Abstract
The fungal strain, Aspergillus niger SA1, isolated from textile wastewater sludge was screened for its decolorization ability for four different textile dyes. It was initially adapted to higher concentration of dyes (10–1,000 mg l−1) on solid culture medium after repeated sub-culturing. Maximum resistant level (mg l−1) sustained by fungal strain against four dyes was in order of; Acid red 151 (850) > Orange II (650) > Drimarene blue K2RL (550) > Sulfur black (500). The apparent dye removal for dyes was seen largely due to biosorption/bioadsorption into/onto the fungal biomass. Decolorization of Acid red 151, Orange II, Sulfur black and Drimarine blue K2RL was 68.64 and 66.72, 43.23 and 44.52, 21.74 and 28.18, 39.45 and 9.33% in two different liquid media under static condition, whereas, it was 67.26, 78.08, 45.83 and 13.74% with 1.40, 1.73, 5.16 and 1.87 mg l−1 of biomass production under shaking conditions respectively in 8 days. The residual amount (mg l−1) of the three products (α-naphthol, sulfanilic acid and aniline) kept quite low i.e., ≤2 in case AR 151 and Or II under shaking conditions. Results clearly elucidated the role of Aspergillus niger SA1 in decolorizing/degrading structurally different dyes into basic constituents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colored wastewater from textile industry is rated as the most polluted in all industrial sectors. It has been estimated that over 10,000 different textile dyes and pigments are in common use and the total world organic colorant production is more than 100,000 tons/year (Pagga and Brown 1986; Easton 1995; McMullan et al. 1995, 2001). Huge amount of dyes in textile sectors are continuously being exhausted in wastewater streams due to their poor adsorbability to the fiber (Wagner 1993; McMullan et al. 2001). Synthetic dyes specially, sulfonated and their related biodegradation products contain structural elements, which are unknown or rare in nature; they not only have a negative aesthetic effect but also resist microbial attack and contribute to aquatic and soil toxicity (Chung et al. 1981; Reid et al. 1984; Rosenkranz and Klopman 1990; Grover et al. 1996; Wang and Yu 1998).
The decolorization of textile waste water is still a major environmental concern because of synthetic dyes which are difficult to be removed by conventional treatment systems (Robinson et al. 2001; Verma et al. 2003; Zhang et al. 2004). However, a perception is growing nowadays in favor of using modern biological techniques in tackling different environmental problems. Biological remediation is considered more efficient in terms of its long lasting benefits and for having almost no harmful effects on environment. In addition, it would be cheaper as compared to other different physicochemical techniques tested (Balan and Monterio 2001; Wallace 2001). Various fungal strains that have proved more efficient in decolorization of textile dyes mostly belonged to the group of White-rot. Dyes are removed by fungi by biosorption (Conatao and Corso 1996; Paymann and Mehnaz 1998; Fu and Viraraghavan 2000) and enzymatic mineralization (degradation) [Lignin peroxidase (LiP), manganese peroxidase (MnP), manganese independent peroxidase (MIP), laccases (Laccs)] (Young and Yu 1997; Ollikka et al. 1998; Podgornik et al. 1999; Wong and Yu 1999; Zheng et al. 1999; Ferreira et al. 2000; Pointing and Vrijmoed 2000; Minussi et al. 2001; Wesenberg et al. 2003; Svobodova et al. 2006). However, one or more of these mechanisms could be involved in color removal, depending on the fungus used.
The four structurally different dyes (Acid red 151, Orange II, Drimarene blue K2RL and Sulfur black) selected in the present research work are known for their high use in textile industry. These dyes, due to their poor adsorbability in textile fiber in comparison with other classes of dyes have a higher exhaustion rate in the wastewater. Due to recalcitrant nature of such dyes in wastewater, the biodecolorization/biodegradation ability of an indigenous fungal isolate, Aspergillus niger SA1 (Ascomycetes) was trialed under different operational conditions.
Experimental
Chemicals
The four textile dyes used in this study were including Acid red (AR) 151 (Di-azo), Orange (Or) II (Mono-azo), Drimarene blue (Db) K2RL (Anthraquinone based Reactive) and Sulfur black (Sb) (Anthraquinone). AR 151 and Or II were purchased from Sigma chemicals Co., while Sb and Db K2RL were obtained from Kohinoor textile mill, Rawalpindi, Pakistan. Chemical compounds and media components used in the study were obtained from BDH laboratory chemical division, Poole, Dorset, England, Sigma chemicals Co., St, Louis and E. Merck, Darmstadt, Germany (Fig. 1).
