (2006), in a study of endophytic fungi in pods of Colophospermum mopane, isolated species of the genera Phoma, Phomopsis and Alternaria, which displayed lignin cellulolytic activity accelerating significantly the dehiscence of pods, enabling effective germination of seeds in arid environments, when the conditions are favorable. Table 2 shows the results obtained after cultivating the endophytic fungi in specific solid medium for the purpose of detection the capacity to produce pectinases, cellulases, xylanases and proteases.
Pectinase
Of the 24 isolates tested, only Aspergillus japonicus and P. glandicola showed pectinolytic activity with ZA of 0.84 (weak) and 0.61 (very strong), respectively. Teixeira et al. (2000), in checking for pectinase production capability by A. japonicus in liquid medium with different concentrations of substrates, noted that the best enzymatic activity of this fungus was obtained under different concentrations of pectin. Yoon et al. (2007), using species of Penicillium for detection of enzymatic activity, noted that P. glandicola displayed weak or no pectinolytic activity, differing in the results found in this study where P. glandicola displayed very strong enzymatic action.
Cellulase
Among the cultures tested, 14 showed cellulolytic activity (53.84%). Fusarium lateritium showed ZA between 0.89 and 0.80 (weak), Nigrospora sphaerica and A. japonicus showed ZAs between 0.79 and 0.70 (strong), the other cultures showed ZA under 0.69 (very strong), with standout for Xylaria sp.1, isolate PF300 and Cladosporium cladosporioides that displayed, respectively, enzymatic indices of 0.20, 0.35 and 0.46. Species of the genera Xylaria, found growing in plant tissues are also reported as potential producers of cellulolytic enzymes (Wei et al. 1996), and also displayed lignin cellulolytic activity when isolated as endophytic in leaf litter of Fagus crenata (Osono and Takeda 2001, 2002). C. cladosporioides when isolated in the soil of an Ecologic Station did not show any cellulolytic activity (Ruegger and Tauk-Tornisielo 2004), differing from the isolate as endophytic in O. fícus-indica. On the other hand, Grandi and Silva (2006) in studies of fungi associated to C. echinata isolated Cladosporium oxysporum decomposing the leaf litter, thus evidencing the genera’s cellulolytic capability.
Xylanase
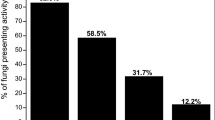
All cultures assessed showed xylanolytic activity, but with very weak ZAs. The production of xylanases by fungi has been reported for a number of species of Aspergillus, Penicillium, Trichoderma, Fusarium, Rhizopus, Cladosporium, Nigrospora and Myrothecium (Saha 2002; Goulart et al. 2005; Butt et al. 2008). Some species of Aspergillus are known as producers of hemicellulolytic enzymes (Juven et al. 1985; Sakellaris et al. 1989). In literature, the majority of species of endophytic fungi are reported as producers of proteases, lipases, cellulases, amylases and pectinases (Silva et al. 2006). However no reports were found of these fungi’s capacity to produce xylanases.
Protease
Of the isolates tested, 21 came out protease-positive (88%). Of these, eight isolates showed very strong ZAs, two strong, eleven isolates between weak and very weak and three showed no degradation halo. Isolate PF103, Phoma tropica, T. aristata and A. terricola stood out as the best producers of the enzyme with ZA varying between 0.31 and 0.39. Braz et al. (2009) detected protease activity in an A. terricola isolate from sugarcane rhizosphere, similar to our results in this study. No studies were found in the literature reporting proteolytic activity of P. tropica and T. aristata. Several fungi have been reported as good producers of proteases, such as Aspergillus and Fusarium (Vishwanatha et al. 2009). A. japonicus and F. lateritium were endophytic isolates of O. fícus-indica in this study, all of which displayed proteolytic activity, despite having very weak ZAs.
Conclusion
This is the first study on the endophytic fungi of forage cactus (O. ficus-indica) from the Caatinga, Brazil. The species A terricola, M. castaneae, P. glandicola, P. tropica and T. aristata are being reported for the first time as endophytic fungi for Brazil. The Cladosporium genus, and the species C. cladosporioides are the most commonly found endophytic fungi in O. ficus-indica. Endophytic fungi in O. ficus-indica show xylanolytic, proteolytic, pectinolytic and cellulolytic activity. The following species indicate possible use in biotechnological applications focused on enzyme production: P. glandicola for pectinases, Xylaria sp. 1 for cellulases, isolate PF103 for proteases and T. aristata for xylanases. This study has contributed to knowledge about the richness of endophytic fungi from Cactaceae of the Caatinga, which has been the subject of few studies, and provides information on the biotechnological potential of these fungi.
