Abstract
Microorganisms that colonize plants require a number of hydrolytic enzymes to help degrade the cell wall. The maize endophyte Acremonium zeae was surveyed for production of extracellular enzymes that hydrolyze cellulose and hemicellulose. The most prominent enzyme activity in cell-free culture medium from A. zeae NRRL 6415 was xylanase, with a specific activity of 60 U/mg from cultures grown on crude corn fiber. Zymogram analysis following SDS-PAGE indicated six functional xylanase polypeptides of the following masses: 51, 44, 34, 29, 23, and 20 kDa. Xylosidase (0.39 U/mg), arabinofuranosidase (1.2 U/mg), endoglucanase (2.3 U/mg), cellobiohydrolase (1.3 U/mg), and β-glucosidase (0.85 U/mg) activities were also detected. Although apparently possessing a full complement of hemicellulolytic activities, cell-free culture supernatants prepared from A. zeae required an exogenously added xylosidase to release more than 90% of the xylose and 80% of the arabinose from corn cob and wheat arabinoxylans. The hydrolytic enzymes from A. zeae may be suitable for application in the bioconversion of lignocellulosic biomass into fermentable sugars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acremonium zeae Gams & Sumner, one of the most prevalent fungal colonists of preharvest corn, produces symptomless infections of corn seeds and has been isolated from the stalks of mature plants [3, 8, 11, 16]. A. zeae has been the subject of recent investigations because of its production of pyrrocidine antibiotics and its potential to serve as a biocontrol agent against mycotoxin-producing fungi [18]. The observation by Harris [5] that, when cultured on artificial media, A. zeae “grew most vigorously on medium containing xylan isolated from maize cobs” suggested that A. zeae might be a source of hemicellulolytic enzymes uniquely adapted for the utilization of maize cell wall components. A. zeae was also found to grow well on the pentose sugars xylose and arabinose and on oat spelt xylan [5; D.T.W., unpublished data], further evidence that it would be a good source of hemicellulolytic enzymes.
Corn fiber is a mixture of corn hulls and residual starch produced during the wet-milling process. It contains about 70% carbohydrate, of which approximately 14% and 35% are in the form of cellulose and hemicellulose, respectively [4]. Its relatively low commercial value, high carbohydrate content, and abundant availability at the wet mills make it an attractive feedstock for fermentation. Recalcitrance of corn fiber hemicellulose to enzymatic hydrolysis, however, poses a significant technical barrier to the commercial use of this feedstock [12]. In the present study, we examined the maize endophtye A. zeae strain NRRL 6415 for the production of hydrolytic enzymes when grown on various lignocellulosic carbon sources. Our objective was to determine if enzyme mixtures from this strain are effective at hydrolyzing lignocellulosic biomass, including corn fiber.
Materials and Methods
Preparation of Cell-Free Culture Supernatants
Acremonium zeae NRRL 6415 was obtained from the ARS Culture Collection maintained at the USDA-ARS National Center for Agricultural Utilization Research, Peoria, Illinois. Culture medium contained the following per liter: 2.8 g NaNO3, 2.0 g KH2PO4, 1.0 g MgSO4 · 7H2O, 0.01 g FeSO4 · 7H2O, and 1% (w/v) carbon source (crude corn fiber [CCF], alkaline hydrogen peroxide-treated corn fiber [AHP-CF], or oat spelt xylan [OSX]). AHP-CF was produced as described by Leathers and Gupta [9]. Cultures were grown at 25°C with shaking at 130 rpm. Cultures were cleared of cells by centrifugation and the supernatant liquid was retained as the cell-free culture supernatant. Protein was determined using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA) against a bovine serum albumin standard.
Assay of Enzymatic Activity
Enzyme assays for polysaccharide and p-nitrophenyl substrates were performed as described previously [2]. For polysaccharide substrates, 1 unit (U) of activity is defined as the amount of enzyme that produces 1 μmol of reducing sugar (glucose equivalent) per minute. For p-nitrophenyl substrates, 1 U of activity is defined as the amount of enzyme that produces 1 μmol of p-nitrophenol per minute. Data for the calculation of kinetic constants were collected under standard assays conditions except for the variation of substrate concentration. K m and V max were determined by evaluation of Lineweaver-Burke plots of initial velocity versus substrate concentration.
