Abstract
In this work, Aspergillus terreus GS28 and Aspergillus flavus CR500 isolated from industrial waste sludge examined for the decolorization of Congo red (CR) dye. The rate of CR decolorization raised due to optimum pH, temperature, carbon, nitrogen, and heavy metals. In the comparative study, A. terreus has the maximum ability (95%) to decolorize CR (≈ 100 mg L−1) as compared with A. flavus (92.96%) under optimized condition after 120 h. GC–MS and FTIR analysis of the fungal-metabolite and fungal-biomass shows bio-degradation and biosorption processes respectively. The degraded products were benzenepropanic (Rt-26.147), 3, 4-diaminonapthelene-1-sulfonic acid, and benzenedicarboxylic acid (Rt-26.660) by A. terreus, and benzenedicarboxylic acid (Rt-41.467) by A. flavus. The phytotoxicity assay revealed that a decrease in toxicity of the degraded product towards the growth and germination rate of two plant seeds compared to CR. Thus, the finding suggests that both the fungi act promising CR remediation candidates, induces restoration of CR polluted wastewater and save soil-land.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The indiscriminate disposal of chemical compounds in water bodies and soil adversely affects the whole biosphere (Bankole et al. 2018; Syafiuddin and Fulazzaky 2021). Synthetic dyes increased their attention because of their broad range of use in different sectors for coloring purposes (Tan et al. 2019). Among synthetic dyes, azo dyes have taken great attention due to their frequent use in the industrial sector because of their stability in nature and less cost production as other groups like anthraquinone dyes (Sen et al. 2016). Subsequently, azo dye contains an azo group of a bond (–N=N–) is joint with benzene or naphthalene rings (Mnif et al. 2016; He et al. 2018). About 10%–15% of dyes can be discharged from coloring industries in the aquatic environment which affects the light penetration, reduces photosynthetic activities, causes eutrophication and interference in the aquatic-ecosystem (Asses et al. 2018). It has an offensive impact on life-forms like allergy and their nature is carcinogens, toxic and mutagenic (Sen et al. 2016; Tan et al. 2019). Benzidine-based anionic dye, CR (Congo red) is the most common and frequently used in textile industries (Mnif et al. 2016; Singh et al. 2021).
Several physicochemical techniques are available for the removal of dye but all the methods are so costly and also release a vast quantity of sludge by which their use is not beneficial at the economic level and not ecofriendly at the industrial level (Sen et al. 2016). The microbial bioremediation processes are ecofriendly suitable method for reducing CR azo dye in wastewater through its degradation in less toxic components (Syafiuddin and Fulazzaky 2021). In the decolorization of dye, fungi contain exclusive features as their biomass (live or dead biomass) is used as adsorption and their supernatant may use as the degradation process (Singh and Dwivedi 2020). Several researchers have investigated and revealed that the fungus have ability to degrade various type of azo dye including Congo red (CR) from waste water (Levin et al. 2010; Almida and Corso 2014; Zhang et al. 2020). Wang et al. (2017) illustrate that Ceriporia lacerates decolorize CR up to 90% at 100 mgL−1. The fungal cell wall/ biomass can remove contaminants from environments through the adsorption process (Tan et al. 2019). The extracellular enzymes of fungi, such as laccase (lac), manganese peroxidase (MnP) and lignin peroxidase (LiP) etc. are also involved in the degradation of the azo dye (Wang et al. 2017; Asses et al. 2018; Iark et al. 2019).
Fungi can achieve maximum dye decolorization or degradation on different parameters such as pH, temperature, time, nutrient sources, dye concentration and carbon–nitrogen sources etc. (Singh and Dwivedi 2020). Keeping this fact, the present study aimed with (i) comparative study of CR decolorization by both fungi at optimized condition (ii) identify the mechanisms of decolorization (degradation/adsorption) process of CR by both native fungal isolates and (iii) phytotoxicity test to confirm the eco-toxicological effect of fungal dye degraded products.
Materials and Methods
Congo red (azo dye) was obtained from Sigma-Aldrich while further chemicals were procured from HiMedia, Pvt. Ltd., Mumbai, India. CR solution of 1000 mg L−1 was prepared, preserved at 4°C and used as a stock solution.