References
Araújo WL, Saridakis HO, Barroso PAV, Aguilar-Vildoso CI, Azevedo JL (2001) Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can J Microbiol 47:229–236
Araújo WL, Marcon J, Maccheroni W Jr, Elsas JDV, Vuurde JWL, Azevedo JL (2002) Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 68:4906–4914
Ates M (1991) Izmir ve civarinda soguk hava depolarinda depolanan elmalarda depolama sirasinda bozukluklardaki kuf florasinin saptanmasi konusunda bir arastirma. MSc thesis, E.Ü. Fen Bilimleri Enstitüsü
Azevedo JL, Maccheroni W Jr, Pereira JO, Araújo WL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biotechnol 3:40–65
Baayen RP, Bonants PJM, Verkley G, Carroll GC, van der Aa HA, de Weerdt M, van Brouwershaven IR, Schutte GC, Maccheroni W Jr, Glienke de Blanco C, Azevedo JL (2002) Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology 92:464–477
Bills GF (1996) Isolation and analysis of endophytic fungal communities from woody plants. In: Redlin SC, Carris LM (eds) Endophytic fungi in grasses and woody plants: systematics, ecology, and evolution. American Phytopathological Society Press, Saint Paul, pp 31–65
Bischoff KM, Wicklow DT, Jordan DB, de Rezende ST, Liu SQ, Hughes SR, Rich JO (2009) Extracellular hemicellulolytic enzymes from the maize endophyte Acremonium zeae. Curr Microbiol 58:499–503
Braz SCM, Souza-Motta CM, Lima DMM, Neves RP, Magalhães OMC (2009) Viability, taxonomic confirmation and enzymatic detection of Acremonium species preserved under mineral oil in the URM Culture Collection. Rev Soc Bras Med Trop 42:63–66
Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Carroll G, Petrini O (1983) Patterns of substrate utilization by some fungal endophytes from coniferous foliage. Mycologia 75:53–63
Cavalcanti MAQ, Oliveira LG, Fernandes MJ, Lima DM (2006) Fungos filamentosos isolados do solo em municípios na região Xingó, Brasil. Acta Bot Bras 20:831–837
Chareprasert S, Piapukiew J, Thienhirun S, Whalley AJS, Sihanonth P (2006) Endophytic fungi of teak leaves Tectona grandis L. and rain tree leaves Samanea saman Merr. World J Microbiol Biotechnol 22:481–486
Chen Juan, Ke-Xing Hu, Hou Xiao-Qiang, Guo Shun-Xing (2011) Endophytic fungi assemblages from 10 Dendrobium medicinal plants (Orchidaceae). World J Microbiol Biotechnol 27:1009–1016
Costa CR (2007) Fungos associados às plantas ornamentais tropicais no Distrito Federal. Universidade de Brasília, Dissertação
Costa IPMW, Cavalcanti MAQ, Fernandes MJS, Lima DMM (2006) Hyphomycetes from soil of an area affected by copper mining activities in the state of Bahia, Brazil. Braz J Microbiol 37:290–295
Dai CC, Tian LS, Zhao YT, Chen Y, Xie H (2010) Degradation of phenanthrene by the endophytic fungus Ceratobasidum stevensii found in Bischofia polycarpa. Biodegradation 21:244–255
Domsch KH, Gams W, Anderson TH (2007) Compendium of soil fungi. IHW-Verlag, Eching
Doolotkeldieva TD (2010) Microbiological control of flour-manufacture: dissemination of mycotoxins producing fungi in cereal products. Microbiol Insights 3:1–15
El Bokhary HA (1999) Osmotolerant and osmophilic fungi from house dust, Riyadh, Saudi Arabia. Saudi J Biol Sci 6:147–155
El Kady IA, Zohri AA, Mohamed EM (1997) Phyllosphere and phylloplane fungi of some herbal plants belonging to Labiatae, Solanaceae an Umbelliferae in Egypt. Qatar Univ Sci J 17:247–264
Ellis MB (1971) Dematiaceus Hyphomycetes. Commonwealth Mycological Institute, Kew
Fischer PJ, Sutton BC, Petrini LE, Petrini O (1994) Fungal endophytes from Opuntia stricta: a first report. Nova Hedwigia 59:195–200