Culture media
Saboraud dextrose broth (SDB) (Merck) and mineral salt media were used in the study. Mineral salt medium was made by adding per liter of distilled water; Acetic Acid (99.9%) 0.150 ml, (NH2)2CO 100.0 mg, KH2PO4 67.0 mg, NaHCO3 840.0 mg, MgSO4.7H2O 38.0 mg, CaCl2 21.0 mg, FeCl3 6H2O 7.0 mg and glucose 6 g. pH of the media was adjusted to 8 by using 0.1M HCl and NaOH. Agar (15 g l−1) was used as solidifying agent in the media when required in the experiments.
Isolation of fungal strain
The fungus was isolated from the diluted sludge sample taken from textile wastewater pond on Saboraud dextrose agar (SDB) plates at 28°C. The fungal isolate was identified as Aspergillus niger SA1 on the basis of its vegetative and reproductive structure using dichotomous keys by the expertise of Pakistan Museum of Natural History, Islamabad, Pakistan.
Preparation of fungal inoculum
Aspergillus niger SA1 was grown in SDB on a rotatory shaking incubator (100 rpm, 28°C) for 8 days and its biomass was then filtered and washed twice with distilled water. Fungal bioimass (pellets), 5 g 100 ml−1 of distilled water was homogenized in a blender for 5 min and later used as inoculum in the experiments.
Adaptation of A. niger SA1 to higher concentration of textile dyes
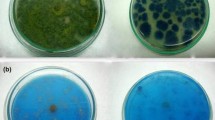
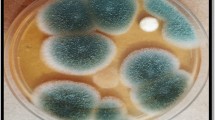
The fungal strain A. niger SA1 was sequentially adapted to higher concentrations (10–1,000 mg l−1) of each dye by being sub-culturing it multiple times on dye containing mineral salt-agar plates at pH 7. Dyes containing plates inoculated with fresh fungal inoculum (5 mm disc) were incubated for 8 days at 28°C. The maximum resistant levels (MRL) beyond which the fungal growth was completely inhibited was marked. Besides rich growth limits (RGL) of the fungal isolate on solid medium containing dyes were also determined. RGL defined as the growth appeared (in diameter) i.e., ≥half and ≤full on dye containing medium compared to one obtained on medium devoid of any dye. The apparent dye removal by the fungal strain was critically examined into/onto the hyphae by microscope and as decolorization zones in media plates.
Decolorization of different textile dyes by A. niger SA1
Decolorization assays of different textile dyes with A. niger SA1 were carried out by taking 100 ml of saboraud dextrose broth or mineral salt media containing 20 mg l−1 of dye in cotton plugged Erlenmeyer flasks. Each experimental flask was inoculated with 0.001% (w/v) fresh inoculum of homogenized fungal biomass. The experiments were carried out in static and shaking (100 rpm) conditions for 8 days at 30°C. A group of three flasks was operated for each dye decolorization assay along with a set of control flasks without fungal inoculum.
Analytical methods
Samples (2 ml each) were drawn from dye containing medium during treatment after every 24 h in plastic cuvettes. Initially, each sample was filtered and centrifuged at 10,000 rpm for the analysis of the supernatant. The residual amount of dyes in each sample was monitored through Shimadzu UV-visible spectrophotometer at their respective wavelengths (λmax) i.e., Db K2RL = 620 nm; Sb = 610 nm; AR 151 = 512 nm; Or II = 480 nm. The initial and final samples in case of AR 151 and Or II were also analyzed by using Agilent 1100 HPLC with a C18 column at a flow rate of 1.5 ml/min. The solvent system (mobile phase) used during analysis of Or II (λmax = 231 nm) was acetonitrile and 0.03 M ammonium carbonate buffer (30:70%) and for AR 151 (λmax = 225 nm), it was acetonitrile and H2O (30:70). Percentage (%) decolorization of dyes in liquid media was determined by using the following formula;
Fungal biomass produced during each experiment was separated out on 8th day from culture filtrate by Whattman filter paper No. 1. It was then dried overnight at 100°C in an oven to calculate the dry weight.
The results obtained during experimentation were expressed in terms of Means and Standard error (SE). Data was defined statistically by ANOVA (Single factor), T-test and LSD test by using Microsoft excel and MSTAT softwares. Correlation between different parameters was calculated by SPSS software. Probability (p-value) less than 0.05 and 0.01 was considered significant and highly significant respectively.
