Abstract
Enzymes are biocatalysts that are widely used in different industries and generate billions of dollars annually. With the advancement of biotechnology, new enzymatic sources are being evaluated, especially microbial ones, in order to find efficient producers. Endophytic fungi are promising sources of biomolecules; however, Amazonian species are still poorly studied as to their enzymatic production potential. In this sense, the production of hydrolases (amylases, lipases, cellulases and pectinases) was evaluated in endophytic fungi isolated from the leaves, roots and stems of açai palms (Euterpe precatoria). A qualitative test was carried out to detect the enzymatic synthesis in each isolate, and the most promising ones were cultivated using submerged fermentation. The enzyme extracts were quantified to determine those with the greatest activity. Cellulolytic and amylolytic extracts showed the highest enzymatic activities and were partially characterized. Among 50 isolates, 82.9% produced pectinase, 58.5% produced cellulase, 31.7% produced amylase, and 12.2% produced lipase. Penicillium sp. L3 was the best producer of amylase and Colletotrichum sp. S1 was the best producer of cellulase in liquid medium cultivation. The amylolytic extract showed the highest enzymatic activity at pH 8.0 and 45 °C, and the cellulolytic extract at pH 5.0 and 35 °C. The cellulase and amylase produced by the endophytes had their molecular masses estimated between 38 and 76 kDa. These results indicate that endophytic fungi from the açai palm can be used as a new source of hydrolytic enzymes, which can be applied in numerous biotechnological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In biotechnology, discoveries in enzymology have triggered important advances for numerous industrial applications (Bilal and Iqbal 2019). Enzymes are macromolecules that are catalysts of high specificity to their substrates, and are used in food, textile, pharmaceutical, cosmetic, cleaning, chemical synthesis industries, among many others. Advantages, such as the ability to reduce reaction time and low energy consumption, make these biocatalysts increasingly attractive to industry (Choi et al. 2015; Singh et al. 2016).
Enzymes of microbial origin are most often used for enabling large-scale production, due to easy manipulation, whether genetic or environmental. Endophytic microorganisms, inhabitants of the interior of plant tissues, have been shown to be potential producers of mainly hydrolytic enzymes (Mendes et al. 2015; Orlandelli et al. 2015; Marques et al. 2018; Matias et al. 2021). The global market value of enzymes was USD 9.9 billion in 2019 and it is expected to grow at a compound annual growth rate of 7.1% until 2027 (Grand View Research 2020).
Hydrolases comprise the group with the greatest industrial application due to factors such as the ability to catalyze biotransformation with high chemo-, regio- and enantioselectivity, and also because they do not depend on the regeneration of cofactors (Moran et al. 2013; Robinson 2015). Among the hydrolases, amylases occupy 25% of the total enzymes used in industry, followed by cellulases (20%), which are known mainly for their applications in the biofuel industry. Lipases and pectinases are used in the production of flavors and beverages, respectively (Moran et al. 2013; Cunha et al. 2016; Gopinath et al. 2017).
Considering the industrial importance of hydrolases, especially in relation to their numerous applications, it is deemed interesting to evaluate the potential of endophytic fungi associated with tropical species, whose studies are still scarce. Since endophytic fungi represent a promising alternative as a source of bioactive molecules (Fadiji and Babalola 2020), this study evaluated the hydrolytic potential of endophytic fungi isolated from açai palms (Euterpe precatoria Mart.), which is an Amazonian species of great economic importance, and known worldwide. In addition, the amylase and cellulase extracts were characterized, intending to reveal its potential industrial applications.
Materials and methods
Microorganisms
Fungi were obtained from leaves, stems, and roots of seedlings of Euterpe precatoria Mart. cultivated in the nursery of the School of Technology at the Amazonas State University (EST/UEA). The fungi were identified at the genus level using micromorphological analysis (Batista et al. 2018) and are deposited in the Microbiological Collections Center at UEA. The reactivation of the fungi was performed in potato dextrose agar (PDA) in a biochemical oxygen demand (BOD) incubator at 28 °C for 7 days. When the growth of fungi was identified, they were subjected to qualitative tests in solid medium to detect hydrolytic activity.
Detection of enzyme production in solid medium
In triplicate, discs of the reactivated mycelial material of 5 mm diameter were transferred to the center of a Petri dish, containing a specific medium for the induction of hydrolytic synthesis. For detection of amylase production, a medium composed of agar (1.8%), starch (1.0%) and phosphate citrate buffer 0.1 M, pH 5.0 was used. For detection of cellulolytic activity, agar (1.8%), carboxymethylcellulose (CMC) (1%) and sodium acetate buffer 0.1 M, pH 5.0 were used. For the detection of pectinolytic activity, agar (1.8%), citrus pectin (1.0%) and sodium acetate buffer 0.1 M, pH 5.0 were used; for the detection of lipolytic activity, peptone (6.0 g/L), NaCl (3.0 g/L), CaCl2.2H2O (0.06 g/L), agar (10.8 g/L) and Tween 80 at 1% (v/v) were used (Souza et al. 2008; Hankin and Anagnostakis 1975).
After the 7 day-incubation period, the plates were stained with iodine 0.1 N (for detection of amylases and pectinases), and lugol (for detection of cellulases) to facilitate the visualization of the degradation halos. The lipolytic activity was detected through the presence of calcium crystals forming around the fungal colony. Halos, indicative of enzyme production, were measured with the aid of a caliper, as were as fungal colonies, for the calculation of the enzyme index (EI), which expresses extracellular enzyme activity. EI was obtained by analyzing the relationship between the diameter of the degradation halo and the average diameter of the fungal colony (Oliveira et al. 2010). Fungi with an EI ≥ 2 were considered suitable for evaluation of hydrolytic production in liquid media.
Enzymatic production in liquid medium
The fungi selected from the enzymatic index were reactivated in tubes containing potato dextrose (BD) medium for 5 days at 28 °C. A spore suspension was prepared (1 × 106 spores/mL) and added to Erlenmeyer flasks containing 50 mL of specific liquid medium to produce each enzyme. Afterwards, the flasks were incubated in shaker. The cultures were performed in triplicate and maintained for 7 days. After cultivation, the fermented broths were filtered, and the cell-free enzyme extracts were used for the determination of enzyme activity.