Temperature optima for xylanase, arabinofuranosidase, and xylosidase activities were determined by varying the assay temperature between 37°C and 85°C under otherwise standard assay conditions. The following discontinuous buffer system was used to determine pH optima for enzyme activities at 37°C: 50 mM sodium acetate (pH 3.5–5.5), 50 mM sodium phosphate (pH 6.0–7.0), 50 mM glycine (8.0–10.0). Temperature stability was determined by incubating cell-free culture supernatants at varying temperatures (37° to 85°C) for 60 min, then measuring residual activity under standard assay conditions.
Zymogram Analysis
Zymogram analysis was performed in 10% SDS-PAGE gels containing 0.1% (w/v) oat spelt xylan polymerized within the gel matrix. Activity was detected as described previously [2].
Hydrolysis of Arabinoxylans
Recombinant β-D-xylosidase (SXA) from Selenomonas ruminantium was produced as described previously [7]. Reactions (1 ml) contained 40 μg of xylan substrate, 360 μg of SXA, and 5 μg of cell-free culture supernatant in 100 mM sodium succinate, pH 5.3. After 14 h at 25ºC, a second dose of cellulase was added, and the mixture incubated for an additional 6 h. Products were analyzed by HPLC as described previously [7].
Results
Hydrolytic Enzyme Activities of Acremonium zeae NRRL 6415
A. zeae NRRL 6415 cultures grown on CCF produced 98 ± 10 μg protein/ml in the cell-free culture supernatant, AHP-CF cultures produced 68 ± 7.0 μg protein/ml, and OSX cultures produced 44 ± 1.0 μg protein/ml. Table 1 lists the specific activities of hydrolytic enzymes present in the extracellullar matrix. The most prominent activity was xylanase, with a specific activity of 60 U/mg from cultures grown on CCF. Other hemicellulolytic activities detected included xylosidase (pNP-X substrate) and arabinofuranosidase (pNP-A substrate). Cellulolytic activity against CMC (representing endoglucanase), pNP-C and avicel (representing cellobiohydrolase), and pNP-G (β-glucosidase) was also detected. No activity against soluble starch (amylase) was detected.
Zymogram analysis indicated that the CCF culture possessed at least six active polypeptides of 51, 44, 34, 29, 23, and 20 kDa (Fig. 1). The OSX culture displayed four active polypeptides (51, 34, 23, and 20 kDa), and the AHP-CF culture only three (51, 34, and 20 kDa).
Zymogram analysis for xylanase activity. Each lane contains 0.5 μg of cell-free culture supernatant. Lane designations are as follows: S, Bio-Rad Precision Plus protein standards; CCF, culture grown on crude corn fiber; AHP, culture grown on AHP-treated corn fiber; OSX, culture grown on oat spelt xylan. The masses of molecular markers (kDa) are listed at the left
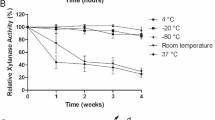
The pH and temperature for optimum xylanase activity in cultures grown on crude corn fiber were pH 5.5 and 65°C. The enzyme had a broad pH range, retaining more than 37% of its maximal activity at 65°C over the pH range from 4.5 to 9.0. At pH 5.5, it retained 68% of maximal activity at 75°C, but enzyme activity was not stable at higher temperatures, losing 23%, 65%, and 98% of its activity following 60 min of incubation at 45, 55, and 65°C, respectively. Temperature and pH optima for the pNP-arabinofuranosidase activity were 70°C and pH 5.0, and the optima for the pNP-xylosidase activity were 65°C and pH 5.5. Kinetic parameters for xylanase, pNP-arabinofuranosidase, and pNP-xylosidase activities from cultures grown on crude corn fiber, AHP-treated corn fiber, and oat spelt xylan are listed in Table 2.