Precaution
Experiments were performed under standard sterilized conditions. Glassware and media used in experiments were properly sterilized before use at 121°C and at 15 psi pressure for 20 min.
Result and discussion
Adaptation of A. niger SA1 to higher concentrations of textile dyes
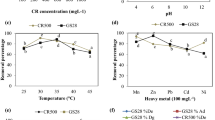
The fungal strain initially adapted to higher concentrations of four dyes on mineral salt–agar plates showed quite high resistance level (MRL) (average = 637.50 ± 77.39 mg l−1) ranging from 500–850 mg l−1. Maximum resistance level (mg l−1) was observed against AR 151 i.e., 850, while 650, 500 and 550 against Or II, Sb and Db K2RL respectively (Fig. 2). Increase in concentration of dyes from 10–1,000 mg l−1 led to decrease in fungal growth (in diameter) on media plates. Besides, the rich growth limits (RGL) of A. niger SA1 was below MRL values against different concentration of each tested dye. In addition, the growth of A. niger SA1 seemed to be correlated with its apparent dye removal efficiency. Similarly, dyes removals on solid medium with related growth of different fungal strains (basidiomycetes) have also been mentioned in different reports (Shah and Nerud 2002; Wesenberg et al. 2003; Machado et al. 2005). Dyes removal by A. niger SA1 was microscopically found more due to biosorption/bioadsorption into/onto fungal hyphae as was reported by Fu and Viraraghavan (2000). However, diffused decolorization halos around fungal colonies specifically with AR 151 also pointed towards extracellular degradation of dyes. Likewise, Balan and Monterio (2001) assigned reduction of a dye (Indigo) intensity in the medium to adsorption as well as extracellular fungal activity. Biosorption was considered as the prime dye removal phenomenon in wood rotting basidiomycetes (Knapp and Newby 1995). Adsorption has been attributed to the electrostatic attraction between the negatively charged dye with positively charged cell wall constituents such as chitin, acidic polysaccharides, lipids, or amino acids (Aksu et al. 1999; Aksu and Tezer 2000). However, increase in concentration (>100 mg l−1) of dye (Reactive blue) at times proved to be toxic, thereby limiting the decolorization activity of Aspergillus spp. (Ramya et al. 2007). Moreover, decolorization of different dyes on solid culture media was noticed considerably slower than radial fungal growth and it occurred between 5–30 days (Pasti-Grigsby et al. 1996; Minussi et al. 2001; Machado et al. 2006). However, fungal colonies previously adapted to azo dyes, showing decolorizing abilities, gave considerably higher results than those taken from the non-treated culture medium (Wataru et al. 1999; Arora and Chander 2004).
Decolorization/degradation of dyes under static and shaking conditions
Screening of A. niger SA1 pre-adapted to higher concentration of dyes, was carried out for the decolorization/degradation of four structurally different dyes in two different media SDB and mineral salt media under static condition (8 days). Decolorization (%) insignificantly differed in two media in all the tested dyes with an exception of Db K2RL [(T-test; 2 sample assuming equal variance) (n = 3) (p < 0.05)]. It was 68.64 and 66.72, 43.23 and 44.52, 21.74 and 28.18, 39.45 and 9.33 in AR 151, Or II, Sb and Db K2RL in SDB and mineral salt media respectively (Fig. 3). So, the use of different media did not bring any major difference in decolorizing of AR 151 and Or II, although, the composition of the two media quite differed. Specifically, the amount of additional carbon source as dextrose sugar in SDB (40 g l−1) was considerably higher than glucose used in mineral salt medium (6 g l−1). This indicated that dyes might have been used as primary nutritional source (specifically carbon) in fungal metabolism compared with other nutrients in culture media. From relatively smaller to major structural differences including class/type and side-groups in dyes can markedly affect decolorization (Sani et al. 1998; Wallace 2001) and that were also noted in the present study.