To induce amylase production, a medium composed of NaNO3 (3.0 g/L), MgSO4.7H2O (0.5 g/L), KCl (5.0 g/L), KH2PO4 (1.0 g/L), FeSO4.7H2O (0.01 g/L), CaCl2 (0.1 g/L) and starch (15.0 g/L), pH 7.0, was used. The fungi were incubated in shaker at 120 rpm and 30 °C (Hegde et al. 2011). To induce cellulase production, a medium composed of KH2PO4 (2.0 g/L), (NH4)2SO4 (1.4 g/L), urea (0.3 g/L), MgSO4.7H2O (0.3 g/L), CaCl2 (0.1 g/L), FeSO4.7H2O (5.0 mg/L), MnSO4.H2O (1.6 mg/L), ZnSO4.7H2O (1.4 mg/L) CoCl2.H2O (1.6 mg/L) and CMC (10.0 g/L), pH 5.0, was used. The fungi were incubated in a shaker at 120 rpm and 28 °C (Kanti and Sudiana 2017). To induce lipase production, a medium composed of NH2NO3 (0.1%), MgSO4.7H2O (0.05%), KH2PO4 (0.1%), peptone (2.0%) and olive oil (1.0%), pH 6.0, was used. The fungi were incubated in shaker at 160 rpm and 28 °C (Nascimento et al. 2015). For induction of pectinase production, culture medium composed of pectin (10.0 g/L), sucrose (10.0 g/L), tryptone (3.0 g/L), yeast extract (2.0 g/L), KCl (0.5 g/L), MgSO4.7H2O (0.5 g/L), MnSO4.5H2O (0.01 g/L), (NH4)2SO4 (2.0 g/L) was used and supplemented with 1 mL of a mineral solution (CuSO4.5H2O, 0.04 g/L; FeSO4, 0.08 g/L; Na2MoO4, 0.08 g/L; ZnSO4, 0.8 g/L; Na2B4O7, 0.004 g/L; MnSO4, 0.08 g/L), pH 6.0. The fungi were incubated in shaker at 160 rpm and 50 °C (Jahan et al. 2017).
Dosage of the enzyme activity
To quantify the amylase and cellulase activity, a standard glucose curve was constructed. For lipase activity, a standard curve of p-nitrophenol (p-NP) was constructed and, for pectinase activity, a standard curve of D-galacturonic acid was used. The measurement of amylolytic and cellulolytic activity was determined from the amount of reducing sugars that were formed during the incubation of 50 μL of the enzyme extract together with 50 μL of the substrate (1% starch, diluted in sodium acetate buffer 0.1 M, pH 6.0 (w/v) for amylase; 1% CMC, diluted in 0.05 M sodium citrate buffer, pH 5.0 (w/v) for cellulase) at 50 °C for 30 min. Then, 100 μL of 3,5-dinitrosalicylic acid (DNS) were added, and the mixture was placed in a water bath at 100 °C for 5 min. After the addition of 800 μL of distilled water, the absorbances were read using a spectrophotometer at 540 nm (Miller 1959).
Lipolytic activity was obtained from the amount of p-nitrophenol formed in the incubation of 1.0 mL of the enzyme extract with 2.0 mL of the substrate at 37 °C. The substrate contained 1 mL of solution A (10 mL of isopropanol with 30 mg of p-NPP) and 9 mL of solution B (2 mL of Tween 80 mixed with 0.5 g of gum arabic in 450 mL of phosphate buffer 0.05 M, pH 7.0). After 15 min, absorbance readings were performed on a spectrophotometer at 410 nm. The level of pectinolytic activity was determined from the amount of D-galacturonic acid formed in the incubation of 50 μL of the enzyme extract with 50 μL of the substrate (citrus pectin at 0.5%) at 37 °C for 40 min, followed by the addition of 200 μL of DNS. After 5 min at 100 °C, 700 μL of distilled water were added and the absorbance was read on a spectrophotometer at 540 nm (Roosdiana et al. 2013; Vasconcelos et al. 2013; Nascimento et al. 2015). A unit of enzymatic activity was defined as the amount of enzyme required to release 1 μmol of glucose or p-nitrophenol, or d-galacturonic acid per minute, under the conditions of the assays.
Commercial enzymes
In order to compare the enzymatic production of endophytic fungi, the following commercially acquired hydrolytic enzymes were used: α-amylase from Aspergillus oryzae (Sigma Aldrich), activity 1.5 U/mg (prepared at 5.0 U/mL); Ceremix® Flex amylase (NovoZymes), activity 1.83 U/mL; Ultraflo® enzyme glucanase (NovoZymes), activity 38.5 U/mL; Aspergillus niger cellulase (Sigma Aldrich), activity 30.9 U/mL; Lipase type VII of Candida rugosa (Sigma Aldrich), activity 700 U/mg (prepared at 5.0 U/mL); and Aspergillus niger pectinase (Sigma Aldrich), activity 2.92 U/mL. The enzymes were prepared as indicated by the manufacturer.
Characterization of the amylolytic and cellulolytic extracts
The filtered enzymatic extracts were assayed to verify their enzymatic activity, as described previously, with variations of the pH of the substrate solution (starch for amylase, and CMC for cellulase). Sodium acetate buffer 50 mM was used for pHs 4.0 and 5.0, and sodium phosphate buffer 50 mM was used for pHs 6.0, 7.0 and 8.0.
To evaluate the optimal temperature, the filtered extracts were also assayed for their enzymatic activity, and the reaction mixtures were incubated at temperatures varying from 30 to 50 °C (Sindhu et al. 2011; Carrasco et al. 2017).
The molecular mass of the amylolytic and cellulolytic extracts was estimated using polyacrylamide gel electrophoresis (SDS-PAGE). After filtration, the extracts were precipitated with trichloroacetic acid and resuspended with acetone. The separating gel was prepared at a concentration of 10% (m/v), and 30 μL of sample being placed in the running gel together with 4 μL of molecular weight marker (Amersham™ ECL™ Rainbow™ Marker—Full Range) with molecular masses of 225 kDa, 150 kDa, 102 kDa, 76 kDa, 52 kDa, 38 kDa, 31 kDa, 24 kDa, 17 kDa and 12 kDa. Finally, the electrophoresis was performed in a Mini-PROTEAN® Tetra Cell system (Bio-Rad) under a current of 200 V, 30 A, 30 W, in Tris/glycine/SDS 1× buffer, pH 8.3. After the run, the gel was stained with Coomassie Brilliant Blue G-250 (Benoliel et al. 2013).