A. terreus GS28 was previously isolated from the mud of carpet industrial area (Singh and Dwivedi 2020) and A. flavus CR500 was isolated from wastewater of electroplating industries (Kumar et al. 2019). Both fungi were molecularly characterized and ITS region sequencing was submitted to genebank NCBI database under the accession number (isolate GS28-MK574833 and isolate CR500-MK450338).
Decolorization experiments at several concentration (100–1000 mg L−1) and time interval (24-168 h) were conducted as illustrated by Singh and Dwivedi (2020) and by taking the absorbance of supernatants at λmax = 497 nm using a spectrophotometer (SYSTRONICS Double beam spectrophotometer 2203). The final dye concentration was calculated (Chakraborty et al. 2013). However, after 168 h, the biomass was collected by filtering culture through Whatman filter paper No. 1 (Singh and Dwivedi 2020) and dried at 60 °C (6 h) followed by weighing on Weighing balance (Sartorius BT 224S). Percent decolorization concentration after treatment was determined by the following formula (Singh et al. 2021).
where Ai = Initial dye concentration before treatment, Af = Final dye concentration after treatment.
To optimize the pH for better removal, the experiment was conducted at various pH (4.0, 6.0, 8.0, 10.0 and 12.0) at 30°C, 120 rpm, for 120 h and 800 mg L−1 of CR containing solution. pH was maintained by 0.1 M NaCl and HCl solution (Singh and Dwivedi 2020).
To examine the role of various temperature (25, 30, 35, 40, 45°C) on removal of CR was analyzed by incubating the both fungi in different conical flask containing CR (800 mg L−1) amended in 50 mL of PDB medium. Temperature was maintained in thermostat incubator shaker (pH 7.0 and 120 rpm in shaking condition) for 120 h (Kumar and Dwivedi 2019).
The consequences of carbon and nitrogen sources on CR decolorization by fungi were analyzed using CR (800 mg L−1) containing 50 mL of PDB medium supplemented with 0.25 g of different carbon (Glucose, Dextrose) nitrogen sources (Peptone, Yeast extract and Beef extract) (Singh et al. 2021). All the treatments were conducted in triplicates in the experimental condition at 30°C, pH 7.0, and 120 rpm for 120 h. Carbon sources were added in a concentration of 0.5% w/v each (Basak et al. 2013).
To analyze the effect of heavy metal on the percent decolorization (degradation and adsorption) of CR were performed. These metal salts (MnSO4, ZnSO4, Pb(NO3)2, CdCl2 and NiSO4) were used with single concentration of 100 mg L−1supplemented with PDB medium added with single concentration of CR (800 mg L−1) at 30°C, pH 7.0,120 rpm for 120 h (Chakraborty et al. 2013; Singh et al. 2021).
Among these heavy metals Mn (II) act as enhancer for the CR decolorization activity, so the various concentration of Mn (50, 100, 150 and 200 mg L−1) were also tested to check the decolorization rate of CR. All experiments were conducted in triplicate and the percentage decolorization was calculated by Eq. (1).
The decolorization efficiency of CR by both fungi was investigated under optimum conditions. The optimized condition was selected from the above experiments. The decolorization assay with A. flavus was performed at pH 6.0 and 800 mg L−1 of CR using glucose (5 gL−1) and Mn (100 mgL−1) amended PDB and incubated at 30°C for 120 h in an incubator shaker at 120 rpm. The same conditions followed for decolorization with A. terreus except for temperature (35 °C) and pH (8.0) and the further process was performed as above discussed and decolorization percent was calculated by Eq. (1) (Singh et al. 2015). After 168 h treatment, biomass obtained from culture broth and dip into 50 ml of distilled water followed by autoclaving at 121°C and 18 psi for 15 min (Singh and Dwivedi 2020). OD of the obtained solution was taken at 497 nm. The adsorption amount was calculated as follows
To assess the reusability of obtained biomass, the biomass obtained at the treatment of only 100 mgL−1 of CR was rinsed with different types of solution (0.1 M HCl and NaOH solution) at room temperature for 1 h separately and subsequently rinsed by distilled water. Different solution rinsed biomass were used of decolorization of 100 mg L−1 of CR prepared in distilled water (30°C, pH 7.0, 48 h and 120 rpm in shaking condition) (Gautam et al. 2018). The adsorption intensity of extracted metabolites was taken at the wavelength 497 nm and decolorization percent was calculated using Eq. (1). Similar work was also performed with the HCl solution. After each chemical treatment process, the biomass was washed multiple times with distilled water until pH value become neutral (7.0) for the filtrate (Sen et al. 2016; Gautam et al. 2018). Then retreated fungal mycelia used for removal of CR dye with the same concentration (100 mg L−1) in 50 ml of an aqueous solution for 48 h. The fungal mycelia were recycled up to two times under the same environmental condition at shaking condition with 120 rpm.