There was observed a significant (p < 0.01) increase in the decolorization of Or II, Sb and Db K2RL in mineral salt medium when experiments were shifted from static to shaking condition, though the results were almost similar in case of AR 151 [(T-test; 2 sample assuming equal variance) (n = 3)]. Decolorization was maximum in Or II, i.e., 78.08% with 1.73 mg l−1 of biomass production. Whereas, it was 67.26, 45.83 and 13.74% with 1.40, 5.16 and 1.87 mg l−1 of biomass in case of AR 151, Sb and Db K2RL respectively in 8 days (Fig. 4). Generally, increase in decolorization efficiency has been linked with shaking due to an increase in mass and oxygen transfer between cells and the medium, factors that optimize the action of oxidative enzymes. Shaking (200 rpm) resulted in an increase in dye decolorization by P. chrysosporium, Trametes versicolor, Bjerkandera spp. BOS55, Bjerkandera fumosa, Kuehneromyces mutabilis, Strofaria rugoso-annulata and T. villosa (Swamy and Ramsay 1999; Jarosz-Wilkolazka et al. 2002; Wesenberg et al. 2003; Machado et al. 2006). However, better dyes’ decolorization abilities were reported in Phlebia tremellosa under static conditions (Kirby et al. 2000). In this context, different reports suggested that the enzymes involved in the decolorization might have been suppressed or altered under agitation (Glenn and Gold 1983; Kuwahara et al. 1984), despite, dyes removal through biosorption proved to be slower under static culture conditions.
Apparently, the phenomenon of biosorption was seen in the removal of dyes from liquid culture medium. Further, reduction of dyes after biosorption onto the biomass indicated biodegradation. HPLC analysis showing declining concentrations of AR 151 and Or II with formation of considerably low residual amount (≤2 mg l−1) of three products (1-naphthol, 4-aminobenzenesulfonic acid (4ABS) and aniline) have favorably supported the idea that mineralization of dyes has occurred (Fig. 4). A general decrease in dyes (like Indigo and Reactive blue) concentration of this kind was more attributed to degradation than adsorption by fungi (Balan and Monterio 2001; Wesenberg et al. 2002; Ramya et al. 2007). The most decolorized dyes, AR 151 and Or II were both azo (Sulfonated) compared to Sb and Db K2RL (Anthraquinone base) in the present study. Similar results (8.7%) as mentioned in case of Sb and Db K2RL were reported in Coloron Black due to its complex chemical structure, higher molecular weight and the presence of inhibitory groups like –NO2 and –SO3Na (Hu and Wu 2001). Contrarily, it was reported in a study that Reactive blue 19 and Reactive blue 49, which are anthraquinone-based structures, were decolorized easily in short period (99% in 8 h) as compared to azo dye Reactive black 5 by Trametes versicolor KCTC 16781 (Kim et al. 2004). This comparative opposite situations clearly reflected different metabolizing properties of the different fungi.
The improvement in decolorization of dyes was apparently associated with increasing amount of fungal biomass under shaking condition. But, insignificant correlation between decolorization (%) of different dyes and biomass production by A. niger SA1 (r = −0.19) (Fig. 4) suggested varying substrate (dyes) specificity of fungus (Wallace 2001). Nonetheless, a substantial varying decrease in biomass with different dyes (at 20 mg l−1) in liquid medium (Fig. 4) indicated that different substituents patterns in dyes and related products (Fig. 1) were causing inhibitory effect on fungal growth. In addition, dyes at specific concentrations were found to create a long lag phase, limiting growth and metabolizing properties of different fungal strains under different culture conditions (Sollai et al. 1996; Albanis et al. 2000; Aksu 2003; Kasinath et al. 2003).
Conclusion
Decolorization/degradation of dyes has been mostly linked with White-rot fungi, however, newly isolated fungal strain, A. niger SA1 belonging to a different group of fungi showed great ability to grow and to decolorize 4 different dyes. Moreover, results proved that decolorization of dyes were more efficient in shaking condition as compared to static one. The production of less amount of degradation products of AR 151 and Or II further validate the role of fungal isolate to be used in bioremediation of dye containing textile effluent.