Galati EM, Mendello MR, Giuffrida D, Miceli N (2001) Anticulcer activity of Opuntia ficus-indica (L.) Mill. (Cactaceae): ultra structural study. J Ethnopharmacol 76:1–9
Gams W (1971) Cephalosporium—artige Schimmelpilze (Hyphomyceto). G. Fisher, Stuttgart
Glienke-Blanco C, Aguilar-Vildoso CI, Vieira MLCV, Barroso PAV, Azevedo JL (2002) Genetic variability in the endophytic fungus Guignardia citricarpa isolated from citrus plants. Genet Mol Biol 25:251–255
Goulart AJ, Carmona EC, Monti R (2005) Partial purification and properties of cellulase-free alkaline xylanase produced by Rhizopus stolonifer in solid-state fermentation. Braz Arch Biol Technol 48:327–333
Grandi RAP, Silva TV (2006) Fungos anamorfos decompositores do folhedo de Caesalpinia echinata Lam. Rev Bras Bot 29:275–287
Haliki A, Dizbay M (1997) Izmir-Bergama yoresindeki bazi tarimsal alanlardan mezofilik toprak mikrofunguslarinin izolasyonu ve mevsimsel dagilimlari. Turk J Biol 21:329–341
Hata K, Atari R, Sone K (2002) Isolation of endophytic fungi from leaves of Pasania edulis and their within-leaf distributions. Mycoscience 43:369–373
Hurst JL, Pugh GJF, Walton DWH (1983) Fungal succession and substrate utilization on the leaves of three South Georgia phanerogams. Br Antarct Surv Bull 58:89–100
Jordaan A, Taylor JE, Rossenkhan R (2006) Occurrence and possible role of endophytic fungi associated with seed pods of Colophospermum mopane (Fabaceae) in Botswana. S Afr J Bot 72:245–255
Juven BJ, Lindner P, Weisslowicz H (1985) Pectin degradation in plant materials by Leuconostoc mesenteroides. J Appl Bacteriol 58:533–538
Kara O, Bolat I (2007) Influence of soil compaction on microfungal community structure in two soil types in Bartin Province, Turkey. J Basic Microbiol 47:394–399
Kumar DSS, Hyde KD (2004) Biodiversity and tissue-recurrence of endophytic fungi from Tripterygium wilfordii. Fungal Divers 17:69–90
Lacaz CS, Porto E, Martins JEC, Heins-Vacarri EM, Melo NK (2002) Tratado de Micologia Médica. Sarvier Press, São Paulo
Larran S, Perelló A, Simón MR, Moreno V (2002) Isolation and analysis of endophytic microorganisms in weat (Triticum aestivum L.) leaves. World J Microbiol Biotechnol 18:683–686
Latch GCM (1993) Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes. Agric Ecosyst Environ 44:143–156
Leal IR, Silva JMC, Tabarelli M, Lacher TE Jr (2005) Mudando o curso da conservação da biodiversidade na Caatinga do Nordeste do Brasil. Megadiversidade 1:139–146
Lee JC, Kim HR, Kim J, Jam YM (2002) Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem 50:6490–6496
Lee S, Mel’nik V, Taylor JE, Crous PW (2004) Diversity of saprobic hyphomycetes on Proteaceae and Restionaceae from South Africa. Fungal Divers 17:91–114
Liberto R, Saparrat MCN (2005) Crecimiento y habilidad decolorante potencial de Hyphomycetes (Deuteromycetes) de Río Santiago sobre medio agarizado suplementado con cromóforos sintéticos. Bol Soc Argent Bot 40:145–150
Mariano RLR, Lira RVI, Silveira EB, Menezes M (1997) Levantamento de fungos endofíticos e epifíticos em folhas de coqueiro no Nordeste do Brasil. I. Frequência da população fúngica e efeito da hospedeira. Agrotópica 9:127–134
Neirotti E, Azevedo JL (1988) Técnicas semiquantitativa de avaliação da produção de celulases em Humicola sp. Rev Microbiol 19:78–81
Nováková A (2009) Microscopic fungi isolated from the Domica Cave system (Slovak Karst National Park, Slovakia). A review. Int J Speleol 38:71–82
Okten S (2008) Airborne fungi and bacteria in indoor and outdoor environment of pediatry unit of Edirne Government Hospital. University Graduate School of Natural and Applied Sciences, Thesis