To investigate the role of enzyme on dye decolorization, fungi (GS28 and CR500) were grown in different concentrations of CR (100, 500 and 1000 mg L−1) containing PDB medium. After 120 h, treated and untreated culture were filtered and kept at 4°C for further analysis. After decolorization, the extracted metabolites were used as crude enzyme extract to examine the extracellular enzyme and obtained biomass used for H2O2 and protein content estimation which involved during dye decolorization by fungi (A. terreus and A. flavus). For the determination of laccase activity, guaiacol was used as a substrate. The laccase activity was determined as described by Srinu et al. (2017) and the value presented in Umin−1 mg protein−1(Adnan et al. 2014).
The manganese peroxidase (Mnp) activity examined on the basis of Srinu et al. (2017). By using, a spectrophotometer (Systronic, 2203), the absorbance was taken at the wavelength of 465 nm and the activity of MnP was computed. Bovine serum albumin used as a standard for Protein content (Lowry et al. 1951) and H2O2 content was determined by Velikova et al. (2000).
For FTIR analysis, CR (200 mg L−1) treated and untreated fungal biomass (A. terreus and A. flavus) was filtered by filter paper (Whatman No.1) and oven-dried for 6 h at 60°C. Obtained dried fungal biomass was crushed with the help of mortal-pestle and mixed with a 1:100 (w/w) ratio of the KBr (potassium bromide). In the chamber of the sample cabin, the obtained pellets were fixed and examined by the FTIR spectrometer (Thermo Scientific Nicole 6700, USA). The FTIR spectra of the sample were scanned in IR wavelength ranged from 400 to 4000 cm−1 (Bankole et al. 2018).
To extract the metabolites for GC–MS analysis, sample were prepared as described He et al. (2018). The extracted product was further dried using a rotatory evaporator. The obtained residues were passed in GC- vial and dried by nitrogen gas as well as suspend in a little amount of methanol which is related to HPLC grade. GC–MS analysis was performed by Gas chromatography-mass spectroscopy [Shimadzu QP-2010 plus with Thermal Desorption System TD 20] (Pandi et al 2018; Singh and Dwivedi 2020).
A phytotoxicity test was conducted to determine the toxicity of fungal treated and untreated CR (100 mg L−1) dye. The seeds of Solanum lycopersicum (tomato) and Triticum aestivum (wheat) were used to analyze the phytotoxicity test. Seeds were surface sterilized using 10% H2O2 content for ten minutes and rinsed with autoclaved distilled water (Srinu et al. 2017). Twenty seeds of each plant were dispersed on Petri-plate at a similar distance and poured with the same volume (10 mL) of autoclave distilled water, untreated CR (100 mg L−1) solution and fungal treated (100 mg L−1) solution separately. The plates were watered on alternate days with 10 mL of sterilized water and fungal treated as well as untreated CR solution and incubated at room temperature. After eight days, the root and shoot length of seedlings was measured and the percent germination rate of seed was calculated by Eq. (3) (Kannan and Upreti 2008).
Each experiment was performed in three-replicates to enhance the analytical accuracy of data. Therefore One Way Analysis of Variance (ANOVA) was used to analyze the obtained data followed by Duncan Multiple Range Test (DMRT; p ≤ 0.05) (post–hoc test) by SPSS software (version 20.0).