Statistical analysis
A completely randomized design was utilized. For solid medium tests, only those samples that presented EI above 2 were used, and the data were organized according to the activity. Variance analyses (ANOVA) were performed using the Minitab® 17.3.1 program (Minitab© Inc. 2003–2006, USA), using the Tukey test for the means, at the level of 5% significance.
Results
Production of hydrolases in solid medium
The percentage of enzymes produced by endophytic fungi from the açai palm is shown in Fig. 1. The production of pectinolytic enzymes in the endophytes was higher than the production of the other enzymes, which indicates the importance of the production of pectinases for the development of some of the fungi associated with E. precatoria. Cellulolytic activity was the second most frequent, followed by amylolytic and lipolytic activity.
The enzyme indices observed after 7 days of incubation are shown in Fig. 2. It was notable that the same endophytic fungus was able to synthesize more than one type of hydrolase. In addition, it was also noted that the fungi isolated from the stems showed higher production levels of enzymes than those of the leaves. The isolate Guignardia sp. S22 presented the highest amylolytic index (3.5), followed by the isolate Penicillium sp. L3 (3.4), as can be seen in Fig. 2a. In Fig. 2b, it is observed that the highest cellulolytic index was obtained by the isolate Guignardia sp. L11 (7.0), followed by Colletotrichum sp. S1 (6.7). As for the pectinolytic index, it is possible to observe in Fig. 2c that the isolates R3 (unknown genus) and Aspergillus sp. R13 were the best producers with an EI of 9.4 and 7.6, respectively. The best lipolytic indices can be seen in Fig. 2d, for which the isolate Penicillium sp. L3 had the highest index (3.9), followed by Guignardia sp. L11 (3.6).
Hydrolytic production in liquid medium
The hydrolytic activity of the enzymatic extracts produced by the endophytic fungi isolated from E. precatoria is shown in Fig. 3. In Fig. 3a, the peak of the amylolytic activity of Penicillium sp. L3 (30.96 U/mL) was seen after 72 h of cultivation, while the isolate Guignardia sp. S22 showed maximum activity on the sixth day (5.86 U/mL). When compared with commercial amylases, it is found that the activity value obtained in the enzyme extract produced by the fungus Penicillium sp. L3 is quite promising, and this isolate demonstrates potential as a new source of amylase.
Hydrolytic activity of enzymatic extracts produced by endophytic fungi isolated from açai palms. a Amylolytic activity of Penicillium sp. L3 (●) and Guignardia sp. S22 (○). b Cellulolytic activity of Colletotrichum sp. S1 (●) and Guignardia sp. L11 (○). c Pectinolytic activity of fungus R3 (●) and Aspergillus sp. R13 (○).d Lipolytic activity of Penicillium sp. L3 (●) and Guignardia sp. L11 (○)
The quantification of cellulolytic activity performed in the enzymatic extracts of the isolates Colletotrichum sp. S1 and Guignardia sp. L11 is shown in Fig. 3b. It is noted that, despite the high EI obtained in solid medium (Fig. 2b), the fungus Guignardia sp. L11 was not able to produce cellulase in liquid medium under the test conditions, with maximum enzymatic activity of 0.79 U/mL after 5 days of cultivation. Colletotrichum sp. S1 showed peak enzymatic activity (15.76 U/mL) after 4 days of cultivation and maintained activity at this level until the end of the experiment. When comparing the enzymatic activity obtained from the S1 fungus with the activities of commercial cellulases, it is perceived that there is a need to optimize the cultivation conditions of the endophyte to increase its cellulolytic production.
The pectinolytic activity of fungal isolates R3 and Aspergillus sp. R13 is shown in Fig. 3c. A similarity can be seen in the pectinase production profile in the two fungi. The isolate R3, whose genus was unidentified, showed maximum activity (0.013 U/mL) near the end of the assay. The R13 isolate showed peak activity on the fifth day (0.011 U/mL). Both isolates produced pectinases with less enzymatic activity than the commercial enzyme.
The quantification of lipolytic activity of enzyme extracts obtained from the isolates Penicillium sp. L3 and Guignardia sp. L11 is shown in Fig. 3d. It is noted that the production of lipase in liquid medium presents similar behavior in the two fungi and has little variation after the fourth day of cultivation. The isolate Penicillium sp. L3 showed the peak of activity at the end of the assay (0.18 U/mL), while the isolate S1 showed higher activity (0.13 U/mL) on the fourth day. On the other hand, the values of enzymatic activity that were obtained were low when compared to the commercial lipase. Therefore, the isolates evaluated here are not remarkable in the production of lipase in liquid medium under these experimental conditions.
Characterization of the amylolytic extract
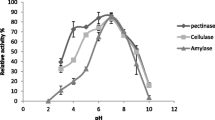
The activity of the amylolytic extract under varying pH and temperature ranges is shown in Fig. 4. High amylase activity can be perceived at different pH values, regardless of the temperature. At 30 °C (Fig. 4a), higher amylase activity was observed at pH 7.0; from 35 to 50 °C (Fig. 4b–e), the enzymatic extract showed higher amylolytic activity at pH 8.0. The best result was observed at 45 °C with a pH value of 8.0 (39.6 U/mL).
Characterization of the cellulolytic extract
The activity of the cellulolytic extract under varying pH and temperature ranges is shown in Fig. 5. It can be noted that at pH 4.0 the cellulolytic activity was low, regardless of the temperature. At 30 °C (Fig. 5a), the highest activities were obtained at pH 6.0, 7.0 and 8.0; while at 35 °C (Fig. 5b), the highest activities were observed at pH 5.0 (12.31 U/mL) and 6.0 (12.07 U/mL). At 40 °C and 45 °C (Fig. 5c, d), the highest activities were found when pHs 6.0, 7.0 and 8.0 were used; and at 50 °C, pHs 5.0, 6.0 and 7.0 were the most adequate for the enzymatic reaction.