Oses R, Valenzuela S, Freer J, Baeza J, Rodriguez J (2006) Evaluation of fungal endophytes for lignocellulolytic enzyme production and wood biodegradation. Int Biodeterior Biodegrad 57:129–135
Osono T, Takeda H (2001) Effects of organic chemical quality and mineral nitrogen addition on lignin and holocellulose decomposition of beech leaf litter by Xylaria sp. Eur J Soil Biol 37:17–23
Osono T, Takeda H (2002) Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 94:421–427
Osterhage C, König GM, Jones PG, Wright AD (2002) 5-Hydroxyramulosin, a new natural product produced by Phoma tropica, a marine-derived fungus from the alga Fucus spiralis. Planta Med 68:1052–1054
Park E, Kahng J, Lee S, Shin K (2000) An anti-inflamatory principle from cactus. Fitoterapia 72:288–290
Paterson R, Russell M (2004) The isoepoxydon dehydrogenase gene of patulin biosynthesis in cultures and secondary metabolites as candidate PCR inhibitors. Mycol Res 108:1431–1437
Patil VA, Mehta BP, Deshmukh AJ (2010) Field evaluation of different fungicides against Phoma leaf spot disease of Indian bean. Int J Pharm Bio Sci 1:1–5
Pereira JO, Carneiro-Vieira ML, Azevedo JL (1999) Endophytic fungi from Musa acuminata and their reintroduction into axenic plants. World J Microbiol Biotechnol 15:37–40
Pérez E, Santos R, Montiel A, Isea F, Marín M, Sandoval L (2003) Método para el muestreo de esporas de hongos en una plantación de guayabo (Psidium guajava L.). Rev Fac Agron (LUZ) 20:52–62
Petrini O, Stone J, Carroll FE (1992) Endophytic fungi in evergreen shrubs in western Oregon: a preliminary study. Can J Bot 60:789–796
Photita W, Lumyong S, Lumyong P, Hyde KD (2001) Endophytic fungi of wild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand. Mycol Res 105:1508–1513
Rakotoniriana EF, Munaut F, Decock C, Randriamampionona D, Iriambololoniaiana M, Rakotomalala T, Rakotonirina EJ, Rabemanantsoa C, Cheuk K, Ratsimamanga SU, Mahillon J, El-Jaziri M, Quetin-Leclercq J, Corbisier AM (2008) Endophytic fungi from leaves of Centella asiatica: occurrence and potential interactions within leaves. Anton Leeuw Int J G 93:26–36
Ruegger MJS, Tauk-Tornisielo SM (2004) Atividade da celulase de fungos isolados do solo da Estação Ecológica de Juréia-Itatins, São Paulo, Brasil. Rev Bras Bot 27:205–211
Saha BC (2002) Production, purification and properties of xylanase from a newly isolated Fusarium proleferatum. Process Biochem 37:1279–1284
Sakellaris G, Nokolaropoulos S, Evangelopoulos AE (1989) Purification and characterisation of an extracellular polygalacturonase from Lactobacillus plantarum train BA 11. J Appl Bacteriol 67:77–85
Samson RA, Frisvad JC (2004) Penicillium subgenus Penicillium: new taxonomics schemes, mycotoxins and other extrolites. Stud Micol 49:1–260
Santamaría J, Bayman P (2005) Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microb Ecol 0:1–8
Santos SFM (2007) Estudo da produção de pectinase por fermentação em estado sólido utilizando pedúnculo de caju como substrato. Tese, Universidade Federal do Rio Grande do Norte
Sarath G, de La Motte RS, Wagner FW (1989) Protease Assay Methods. In: Beynon RJ, Bonde JS (eds) Proteolytics enzymes: an pratical approach. Oxford University Press, Oxford, pp 25–54
Scheinvar L (1995) Taxonomy of utilized Opuntia. In: Barbera G, Inglese P, Pimienta-Barrios E (eds) Agro-ecology, cultivation and uses of cactus pear. Food and Agriculture Organization, Roma, pp 20–27
Schoenlein NC, Corso CR, Schoenlein-Crusius IH, de Souza JI, Oliveira LHS (2008) Fungos anamorfos do solo da região dos lagos no Município de Santa Gertrudes, SP, Brasil. Rev Bras Bot 31:667–678
Schoenlein-Crusius IH, Milanez AI (1998) Fungos microscópicos da Mata Atlântica de Paranapiacaba, São Paulo, Brasil1. Rev Bras Bot 21:73–79