Results and Discussion
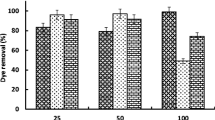
In this work, Congo red (CR) decolorization efficiency by A. terreus and A. flavus were investigated. Both the fungi play a crucial role in CR decolorization from lower concentration to higher concentration (100–1000 mg L−1) within 168 h at shaking condition of 120 rpm and results are shown in Fig. 1a. Maximum decolorization activities were recorded at 100 mg L−1 of CR (95% – A. terreus and 92.96% – A. flavus) by both the fungi. The decolorization rate was decreased drastically (p < 0.05; DMRT) with an increase in CR concentration up to 1000 mg L−1 and was reduced to 78.18 and 73.81% by A. terreus and A. flavus respectively. The fungal biomass and dye concentration was opposite to each other as dye concentrations were increased the fungal biomass was decreased from 100 to 1000 mg L−1 of CR (Fig. 1a). Result indicated that both fungi might feel stress condition at higher concentration by which their growth was inhibited and also released few extracellular enzymes (such as manganese peroxidase (MnP), lignin peroxidase (LiP) and laccase) which involved in the CR decolorization via degradation (Table 1) (Srinu et al. 2017). Both fungi were used for the study but A. terreus was more efficient as compare to A. flavus for decolorization of CR. For the decolorization of dye, both (adsorption and degradation) mechanism were involved (Fig. 1f). The growth of fungal strain was reduced as dye concentration was increased because of increasing stress and the degradation process become slower due to a reduction in the number of enzymes (Wang et al. 2017; Tan et al. 2019). Similarly, the dye decolorization in a liquid medium with different fungi has also been reported in several works (Sen et al. 2016; Bankole et al. 2018; Syafiuddin and Fulazzaky 2021). The decolorization capacity of GS28 and CR500 was higher than Alternaria alternata CMERI F6 for which the decolorization efficiency was 78% at 800 mg L−1 (Chakraborty et al. 2013). Similar work has been done in the report of Bosco et al. (2016) but their decolorization efficiency of CR was less than A. terreus. Both fungi have enormous differences for decolorization of CR by their enzymatic activity and adsorption rate.
a Percent removal of CR (100–1000 mgL−1) and Obtained biomass (gmL−1) after decolorization by Aspergillus flavus-CR500 and Aspergillus terreus-GS28 after 168 h b Percent removal of CR (800 mgL−1) at different pH c at several temperature d different heavy metal with single concentration (100 mgL−1) e Percent decolorization of CR (800 mgL−1) under the various concentration of Carbon and nitrogen sources (PDB, PDB + P, PDB + Y, PDB + G, PDB + D and PDB + B) with the concentration of 5 gmL−1 f De decolorization, Ad adsorption and Dg degradation percent of CR at several concentration by A. flavus and A. terreus. Bar above the graph denotes standard deviation (n = 3). Same letters above the graph denotes no significant differences using DMRT (p ≤ 0.05)
The removal rate of CR was influenced by various pH (4.0, 6.0, 8.0, 10.0 and 12.0) observed in the present study. The rate of CR decolorization and obtained biomass was affected by the pH of the CR-containing solution (Chakraborty et al. 2013). The highest decolorization of CR 800 mg L−1 by A. terreus was showed at the pH of 8.0 was recorded which corresponds to 92.35% and A. flavus treated showed maximum decolorization (92.60%) at 6.0 pH at the temperature of 30°C, 120 rpm within 120 h (Fig. 1b). An interesting observation was found in this study that maximum CR (800 mg L−1) decolorization was observed at slightly acidic (6.0) for A. flavus and slightly alkaline (8.0) condition for A. terreus and this could happen due to copping behavior of fungi. The pH of the medium is a critical environmental factor to affect the azo biodegradation (Asses et al. 2018; Singh et al. 2021) and the degradation of dyes depends on [H+] ions (Basak et al. 2013). During the adsorption process, the structure of the dye molecule and mycelial surface charge is also affected by pH value. The pH value and isoelectric point of mycelia interacted with each other. As mycelia surface showed negative charges which reflect the pH value is more than the isoelectric point of mycelia and when mycelia surface shows positive charges which reflect the pH value is less than isoelectric point of mycelia (Basak et al. 2013). A strong acidic or alkaline condition, decolorization rate was slow due to the existence of the same charge on mycelia and dye molecule that cause repulsion between them (Asses et al. 2018). The pH values from 6.0 to 8.0 were more suitable for better decolorization (Wang et al. 2017).