Molecular mass profile
The protein profile of the amylolytic and cellulolytic extracts is presented in Fig. 6. The amylolytic extract obtained from the endophytic fungus Penicillium sp. L3 revealed three major bands that had molecular masses between 38 and 76 kDa. The cellulolytic extract produced by the endophytic fungus Colletotrichum sp. S1 presented four bands, with molecular masses between 38 and 52 kDa.
Discussion
Filamentous fungi are known for their ability to grow in different environments, using simple or complex substrates. These microorganisms have been used for the synthesis of several types of enzymes used in industrial applications, mainly hydrolases (Cortez et al. 2017). In recent years, interest in endophytic fungi as new sources of hydrolytic enzymes has increased (Marques et al. 2018; Matias et al. 2021) since these are microorganisms recognized for their potential for synthesis of active metabolites. In addition, fungi associated with different hosts have varied hydrolytic potential, since the production of enzymes in endophytes is associated with colonization of the plant surface. This helps in the hydrolysis of the plant cell wall, as well as serving as direct protection against pathogens (Fadiji and Babalola 2020). Endophytic fungi isolated from açai palms showed versatility in the production of hydrolytic enzymes in solid media. The fungus Colletotrichum sp. S1, isolated from the stem, presented production of amylase, cellulase and pectinase, which are enzymes that are directly involved in the degradation of the plant cell wall. Moreover, the fact that species of this genus can synthesize pectinases is considered a characteristic indicator of the causative agent of anthracnose (Marchi et al. 2009).
The fungus Guignardia sp. L11, which was isolated from the leaf, was able to produce amylase, cellulase and lipase in solid medium. Romão et al. (2011) evaluated enzymatic profiles of the endophyte G. mangiferae and the pathogen G. citricarpa, and found that the pathogenic species synthesized a greater amount of hydrolytic enzymes.
Pectinolytic enzymes were the most produced in solid media, and 83% of the evaluated fungal isolates demonstrated the ability to synthesize these enzymes. The fact that most isolates from açai palms present synthesis of amylases, cellulases and pectinases is not unusual since the action of these enzymes generates assimilable glucose monomers for the host and for the fungus (Lopez and Pereira 2010). In addition, the high presence of synthesized pectinases may suggest the importance of pectinolytic synthesis in some part of the life cycle of the isolates of the açai palm (Bezerra et al. 2012).
In a study conducted by Sandri et al. (2013), which involved 60 isolates obtained from decomposing plant tissues, the pectinolytic levels were compared with those produced by the strains of A. niger T0005007-2 and A. oryzae IPT 301, which are known for being pectinase producers. The authors found that 81% of the isolates presented pectinolytic activity, and this is a similar result to that obtained in the present study. Furthermore, 3% of the isolates presented enzymatic activity similar to that of the standard fungus, and the highest activity (74 U/mL in 96 h) was obtained using an isolate of the genus Aspergillus.
Fungi belonging to the genus Aspergillus, as is the case of the R13 isolate, are microorganisms that are capable of synthesizing enzymes that assist in the degradation of various food products. Therefore, these microorganisms have large-scale biotechnological potential for the synthesis of polygalacturonase, which in turn is used in the clarification of juices (Orlandelli et al. 2015; Gulhane et al. 2016).
Of all the fungi evaluated, 32% were able to produce amylase in solid medium. Amylases hydrolyze glycosidic bonds, which result in sugars and dextrins from starch degradation and can be used in different industrial sectors (Norouzian et al. 2006). These enzymes are obtained from endophytic fungi associated with different hosts. Onofre et al. (2011) identified the potential of the endophytic fungus Colletotrichum gloeosporioides, which was isolated from Baccharis dracunculifolia, as a good producer of amylases. El-Gendy (2012) evaluated the production of glucoamylse by Aspergillus sp. JAN-25, an endophytic isolate of Dendronephtha hamprechii. Hegde et al. (2011) observed the production of amylase from endophytic fungi isolated from Calophyllum inophyllum, for which the greatest enzymatic activity was presented by a Penicillium, as also observed in the present study. The endophytic isolate of E. precatoria, Penicillium sp. L3, shows promise in the production of amylase in liquid medium, corroborating studies that demonstrate that Penicillium species are important hydrolase producers (Li and Zong 2010; Schneider et al. 2014; Rodrigues et al. 2015; Boratyński et al. 2018).
The submerged fermentation method favored the amylolytic synthesis of the isolate Penicillium sp. L3, which can be explained by the fact that the nutrients were more readily available, as well as due to the supply of oxygen through agitation. In addition, the genus Penicillium has been described as a good producer of α and β-amylases. Species of this genus have even managed to use alternative substrates for their growth, such as soybean residues, barley husks, wheat bran and corn husk (Dar et al. 2015; Cunha et al. 2016; Gopinath et al. 2017), which can improve the economic viability of the bioprocess.
On the other hand, lipase production in the endophytes of E. precatoria was low. Only 12% of the isolates synthesized the enzyme in a solid medium, and in a liquid medium the production was incipient. Studies involving endophytic fungi demonstrate the ability of these microorganisms to produce lipases. Shubha and Srinivas (2017) found that 23% of fungi isolated from Cymbidium aloifolium produced the enzyme, which is double that observed in the present study.
Species of the genera Aspergillus and Penicillium are known to be producers of lipases that are used in industry (Li and Zong 2010; Abdel-Azeem et al. 2019). This fact reinforces the potential observed in lipolytic detection assays in solid medium of isolates L3 and R13, which belong to the genera Penicillium and Aspergillus, respectively (Silva et al. 2006; Lopez and Pereira 2010; Matias et al. 2021). According to Cortez et al. (2017), Penicillium species can synthesize high levels of lipase, especially the species P. cyclopium, P. citrinum, P. roqueforti and P. fumiculosum. Therefore, the endophytic fungi Penicillium sp. L3 should be further evaluated under different submerged cultivation conditions since it showed promising results in the solid medium assay.
Nascimento et al. (2015) also state that the fungi that presented the highest lipolytic index were not able to produce the enzyme in liquid media. The authors performed the evaluation of lipolytic production in fungal isolates obtained from the fruit known as “macaúba” (Acrocomia aculeata (Jacq) Lood. Ex Mart.). Of the 19 isolates, four presented lipolytic activity in the qualitative assay and, in quantitative assays, the isolates that showed the highest enzymatic activities were not the same four as those from the qualitative assay. Thus, evaluating all the isolates in lipolytic liquid medium would be an interesting way to observe the activity they may present, and therefore, avoid the possibility of not identifying the best producers.