Serda SK, Yucel A (2002) Phospholipase and protease activities in clinical Candida isolates with reference to the sources of strains. Mycoses 45:160–165
Silva JMC, Tabarelli M, Fonseca MT, Lins LV (orgs.) (2004) Biodiversidade da Caatinga: áreas e ações prioritárias para a conservação. Ministério do Meio Ambiente, Brasília
Silva RLO, Luz JS, Silveira EB, Cavalcante UMT (2006) Fungos endofíticos em Annona spp.: isolamento, caracterização enzimática e promoção do crescimento em mudas de pinha (Annona squamosa L.). Acta Bot Bras 20:649–655
Sinclair RCD (1990) A taxonomic study of some saprophytic hyphomycetes of southern Africa. University of Pretoria, Thesis
Siqueira MV, Braun U, Souza-Motta CM (2008) Corynespora subcylindrica sp. nov., a new hyphomycete species from Brazil and a discussion on the taxonomy of corynespora-like genera. Sydowia 60:113–122
Stone JK, Polishook JD, White F Jr (2004) Endophytic Fungi. In: Muller JM, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic Press, San Diego, pp 241–270
Suryanarayanan TS, Wittlinger SK, Faeth SH (2005) Endophytic fungi associated with cacti in Arizona. Mycol Res 109:635–639
Suto M, Takebayashi M, Sait K, Tanaka M, Yokota A, Tomita F (2002) Endophytes as produces of xylanase. J Biosci Bioeng 93:88–90
Sutton BC (1980) The Coelomycetes: fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, Sano T, Shirouzu T, Hosoya T (2009) Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Stud Mycol 64:175–209
Teixeira JC, Evangelista AR, Perez JRO, Trindade IACM, Moron IR (1999) Cinética da digestão ruminal da palma forrageira (Nopalea cochenillifera (L.) Lyons (Cactaceae) em bovinos e caprinos. Ciência e Agrotecnologia 23:179–186
Teixeira MFS, Filho JLL, Durán N (2000) Carbon sources effect on pectinase production from Aspergillus japonicus 586. Braz J Microbiol 31:286–290
Topal S (1998) Turkiye’nin dominant mikoflorasiyla kultur kolleksiyon merkezi olusturulmasi. J Kukem 21:69–88
Uenojo M, Pastore GM (2006) Isolamento e seleção de microrganismos pectinolíticos a partir de resíduos provenientes de agroindústrias para produção de aromas frutais. Ciência Tecnologia Alimentar 26:509–515
Vishwanatha KS, Rao A, Singh SA (2009) Characterization of acid protease expressed from Aspergillus oryzae MTCC 5341. Food Chem 114:402–407
Wang Y, Dai C-C (2011) Endophytes: a potencial resource for biosynthesis, biotranformation, and biodegradation. Review article. Ann Microbiol 61:207–215
Wang FW, Jiao RH, Cheng AB, Tan SH, Song YC (2007) Antimicrobial potentials of endophytic fungi residing in Quercus variabilis and brefeldin a obtained from Cladosporium sp. World J Microbiol Biotechnol 23:79–83
Wei DL, Kirimur K, Usami S, Lin TH (1996) Purification and characterization of an extracellular β-glucosidase from the wood-grown fungus Xylaria regalis. Curr Microbiol 33:297–301
Xing X, Guo S, Fu J (2010) Biodiversity and distribution of endophytic fungi associated with Panax quinquefolium L. cultivated in a forest reserve. Symbiosis 51:161–166
Yoon JH, Hong SB, Ko SJ, Kim SH (2007) Detection of extracellular enzyme activity in Penicillium using chromogenic media. Mycobiology 35:166–169
Acknowledgments
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for Brazil, and Federal University of Pernambuco (UFPE) for funding this Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bezerra, J.D.P., Santos, M.G.S., Svedese, V.M. et al. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol 28, 1989–1995 (2012). https://doi.org/10.1007/s11274-011-1001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-1001-2