Temperature is a most influencing factor that affects the growth of fungi and the rate of degradation/adsorption mechanism (Adnan et al. 2014). The effects of various temperatures (25 to 45°C) were examined for CR decolorization at 800 mg L−1. Both fungi can decolorize CR up to 35°C while maximum CR decolorizes were 88.48% at 35°C by A. terreus and 91.02% by A. flavus at 30°C (Fig. 1c). An increase in temperature above 35°C, the decolorization ability of both fungi was reduced due to a decrease in their growth, biomass as well as metallic activity. The result showed that optimum temperature (25–35°C) was favorable for dye decolorization by fungi. However, optimum temperature plays an important role to enhance the microbial metabolic activity and their growth by which metabolic activity constantly depends on the enzymatic activity of microbes (Sen et al. 2016). Thus in the adsorption process, dye molecule surface and kinetic energy are enhanced by optimum temperature and in the degradation mechanism, optimum temperature involved in the increasing production of enzymes that participate in dye decolorization (Zhang et al. 2020). It showed that a high temperature reduces cell viability and inactivation of enzyme which is responsible for CR decolorization (Singh and Dwivedi 2020).
The CR decolorization activity in 50 mL of PDB medium supplemented with different carbon and nitrogen sources (Yeast extract, Peptone, Beef extract, Dextrose, and Glucose) was studied with a single concentration of (800 mg L−1) CR at the temperature of 30 °C, pH 7.0 and 120 rpm for 168 h. PDB supplemented with glucose medium showed maximum (90.59%) decolorization while PDB supplemented dextrose showed minimum (78.61%) decolorization by A. flavus and PDB supplemented with dextrose showed maximum decolorization (92.19%) and beef extract showed less (74.28%) decolorization by A. terreus (Fig. 1e). The result of the present work showed that among all carbon and nitrogen sources, glucose is an excellent carbon source for the removal of CR by both fungi. Usually, carbon sources increase the growth of microbe and relocate the reducing equivalents to the dye for the cleavage of –N=N– (azo bond) (Kumar et al. 2009; Zhang et al. 2020) while less amount of carbon–nitrogen sources in PDB medium allow the slow rate of biomass production and decrease fungal metabolic activity which is also essential for the dye degradation (Kumar et al. 2009). Degradation/decolorization process of azo dye by microbe depends on the types and presence of carbon–nitrogen sources due to their activities which play the role of electron donor in the azo dye decolorization process (Singh et al. 2021).
The existence of heavy metals in the PDB medium affects the rate of CR decolorization extensively at the level of p ≤ 0.05 followed by DMRT (Fig. 1d). However, the chosen metals were Mn(II); MnSO4, Zn(II); ZnSO4, Pb(II); Pb(NO3)2, Cd(II); CdCl2 and Ni(II); NiSO4 used to investigate the percent decolorization ability with 100 mgL−1 single concentration. A. flavus decolorized maximum CR (93.33%) with the presence of Mn and less decolorization showed with Cd- 68.21% and Ni- while A. terreus decolorized maximum under the presence of Zn (94.71%) and least decolorization (61.79%) occurred with the presence of Ni (Fig. 1d). Results exposed that the decolorization via degradation of CR azo dye was enhanced in the presence of manganese which might be due to Mn(II) induced MnP activity (Gao et al. 2010; Singh and Dwivedi 2020). In general, the toxicity of heavy metal present in wastewater can influence the fungal growth and decolorization ability (Kumar et al. 2019). Cd and Ni also have been reported to cause toxicity to the fungi by inducing reactive oxygen species production that possibly reduces the growth of A. terreus and A. flavus which led to a decrease in CR decolorization (Kumar et al. 2019). While in the present study, isolate A. terreus and A. flavus have a proficient ability to decolorize CR with the presence of some specific heavy metal (Mn(II) and Zn(II)). Results demonstrated that both fungi can be used for the handling of dye-containing wastewater co-contaminated with heavy metal.