Although the isolates S22 and L11 (Guignardia sp.) had high amylolytic and cellulolytic indices, respectively, both had low activity in the first hours of the liquid medium cultivation. In this case, it is possible to suppose that a longer period would be required for synthesis, since fungi of the genus Guignardia sp. have a growth that is characteristically slow. Another factor that may have influenced enzymatic synthesis was the variation in glucose levels, which can lead to catabolic repression (Ahmed et al. 2009; Romão et al. 2011; Moran et al. 2013).
The production of cellulase in solid medium was 58% among the fungal isolates of the açai palm. The best producer of cellulase in liquid medium was the fungus Colletotrichum sp. S1, which was a stem isolate. For this isolate, the cellulolytic activity increased in parallel to the growth of the fungus, until the fourth day of cultivation. The same was observed in a study involving C. capsici, which presented maximum cellulolytic synthesis on the tenth day, which, however was then followed by a sudden reduction, indicating the complete consumption of cellulose as a carbon source (Anand et al. 2008; Peeran et al. 2014).
Most industrial cellulases are derived from fungi such as Trichoderma, Aspergillus, and Penicillium. However, the universal ability of plant pathogens is that they can produce a variety of cell wall polysaccharide-degrading enzymes, which include cellulases, for cleavage of glycosidic bonds present in wall polysaccharides of the plant tissues. Therefore, Colletotrichum sp. can be considered an important source of hydrolases (Zhou et al. 2020).
The cellulolytic and amylolytic extracts produced by Colletotrichum sp. S1 and Penicillium sp. L3, respectively, were partially characterized in relation to the optimal pH and temperature. The determination of the pH and temperature at which the extracts present the highest enzymatic activity indicates the possible industrial applications for these enzymes. The cellulolytic extract produced by Colletotrichum sp. S1 maintained its enzymatic activity above 10 U/mL at a pH range that varied from 5.0 to 8.0, regardless of the temperature. Cellulases that prove to be thermostable have important applications, such as in the degradation of microcrystalline cellulose, which is insoluble in water due to its highly compact structure (Delatorre et al. 2010). They can also be used in the degradation of hemicellulose into gums, a process carried out in a pH range between 4.0 and 5.0, and in a temperature range between 40 and 45 °C (Almeida et al. 2013), as well as in the production of feed for ruminants, in which the cellulase needs to be activated at a temperature between 39 and 42 °C (Cysneiros et al. 2013). The cellulolytic extract, however, did not present activity at pH 4.0, which is different from cellulases synthesized by fungi such as Fusarium verticillioides and Aspergillus terreus, which are generally acidophilic (Almeida et al. 2013; Narra et al. 2014).
Zimbardi et al. (2013) optimized the production of β-glucosidases in a strain of Colletotrichum graminicola fermented on a solid substrate, in which wheat bran was considered the best carbon source for inducing the enzymatic activity (109.7 U/mL). The authors found an optimal pH of 4.9 and temperature of 64.6 °C. In a recent study, Zhou et al. (2020) used the endoglucanase gene (CoCel5A) from Colletotrichum orchidophilum in a recombinant Pichia pastoris. After purification, the endoglucanase CoCel5A exhibited optimal activity at 55–75 °C and high thermostability, with the highest activity detected betweeen pHs 4.0–5.0, and excellent pH stability bet pHs 3.0–6.0. Thus, it seems that, as observed in the present study, cellulases produced by Colletotrichum species are thermostable, which is an advantage for industrial applications.
The amylolytic extract produced by the endophytic fungi Penicillium sp. L3 showed the highest enzymatic activity at pH 8.0 and at 45 °C. In the study of Sindhu et al. (2011), which evaluated the activity of α- amylases from Penicillium janthinellum NCIM 49,960, at pH values of between 4.0 and 10.0, and temperatures between 30 and 80 °C, the optimal pH was 5.0, although the enzyme remained stable in the range of 4.0 to 8.0. The optimal temperature was found to be 50 °C, although the enzyme continued to present high activity in the range between 40 and 60 °C. Thus, there appears to be a similarity in the properties of the amylases obtained from Penicillium sp., and this makes them attractive for industrial applications.
The amylolytic extract produced by the Amazonian isolate may be applied in the saccharification stage of beer production, which is carried out at temperatures between 50 and 70 °C. In addition, the extract has possible applications in food processes such as the production of sweeteners, which is generally carried out at pHs of between 4.0 and 6.0 and at a temperature below 60 °C. It can also be used for the production of glucose syrups, which use pHs that range between 7.0 and 8.0, and temperatures between 40 and 70 °C, among other industrial applications (Bon et al. 2008; Damodaran et al. 2008).
The amylolytic extract produced by Penicillium sp. L3 presented two bands with molecular masses between 38 and 52 kDa. The molecular mass of α-amylases from different microbial sources are usually between 10 and 210 kDa, although most are included in the range of 30 to 70 kDa (Janec̆ek 1997; Espinel and López 2009; Baltas et al. 2016). Exoamylases, such as β-amylases, usually have a molecular mass in the range of 22 to 50 kDa; α-glycosidases between 110 and 154 kDa; and glycoamylase between 70 and 76 kDa (Soro et al. 2007; Sagu et al. 2015). Therefore, purification of the extract is necessary, since it enables the determination of the type of amylase produced by the endophytic isolate.
The cellulolytic extract produced by the fungus Colletotrichum sp. S1 presented protein bands with molecular masses between 38 and 52 kDa. The cellulase family includes several enzymes, such as endoglucanases, cellobiohydrolases and beta-glucosidases (Zimbardi et al. 2013). Endoglucanases present molecular masses of between 22 and 50 kDa, while β-glucosidases have molecular masses of between 165 and 182 kDa; and, in exoglucanases, they are between 66 and 72 kDa (Benoliel et al. 2013). The number of bands in the enzyme extract indicates the variety of proteins that were produced. Proteins are synthesized according to the environment to which the microorganism is submitted, thus making the synthesis of different hydrolases possible. If one considers the bands found in the electrophoretic profile of the enzymatic extract produced by the endophyte isolated from the açai palm, it is possible to suggest that the cellulase produced is an endoglucanase. However, it is important to purify and further confirm the type of cellulase that is produced by the fungal isolate.