Hydrolysis of Purified Arabinoxylans
Analysis of the hydrolysis products of wheat arabinoxylan indicated that xylobiose accumulated in the reaction in addition to the monosaccharides arabinose and xylose (data not shown). Addition of an exogenous recombinant xylosidase (SXA) converted the accumulated xylobiose to xylose. Table 3 lists the percentages of sugars released from isolated arabinoxylans by A. zeae cell-free culture supernatant supplemented with SXA.
Discussion
The heterogeneous structure of hemicellulose requires a complex set of enzymatic activities for complete hydrolysis [13]. Endophytes represent a rich and diverse source of xylanase producing microorganisms [17], and as one of the most prevalent colonists of preharvest corn, A. zeae may be a source of enzymes uniquely suited for utilization of the corn cell wall. Although other species of Acremonium have previously been studied for production of cellulolytic enzymes [6, 20], there are few data available on the production of hemicellulases. A fungal culture (strain Y-94) identified as ‘Acremonium cellulolyticus’ nom. inval. [21] had been shown to produce large amounts of cellulolytic enzymes but only 3.4 U/mg xylanase [10, 20]. In the present study, A. zeae NRRL 6415 produces more xylanolytic than cellulolytic activity, but with a specific activity of xylanase almost 20-fold higher than that reported for A. cellulolyticus strain Y-94. It should be noted, however, that Y-94 produced over 70-fold more protein in culture broth than A. zeae NRRL 6415. Improvement of the growth medium enhanced production by Y-94 approximately 10-fold [20], and thus, significant increases in A. zeae enzyme yield could be achieved by optimizing the culture conditions.
Zymogram analysis suggests that A. zeae produces multiple xylanases. Consistent with the relative amounts of xylanase activity, six active polypeptides were observed in cultures grown on crude corn fiber, compared with four in cultures grown on oat spelt xylan and only three in cultures grown on AHP-treated corn fiber. Production of multiple xylanases is a common occurrence in microorganisms and may reflect the need for a suite of enzymes with different substrate specificities to efficiently hydrolyze xylan [1, 10, 19]. From the present data, one cannot conclude whether the different polypeptides are the products of distinct genes or artifacts of posttranslational modifications (such as glycosylation or proteolysis) of a single enzyme. To date, there are no genome data available for any Acremonium species, and therefore further genetic analysis is warranted to determine the number and expression levels of each Acremonium xylanase.
A number of other extracellular hydrolytic enzyme activities were produced by A. zeae. Although xylosidase activity is detected in the A. zeae preparations, there may not be sufficient activity, or the A. zeae enzyme might not have the proper substrate specificity, to fully hydrolyze the wheat arabinoxylans. Thus, an exogenous xylosidase was needed to hydrolyze the substrate to monomeric sugars. This is consistent with the reported synergism between endo-1,4-xylanases and α-L-arabinofuranosidases from commercial cellulases when used to hydrolyze wheat arabinoxylan [14, 15]. While Sorensen and colleagues previously reported yields of arabinose and xylose from wheat arabinoxylan of 46% and 89%, respectively [14], it should be noted that these numbers are saturation yields, resulting from hydrolysis of at least 2.5 wt% dry matter. In the present study, the use of a low concentration of arabinoxylan was intended to avoid product inhibition of the enzymes and mimic the low product concentrations likely to be encountered in a simultaneous saccharification and fermentation process. Direct comparison of the A. zeae activities to those reported previously is therefore difficult, but the data suggest that A. zeae enzymes may prove suitable for application in a simultaneous saccharification and fermentation process.
In conclusion, evidence is offered that the maize endophyte Acremonium zeae NRRL 6415 may be uniquely adapted for the utilization of maize cell wall components. It produces a full complement of hemicellulolytic enzymes capable of hydrolysis of arabinoxylans from several industrially important feedstocks. Further investigation of the production and properties of A. zeae hemicellulases is warranted to facilitate development of enzymes to convert agricultural biomass into fuels and chemicals.