In the present study, different concentration (50, 100, 150 and 200 mg L−1) of Mn(II) was also used to check the maximum decolorization rate of CR (Fig. S2a). Maximum decolorization (93.66% and 90.52%) showed in PDB supplemented with 100 mg L−1 of Mn by A. flavus and 150 mg L−1 by A. terreus. While as increasing concentration of Mn decrease the decolorization rate of CR (76.96% by A. flavus and 71.76% by A. terreus at 200 mg L−1 of Mn). Increasing concentration of heavy metal inhibits the growth and enzymatic activity of microbe by which decolorization rate is also affected (Chakraborty et al. 2013; Singh et al. 2021).
Under optimum conditions, both fungi (A. terreus and A. flavus) illustrated better results within 120 h (Fig. S2b). The decolorization of CR (800 mgL−1) by A. terreus was at 35°C, pH 8.0, 5 gm L−1 of dextrose and 150 mg L−1 of Mn in shaking condition for 120 h and the decolorization rate of CR was 96.40%. However the optimum condition for luxurious growth and highest decolorization rate of CR by A. flavus was at 30°C, pH 6.0, 5 gm L−1 of glucose and 100 mg L−1 of Mn in shaking condition for 120 h and the decolorization rate was 95.24%. Same work has been reported by which showed that maximum decolorization (> 90%) and 99.9% decolorization by Alternaria alternata under optimum condition (Chakraborty et al. 2013) Optimum condition is better for the growth of microbes, enzymatic activity and it increases both adsorption and degradation process for excellent decolorization activity (He et al. 2018).
Incubation time is the most influencing factor for decolorization (adsorption and degradation) of dye and biomass production (Asses et al. 2018). Results revealed that as contact time of CR with both (A. terreus and A. flavus) fungi increase than their rate of decolorization increase due to their increasing biomass and enzymes involvement (Fig. S2b). After 24 h, 800 mg L−1 of CR decolorized 40.62% by A. terreus and 34.66% by A. flavus under optimized condition while after 120 h, CR decolorize up to 96.40% by A. terreus and 95.24% by A. flavus. Both fungi play a unique role in CR decolorization with regard to incubation time. Incubation time and shaking conditions amplify the mixing of oxygen which exists in the PDB medium thus helps to promote the growth of fungi for decolorization (Kumar et al. 2009; Tan et al. 2019). Time course is also important for the increase in the enzymatic activity, metabolic rate, (laccase, MnP and liP) and biomass content for degradation and adsorption of CR.
The finding of the present work examined that adsorption via degradation process involves dye decolorization by both (A. terreus and A. flavus) fungi. Adsorption of CR by A. flavus was 45.70% at lower concentration (100 mg L−1) and 18.91% at higher concentration (1000 mg L−1) of CR (Fig. 1f) and almost similar results were recorded for CR adsorption by A. terreus (Fig. 1f). In the report of Sen et al. (2016), Biosorption activity depends on the functional group and cell surface area of fungi which have a great correlation with their adsorption mechanism (Zhang et al. 2020). Several reports highlighted that fungal biomass is involved as an adsorption process in the removal of dye (Almida and Corso 2014; Wang et al. 2017). In microbes, fungal cell walls has charged functional groups (active sites) of different varieties such as carboxyl (–COOH), hydroxyl (–OH), phosphate (PO43−) and others. Heteropolysaccharide releases by fungi (Mahmud et al. 2021), which play important role in the adsorption process for dye decolorization (Vitor and Corso 2008). The amount of fungal biomass decreased with increasing concentration of dye which reflects that the adsorption rate might be decreased due to less availability of surface functional group in fungi and reduces CR adsorption (Cheng et al. 2012; Syafiuddin and Fulazzaky 2021).