Conclusion
The endophytic fungi of the açai palm presented significant potential for the production of hydrolytic enzymes. Most of the isolates synthesized the four types of enzymes, which indicates the versatility of these fungi, as these can be used as new sources of hydrolytic enzymes. Pectinolytic activity was most evident in fungi grown in solid medium, while cellulolytic, amylolytic and lipolytic activities were less frequent. On the other hand, in liquid media, pectinolytic activity was low, as was lipolytic activity, and an optimization of the cultivation conditions is necessary in order to obtain greater production of these enzymes. The isolate Penicillium sp. L3 showed higher amylolytic activity in liquid medium, which is comparable to that of the commercially acquired enzyme. The fungus Colletotrichum sp. S1 showed the highest cellulolytic activity among the açai palm isolates. In this study, the production of these two enzymes appears to be very promising, and further studies of these fungi and other possible applications of the hydrolases produced are necessary.
The amylase synthesized by the isolate Penicillium sp. L3 presents greater activity when incubated at 45 °C and pH 8.0. In addition, the amylolytic extract showed stability at pHs from 4.0 to 8.0 and at temperatures between 30 °C and 50 °C. The cellulolytic extract, synthesized by the isolate Colletotrichum sp. S1, showed higher activity at pH 5.0 and 35 °C and, although this extract has been shown to be slightly less tolerant to pH variations, when compared to amylase, its activity showed thermostability, which allows various applications in industrial processes.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abdel-Azeem AM, Abdel-Azeem MA, Abdul-Hadi SY, Darwish AG (2019) Aspergillus: biodiversity, ecological significances, and industrial applications. In: Mishra S, Singh S, Gupta A, Yadav A (eds) Recent advancement in white biotechnology through fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-10480-1_4
Ahmed S, Bashir A, Saleem H, Saadia M, Jamil A (2009) Production and purification of cellulose-degrading enzymes from a filamentous fungus Trichoderma harzianum. Pak J Bot 41:1411–1419
Almeida MN, Falkoski DL, Guimarães VM, Ramos HJO, Visser EM, Maitan-Alfenas GP, Rezende ST (2013) Characteristics of free endoglucanase and glicosydases multienzyme complex from Fusarium verticillioides. Bioresour Technol 143:413–422. https://doi.org/10.1016/j.biortech.2013.06.021
Anand T, Bhaskaran R, Raguchander T, Karthikeyan G, Rajesh M, Senthilraja G (2008) Production of cell wall degrading enzymes and toxins by Colletotrichum capsici and Alternaria alternata causing fruit rot of chillies. J Plant Prot Res 48:437–451. https://doi.org/10.2478/v10045-008-0053-2
Baltas N, Dincer B, Ekinci AP, Kolayli S, Adiguzel A (2016) Purification and characterization of extracellular α-amylase from a thermophilic Anoxybacillus thermarum A4 strain. Braz Arch Biol Technol 59:1–14. https://doi.org/10.1590/1678-4324-2016160346
Batista BN, Silva IR, Rapôso NVM (2018) Isolation and antimicrobial activity evaluation of endophytic fungi of açaizeiro. Rev Fitos 12:161–174. https://doi.org/10.5935/2446-4775.20180015
Benoliel B, Torres FA, Moraes LM (2013) A novel promising Trichoderma harzianum strain for the production of a cellulolytic complex using sugarcane bagasse in natura. Springerplus 2:656. https://doi.org/10.1186/2193-1801-2-656475
Bezerra JDP, Santos MGS, Svedese VM, Lima DMM, Fernandes MJS, Paiva LM, Souza-Motta CM (2012) Richness of endophytic fungi isolated from Opuntia fícus-indica Mill. (Cactaceae) and preliminar screening for enzyme production. World J Microbiol Biotechnol 28:1989–1995. https://doi.org/10.1007/s11274-011-1001-2
Bilal M, Iqbal HMN (2019) Chemical, physical, and biological coordination: an interplay between materials and enzymes as potential for immobilization. Coordin Chem Rev 388:1–23. https://doi.org/10.1016/j.ccr.2019.02.024
Bon EPS, Ferrara MA, Corvo ML, Vermelho AB, Paiva CLA, Alencastro RB, Coelho RRR (2008) Enzimas em biotecnologia: produção, aplicações e mercado. Interciência, Rio de Janeiro
Boratyński F, Szczepańska E, Grudniewska A, Gniłka R, Olejniczak T (2018) Improving of hydrolase biosythesis by solid-state fermentation of Penicillium camemberti on rapeseed cake. Sci Rep 8:10157. https://doi.org/10.1038/s41598-018-28412-y
Carrasco M, Alcaíno J, Cifuentes V, Baeza M (2017) Purification and characterization of a novel cold adapted fungal glucoamylase. Microb Cell Fact 16:75. https://doi.org/10.1186/s12934-017-0693-x
Choi JM, Han SS, Kim HS (2015) Industrial applications of enzyme biocatalysis: current status and future aspect. Biotechnol Adv 33:1443–1454. https://doi.org/10.1016/j.biotechadv.2015.02.014
Cortez DV, Castro HF, Andrade GSS (2017) Potential catalytic of mycelium-bound lipase of filamentous fungi in biotransformation processes. Quim Nova 40:85–96. https://doi.org/10.21577/0100-4042.20160163
Cunha JRB, Santos FCP, Assis FGV, Leal PL (2016) Cultivation of Penicillium spp. in soybean crop residues for production of cellulase, protease and amylase. Rev Ceres 63:597–504. https://doi.org/10.1590/0034-737x201663050002
Cysneiros CSS, Ferreira RN, Oliveira MA, Favoretto AO, Arnhold E, Ulhoa CJ (2013) Production, characterization and evaluation of fibrolytic enzymes on digestibility of forage maize. Cienc Anim Brasileira 14:426–435. https://doi.org/10.5216/cab.v14I4.19491
Damodaran S, Parkin KL, Fanema OR (2008) Química de alimentos de Fennema, 4th edn. Artmed, Porto Alegre