References
Aksu Z (2003) Reactive dye bioaccumulation by Saccharomyces cerevisiae. Process Biochem 38(10):1437–1444
Aksu Z, Tezer S (2000) Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: effect of temperature. Process Biochem 36:431–439
Aksu Z, Calik A, Dursun AY, Demircan Z (1999) Biosorption of iron III cyanide complex anions to R. arrhizus: application of adsorption isotherms. Process Biochem 34:483–491
Albanis TA, Hela DG, Sakellarides TM, Danis TG (2000) Removal of dyes from aqueous solutions by adsorption on mixtures of fly ash and soil in batch and column techniques. Global Nest Int J 2(3):237–244
Arora DS, Chander M (2004) Decolorization of diverse industrial dyes by some Phlebia spp. and their comparison with Phanerochaete chrysosporium. J Basic Microbiol 44(5):331–338
Balan DSC, Monterio RTR (2001) Decolorization of textile dye indigo by lignolytic fungi. J Biotechnol 89:141–145
Chung KT, Fluk GE, Andrews AE (1981) Mutagenicity testing of some commonly used dyes. App Environ Microbiol 42(4):641–648
Conatao M, Corso CR (1996) Studies of adsorptive interaction between Aspergillus niger and the reactive azo dye procion blue MX-G. Eclet Quim 21:97–102
Easton JR (1995) The dye maker’s view. In: Cooper P (ed) Color in dyehouse effluent. Society of Dyers and Colorists, Bradford, pp 9–21
Ferreira VS, Magalhaes DB, Kling SH, da Silva JG, Bon EPS (2000) N-demethylation of methylene blue by lignin peroxidase from Phanerochaete chrysosporium: Stoichiometric relation for H2O2 consumption. Appl Biochem Biotechnol 84–86:255–265
Fu YZ, Viraraghavan T (2000) Removal of a dye from aqueous solution by the fungus Aspergillus niger. Wat Qual Res J Can 35:95–111
Glenn JK, Gold MH (1983) Decolorization of several polymeric dyes by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 45:1741–1747
Grover IS, Kaur A, Mahajan RK (1996) Mutagenicity of some dye effluents. Nat Acad Sci Lett India 19(7–8):149–158
Hu T, Wu SC (2001) Assessment of the effect of azo dye Rp2B on the growth of nitrogen fixing cyanobacterium Anabena spp. Biores Technol 77:3–95
Jarosz-Wilkolazka A, Kochmanska-rdest J, Malarczyk E, Wardas W, Leonowicz A (2002) Fungi and their ability to decolorize azo and anthraquinonic dyes. Enzyme Microb Technol 30:566–572
Kasinath A, Novotny C, Svobodova K, Patel KC, Sasek V (2003) Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed bed reactor. Enzyme Microbiol Technol 32:167–173
Kim K, Lee Y, Yang J, Lee B, Park C, Kim S (2004) Decolorization of dye solutions by a membrane bioreactor (MBR) using White-rot fungi. Desalination 168:287–293
Kirby N, Marchant R, McMullan G (2000) Decolorization of synthetic textile dyes by Phlebia tremellosa. FEMS Microbiol Lett 188:93–96
Knapp JS, Newby PS (1995) Decolorization of dyes by wood rotting basidiomycetes fungi. Wat Res 29:1807–1809
Kuwahara M, Glenn KJ, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Machado KMG, Matheus DR, Bononi VLR (2005) Ligninolytic enzymes production and Remazol brilliant blue R decolorization by tropical Brazilian basidiomycetes fungi. Braz J Microbiol 36:246–252
Machado KMG, Compart LCA, Morais RO, Rosa LH, Santos MH (2006) Biodegradation of reactive textile dyes by Basidiomycetous fungi from Brazilian ecosystems. Braz J Microbiol 37:481–487
McMullan G, Poonam N, Franklin S, Oxspring D (1995) Bioremediation and chemical analysis of textile industry wastewater. Biotechnol Lett 17:76–764
McMullan G, Meehan C, Conneely A, Nirby N, Robinson T, Nigam P, Banat IM, Marchant SWF (2001) Mini review: microbial decolorization and degradation of textile dyes. Appl Microbiol Biotechnol 56:81–87
Minussi RC, de Moraes SG, Pastore GM, Duran N (2001) Biodecolorization screening of synthetic dyes by four White-rot fungi in a solid medium: possible role of siderophores. Lett in Appl Microbiol 33:21–25
Ollikka P, Harjunpaa T, Palmu K, Mantsala P, Suominen I (1998) Oxidation of croein orange G by lignin peroxidase iso-enzyme. Kinetics and effects of H2O2. Appl Biochem Biotechnol 75:307–321
Pagga U, Brown D (1986) The degradation of dyestuffs: Part II, Behaviour of dyestuffs in aerobic biodegradation test. Chemosphere 15(4):479–491