References
Adelsberger H, Hertel C, Glawischnig E, Zverlov VV, Schwarz WH (2004) Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150:2257–2266
Bischoff KM, Rooney AP, Li X, Liu S, Hughes SR (2006) Purification and characterization of a family 5 endoglucanase from a moderately thermophilic strain of Bacillus licheniformis. Biotechnol Lett 28:1761–1765
Fisher PJ, Petrini O, Lappin-Scott HM (1992) The distribution of some fungal and bacterial endophytes in maize (Zea mays L.). New Phytol 122:299–305
Grohmann K, Bothast RJ (1997) Saccharification of corn fibre by combined treatment with dilute sulphuric acid and enzymes. Process Biochem 32:405–415
Harris MR (1936) The relationship of Cephalosporium acremonium to the black-bundle disease of corn. Phytopathology 26:965–980
Ikeda Y, Hayashi H, Okuda N, Park EY (2007) Efficient cellulase production by the filamentous fungus Acremonium cellulolyticus. Biotechnol Prog 23:333–338
Jordan DB (2008) β-d-Xylosidase from Selenomonas ruminantium: catalyzed reactions with natural and artificial substrates. Appl Biochem Biotechnol 146:137–149
King SB (1981) Time of infections of maize kernels by Fusarium moniliforme and Cephalosporium acremonium. Phytophathology 71:796–799
Leathers TD, Gupta SC (1996) Saccharification of corn fiber using enzymes from Aureobasidium sp. strain NRRL Y-2311-1. Appl Biochem Biotechnol 59:337–347
Mitsuishi Y, Takashi Y, Yagaisawa M, Takasaki Y (1987) Purification and properties of thermostable xylanases from mesophilic fungus strain Y-94. Agr Biol Chem 51:3207–3213
Reddy CS, Holbert JR (1924) The black-bundle disease of corn. J Agr Res 27:177–206
Saha BC, Bothast RJ (1999) Pretreatment and enzymatic saccharification of corn fiber. Appl Biochem Biotechnol 76:65–77
Saha BC, Bothast RJ (2000) Enzymes in biotechnology. In: Lederberg J (ed) Encyclopedia of microbiology, vol 2. Academic Press, San Diego, CA, pp 222–235
Sorensen HR, Pedersen S, Meyer AS (2006) Optimization of reaction conditions for enzymatic viscosity reduction and hydrolysis of wheat arabinoxylan in an industrial ethanol fermentation residue. Biotechnol Prog 22:505–513
Sorensen HR, Pedersen S, Vikso-Nielsen A, Meyer AS (2005) Efficiencies of designed enzyme combinations in releasing arabinose and xylose from wheat arabinoxylan in an industrial ethanol fermentation residue. Enzyme Microb Technol 36:773–784
Sumner DR (1968) Ecology of corn stalk rot in Nebraska. Phytopathology 58:761–765
Suto M, Takebayashi M, Saito K, Tanaka M, Yokota A, Tomita F (2002) Endophytes as producers of xylanase. J Biosci Bioeng 93:88–90
Wicklow DT, Roth S, Deyrup ST, Gloer JB (2005) A protective endophyte of maize: Acremonium zeae antibiotics inhibitory to Aspergillus flavus and Fusarium verticillioides. Mycol Res 109:610–618
Wong KKY, Tan LUL, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Yamanobe T, Mitsuishi Y, Takasaki Y (1987) Isolation of a cellulolytic enzyme producing microorganism, culture conditions and some properties of the enzymes. Agr Biol Chem 51:65–74
Yamanobe T, Mitsuishi Y, Takasaki Y (1985) Method for manufacture of cellulase. U.S. Patent 4562150
Acknowledgment
The authors wish to thank Eric Hoecker, Jay Braker, Imran Khan, and Jacob Brown for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of a trade name or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Bischoff, K.M., Wicklow, D.T., Jordan, D.B. et al. Extracellular Hemicellulolytic Enzymes from the Maize Endophyte Acremonium zeae . Curr Microbiol 58, 499–503 (2009). https://doi.org/10.1007/s00284-008-9353-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9353-z