Dar GH, Kamili AN, Nazir R, Bandh SA, Jan TR, Christi MZ (2015) Enhanced production of α-amylase by Penicillium chrysogenum in liquid culture by modifying the process parameters. Microb Pathogenesis 88:10–15. https://doi.org/10.1016/j.micpath.2015.07.016
Delatorre AB, Ladeira SA, Andrade MVV, Barbosa JB, Martins MLL (2010) Micro-organismos termofílicos e enzimas termoestáveis de importância comercial. Perspectivas 4:132–145
El-Gendy MMAA (2012) Production of glucoamylase by marine endophytic Aspergillus sp. JAN-25 under optimized solid-state fermentation conditions on agro residues. Aust J Basic Appl Sci 6:4154
Espinel E, López E (2009) Purification and characterization of a-amylase from Penicillium commune produced by solid state fermentation. Rev Colomb Qui 38:191–208
Fadiji AE, Babalola OO (2020) Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol 8:467. https://doi.org/10.3389/fbioe.2020.00467
Gopinath SCB, Anbu P, Arshad MKM, Lakshmipriya T, Voon CH, Hashim U, Chinni S (2017) Biotechnological process in microbial amylase production. BioMed Res Int 2017:1272193. https://doi.org/10.1155/2017/1272193
Grand View Research (2020) Enzyme’s market size, share & trends analysis report by application (industrial enzymes, specialty enzymes), by product (carbohydrase, proteases, lipases), by source, by region, and segment forecasts, 2020–2027. https://www.grandviewresearch.com/industry-analysis/ enzymes-industry. Accessed 15 April 2021
Gulhane PA, Gomashe AV, Patne M (2016) Endophytic fungi: a source of novel enzymic antioxidants and biologically active secondary metabolites. Int J Recent Sci Res 7:8226–8231
Hankin L, Anagnostakis SL (1975) The use of solid media for detection of enzyme production by fungi. Mycologia 67:597–607. https://doi.org/10.2307/3758395
Hegde SV, Ramesha A, Srinvas C (2011) Optimization of amylase production from an endophytic fungi Discosia sp. isolated from Calophyllum inophyllum. J Agric Technol 7:805–813
Jahan N, Shahid F, Aman A, Mujahid TY, Qader SAU (2017) Utilization of agro waste pectin for the production of industrially important polygalacturonase. Heliyon 3:e00330. https://doi.org/10.1016/j.heliyon.2017.e00330
Janec̆ek S̆ (1997) α-amylase family: molecular biology and evolution. Prog Biophys Mol Biol 67:67–97. https://doi.org/10.1016/S0079-6107(97)00015-1
Kanti A, Sudiana IM (2017) Ethanol production using cellulolytic, xylanolytic and fermentative yeast on cassava waste. In: 1st SATREPS Conference Proceedia, p 39–52
Li N, Zong M-H (2010) Lipases from the genus Penicillium: production, purification, characterization and applications. J Mol Catal B 66:43–54. https://doi.org/10.1016/j.molcatb.2010.05.004
Lopez AMQ, Pereira DST (2010) Interaction between Colletotrichum gloeosporioides and ecotypes of sugar apple. Bragantia 69:105–114. https://doi.org/10.1590/S0006-87052010000100015
Marchi CE, Dornelas FC, Araújo GLR, Rodrigues FL, Freitas BM, Trentin RA, Jerba VF (2009) Pectinolytic activity of Colletotrichum gloeosporioides and its relationship with aggressiveness to Stylosanthes spp. Bragantia 68:423–433. https://doi.org/10.1590/S0006-87052009000200017
Marques NP, Pereira JC, Gomes E, Da Silva R, Araújo AR, Ferreira H, Rodrigues A, Dussán KJ, Bocchini DA (2018) Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind Crop Prod 122:66–75. https://doi.org/10.1016/j.indcrop.2018.05.022
Matias RR, Sepúlveda AMG, Batista BN, De Lucena JMVM, Albuquerque PM (2021) Degradation of Staphylococcus aureus biofilm using hydrolytic enzymes produced by Amazonian endophytic fungi. Appl Biochem Biotech. https://doi.org/10.1007/s12010-021-03542-8
Mendes MMGS, Pereira SA, Oliveira RL, Silva LAO, Duvoisin Junior S, Albuquerque PM (2015) Screening of Amazon fungi for the production of hydrolytic enzymes. Afr J Microbiol Res 9:741–748. https://doi.org/10.5897/AJMR2014.7158
Miller GL (1959) Use of dinitrosalicyllic acid for determination of reducing sugar. Anal Chem 11:426–428. https://doi.org/10.1021/ac60147a030
Moran LA, Horton HR, Scrimgeour KG, Perr MD (2013) Principles of biochemistry, 5th edn. Pearson, New York
Narra M, Dixit G, Divecha J, Kumar K, Madamwar D, Shah AR (2014) Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Int Biodeterior Biodegrad 88:150–161. https://doi.org/10.1016/j.ibiod.2013.12.016
Nascimento CS, Dos Santos VL, Andrade MHC (2015) Análise da produção de protease e lipase por fungos filamentosos isolados do fruto da macaúba (Acrocomia aculeata (Jacq) Lood. ex Mart). Blucher Chem Eng Proc 1:336–343. https://doi.org/10.5151/chemeng-cobeq2014-0222-26465-171722
Norouzian D, Akbarzadeh A, Scharer JM, Moo Young M (2006) Fungal glucoamylases. Biotechnol Adv 24:80–85. https://doi.org/10.1016/j.biotechadv.2005.06.003
Oliveira AN, Flor NS, Oliveira LA (2010) Influence of pH and temperature on amylolytic activity of rhizobia isolated from Amazonian soils. Acta Amazon 40:401–404. https://doi.org/10.1590/S0044-59672010000200019
Onofre SB, Steilmnn P, Bertolini J, Rotta D, Sartori A, Kagimura FY, Groff AS, Mazzali L (2011) Amylolytic enzymes produced by the fungus Colletotrichum gloeosporioides in rice semi-solid fermentation. J Yeast Fungal Res 2:28–32. https://doi.org/10.5897/JYFR11.003
Orlandelli RC, Almeida TT, Alberto RN, Polonio JC, Azevedo JL, Pamphile JA (2015) Antifungal and proteolytic activities of endophytic fungi isolated from Piper hispidum Sw. Braz J Microbiol 46:359–366. https://doi.org/10.1590/S1517-838246220131042
Peeran MF, Kuppusami P, Thiruvengadam R (2014) Pathogenesis of Colletotrichum lindemuthianum the incitant of anthracnose disease in beans mediated by macerating enzymes. The Bioscan 9:295–300