Pasti-Grigsby MB, Burke NS, Goszczynski S, Crawford DL (1996) Transformation of azo Dye Isomers by Streptomyces chromofuscus A11. Appl Environ Microbiol 62(5):1814–1817
Paymann MA, Mehnaz MA (1998) Decolorization of textile effluent by Aspergillus niger (marine and terrestrial). Fresen Environ Bull 7:1–7
Podgornik H, Gregic I, Perdih A (1999) Decolorization rate of dyes using lignin peroxidases of Phanerochaete chrysosporium. Chemosphere 38:1353–1359
Pointing SB, Vrijmoed LLP (2000) Decolorization of azo and triphenylmethane dyes by Pycnoporus sanguineus producing laccase as the sole phenol oxidase. World J Microbiol Biotechnol 16:317–318
Ramya M, Anusha B, Kalavathy S, Devilaksmi S (2007) Biodecolorization and biodegradation of Reactive Blue by Aspergillus spp. Afr J Biotechnol 6(12):1441–1445
Reid TM, Morton KC, Wang CY, King CM (1984) Mutagenicity of azo dyes following metabolism by different reductive/oxidative systems. Environ Mutagen 6:705–717
Robinson T, Chandran B, Nigam P (2001) Studies on the decolorization of an artificial textile-effluent by White-rot fungi in N-rich and N-limited media. Appl Microbiol Biotechnol 57(5–6):810–813
Rosenkranz HS, Klopman G (1990) Structural basis of the mutagenicity of 1-amino-2-naphthol-based azo dyes. Mutagenesis 5(2):137–146
Sani RK, Azmi W, Banerjee UC (1998) Comparison of static and shake culture in the decolorization of textile dyes and dye effluents by Phaenerochaete chrysosporium. Folia Microbiol 43:85–88
Shah V, Nerud F (2002) Lignin degrading system of White-rot fungi and its exploitation for dye decolorization. Review. Can J Microbiol 48:857–870
Sollai F, Curreli N, Porcu MC, Rescigno A, Rinaldi AC, Rinaldi A et al (1996) Effects of some substituted anthraquinones and anthrones on laccase production in Pleurotus sajor-caju. Biochem Arch 12:7–12
Svobodova K, Erbanova P, Sklenar J, Novotny C (2006) The role of Mn-dependent peroxidase in dye decolorization by static and agitated culture of Irpex lacteus. Folia Microbiol 51(6):573–578
Swamy J, Ramsay JA (1999) The evaluation of White-rot fungi in the decolorization of textile dyes. Enzyme Microbial Technol 24:130–137
Verma P, Baldrian P, Nerud F (2003) Decolorization of structurally different synthetic dyes using cobalt (II)/ascorbic acid/hydrogen peroxide system. Chemosphere 50:975–979
Wagner S (1993) Improvement in products and processing to diminish environmental impact. COTTECH Conference, Raleigh NC, 11–12 November 1993
Wallace TH (2001) Biological treatment of synthetic dye water and an industrial textile wastewater containing azo dye compounds. MSc Thesis. Faculty of Virginia Polytechnic Institute and State University, USA
Wang Y, Yu J (1998) Adsorption and degradation of synthetic dyes on the mycelium of Trametes versicolor. Wat Sci Technol 38:232–237
Wataru S, Miyashita T, Yokoyama J, Arai M (1999) Isolation of azo dye degrading microorganisms and their application to white discharge printing of fabric. J Biol Sci Bioeng 88(5):577–581
Wesenberg D, Buchon F, Agathos SN (2002) Degradation of dye containing textile effluent by the agaric White-rot fungus Clitocybula dusenii. Biotechnol Lett 24:989–993
Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22:161–187
Wong YX, Yu J (1999) Laccase catalyzed decolorization of synthetic dyes. Wat Res 33:3512–3520
Young L, Yu J (1997) Lignin catalyzed decolorization of synthetic dyes. Wat Res 31:1187–1193
Zhang F, Yediler A, Liang X, Kettrup A (2004) Effects of dye additives on the ozonation process and oxidation by products: a comparative study using hydrolyzed CI Reactive red 120. Dyes Pigments 60:1–7
Zheng Z, Levin RE, Pinkham JL, Shetty K (1999) Decolorization of polymeric dyes by a novel Penicillium isolate. Proc Biochem 34:31–37
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ali, N., Hameed, A., Ahmed, S. et al. Decolorization of structurally different textile dyes by Aspergillus niger SA1. World J Microbiol Biotechnol 24, 1067–1072 (2008). https://doi.org/10.1007/s11274-007-9577-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9577-2