Robinson PK (2015) Enzymes: principles and biotechnological applications. Essays Biochem 59:1–41. https://doi.org/10.1042/bse0590001
Rodrigues MLF, Silva EA, Borba CE, Oliveira ACD, Kruger C, Raimundo RW, Silva LP, Rodriques MF, Stuani BT (2015) Hydrolytic enzymes production by the endophytic fungus Penicillium sp. isolated from the sheets of Ricinus communis L. Rev Bras Energias Renováveis 4:129–145. https://doi.org/10.5380/rber.v4i2.42839
Romão AS, Sposito MB, Andreote FD, Azevedo JL, Araujo WL (2011) Enzymatic differences between the endophyte Guignardia mangiferae (Botryosphaeriacceae) and the citrus pathogen G. citricarpa. Genet Mol Res 10:243–252. https://doi.org/10.4238/vol10-1gmr952
Roosdiana A, Prasetyawan S, Mahdi C, Sutrisno S (2013) Production and characterizarion of Bacillus firmus pectinase. J Pure Appl Chem Res 2:35–41. https://doi.org/10.21776/ub.jpacr.2013.002.01.111
Sagu ST, Nso EJ, Hornann T, Kapseu C, Rawel HM (2015) Extraction and purification of beta-amylase from stems of Abrus precatorius by three phase partitioning. Food Chem 183:144–153. https://doi.org/10.1016/j.foodchem.2015.03.028
Sandri IG, Lorenzoni CMT, Fontana RC, Silveira MM (2013) Use of pectinases produced by a new strain of Aspergillus niger for the enzymatic treatment of apple and blueberry juice. Food Sci Technol 51:469–475. https://doi.org/10.1016/j.lwt.2012.10.015
Schneider WDH, Dos Reis L, Camassola M, Dillon AJP (2014) Morphogenesis and production of enzymes by Penicillium echinulatumin response to different carbon sources. BioMed Res Int 2014:1–10. https://doi.org/10.1155/2014/254863
Shubha J, Srinivas C (2017) Diversity and extracellular enzymes of endophytic fungi associated with Cymbidium aloifolium L. Afr J Biotechnol 16:2248–2258. https://doi.org/10.5897/AJB2017.16261
Silva RLO, Luz JS, Silveira EB, Cavalcante UMT (2006) Endophytic fungi of Annona spp.: isolation, enzymatic characterization of isolates and plant growth promotion in Annona squamosa L. seedlings. Acta Bot Bras 20:649–655. https://doi.org/10.1590/S0102-33062006000300015
Sindhu R, Suprabha GN, Shashidhar S (2011) Purification and characterization of α-amylase from Penicillium janthinellum (NCIM 4960) and its application in detergent industry. Biotechnol Bioinform Bioeng 1:25–32
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6:174. https://doi.org/10.1007/s13205-016-0485-8
Soro RY, Diopoh JK, Willemot RM, Combes D (2007) Enzymatic synthesis of polyglucosylfructosides from sucrose alone α-glucosidase isolated from the digestive juice of Archachatina ventricose (Achatinideae). Enzyme Microb Technol 42:4451. https://doi.org/10.1016/j.enzmictec.2007.07.024
Souza HQ, Oliveira LA, Andrade JS (2008) Screening of basidiomycetes from amazonia for the production of biotechnological interest enzymes. Ciencia Tecnol Alime 28:116–124. https://doi.org/10.1590/S0101-20612008000500019
Vasconcelos NM, Pinto GAS, Aragão FAS (2013) Determination of reducing sugars by 3,5-dinitrosalicylic acid: historical development of the method and establishment of a protocol to the Laboratory of Bioprocess. Boletim de Pesquisa e Desenvolvimento 88. Embrapa Agroindústria Tropical, Fortaleza
Zhou H-Y, Zhou J-B, Yi X-N, Wang Y-M, Xue Y-P, Chen D-S, Cheng X-P, Li M, Wang H-Y, Chen K-Q, Liu Z-Q, Zheng Y-G (2020) Heterologous expression and biochemical characterization of a thermostable endo-β-1,4-glucanase from Colletotrichum orchidophilum. Bioproc Biosyst Eng 44:67–79. https://doi.org/10.1007/s00449-020-02420-7
Zimbardi AL, Sehan C, Meleiro LP, Souza FHM, Masui DC, Nozawa MSF, Guimarães LHS, Jorge JA, Furriel RPM (2013) Optimization of β-glucosidase, β-xylosidase and xylanase production by Colletotrichum graminicolaunder solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol Sci 14:2875–2902. https://doi.org/10.3390/ijms14022875
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES and the Fundação de Amparo à Pesquisa do Estado do Amazonas—FAPEAM for the financial support of this study, the Chemistry Applied to Technology Research Group for the infra-structure availability, and Mr. Matthew Miller for the revision of the English language.
Funding
This work was supported by a research grant from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES (Pro-Amazônia Program, Project 52, and Financial Code 001), and by the Fundação de Amparo à Pesquisa do Estado do Amazonas—FAPEAM.
Author information
Authors and Affiliations
Contributions
BNB performed the fungal hydrolases production, carried out the enzymatic activity testing, the enzymatic extracts characterization and participated in data analysis and drafted the manuscript. RRM carried out the initial screening of Euterpe precatoria endophytic fungi that produced hydrolases and helped in the hydrolases production in liquid medium. RLO participated in the design of the study, guided the enzymatic extracts characterization, participated in the analysis and interpretation of data and contributed to the review of the manuscript. PMA conceived the study, and participated in its design and coordination, carried out the analysis and interpretation of hydrolases production, and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Batista, B.N., Matias, R.R., Oliveira, R.L.e. et al. Hydrolytic enzyme production from açai palm (Euterpe precatoria) endophytic fungi and characterization of the amylolytic and cellulolytic extracts. World J Microbiol Biotechnol 38, 30 (2022). https://doi.org/10.1007/s11274-021-03217-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03217-w