In the present degradation process also involved in CR decolorization as concentration increases degradation process increased (100–1000 mg L−1 = 46.76–55.23% by A. flavus and 49.55–58.42% by A. terreus) compared to adsorption process (Fig. 1f). The changes found after the decolorization process specified the transformation of new products which is formed after the degradation process by enzymes. The increasing concentration of dye increase laccase activity and decrease MnP activity (Table 1) and which indicates that degradation was also involved in decolorization of CR by A. terreus and A. flavus. During the biodegradation process, fungi released enzymes (azo-reductases, peroxidases and laccases) which have great concern for the breakdown of azo bond and aromatic ring which is present in azo dye (Singh and Dwivedi 2020). The biodegradation process involves the change of additional organic molecules without a change in the main structure of the dye compound as well as a breakdown of the complex dye molecules in mineral forms (Asses et al. 2018).
For the reusability assay, a block of 5 mm diameter of fungal culture was used to inoculate the CR (100 mg L−1) amended PDB medium and incubated for 168 h at 30°C, pH 6.0. The filtered biomass was again used for decolorization at the concentration of 100 mg L−1 of CR. The regeneration study of fungal biomass (A. terreus and A. flavus) demonstrated good results by desorption with 0.1 M of NaOH in comparison to 0.1 M HCl. It might be occurred due to the acidic nature of CR dye which makes that it highly soluble in alkaline solution. CR Adsorption was to be 71.40% and 46.86% by CR500 and 68.03% and 44.13% by A. terreus in 2nd and 3rd reusability cycle respectively by treating with 0.01 M NaOH solution as a desorption agent. However, 67.62% and 42.59% decolorization was recorded by A. flavus and 65.33% and 45.72% by A. terreus in the 2nd and 3rd cycle with the treatment of 0.1 M HCl solution (Table S1). A similar reusability essay has been done in the previous report (Wang et al. 2017) where only II cycle of regenerative ability of mycelia had been done for dye treatment while in this study III cycle of reusability of fungal biomass for CR decolorization has been done. The nature of azo dye reflects the desorption ability which adheres to the adsorbent surface (Tan et al. 2019). Formation of the weak bond (such as hydrogen bond and ionic bond) among dye and adsorbent may conduct to simply and higher desorption (Syafiuddin and Fulazzaky 2021).
High laccase and low MnP activity were detected at the end of degradation of the CR after 168 h treated with A. terreus while low laccase and high MnP activity were found after degradation of CR treated with A. flavus. In this study, laccase enzyme increased (1.118 ± 0.016, 1.265 ± 0.028 and 1.514 ± 0.022 U min−1 mg protein −1) with increasing concentration of CR (100, 500 and 1000 mg L−1) treated by A. terreus and decrease with increasing concentration of CR treated with A. flavus showed in Table 1. Results revealed that both fungi degrade CR dye but their enzymatic activities changed because of dye nature and behavior of fungi. Synthetic dye degradation by releasing oxido-reductases like laccase, MnP and LiP enzymatic activity through fungi has been highlighted in various research papers (Pandi et al. 2018; He et al. 2018). Literature supported that fungi have extensive reaction potential as well as wide substrate specificity together with no conditions for co-factor by which they degrade azo dye due to their enzymatic activity (Cheng et al. 2012).In this investigation, extracellular (laccase) enzyme effectively involved in the degradation of CR by GS28 and Mnp by CR500 which demonstrated that Parent CR dye compound change in the less toxic compound by oxido-reductase enzymes (Singh and Dwivedi 2020). If the degradation of CR done by laccase enzyme, it directly cleavage the azo groups by non-specific free radical mechanism and no aromatic amines formation occurred after cleavage of azo bond (Zhang et al. 2020). Similarly, MnP enzymes occurred in the family of ligninolytic peroxidase which also able to mineralize azo dye by redox reaction and oxidize phenolic compound due to alteration of Mn2+ in Mn3+ (Sen et al. 2016). Whereas there are few reports found that under in vitro decolorization studies, two or more ligninolytic enzymes are involved in which laccase possibly collaborate with MnP for dye decolorization (Levin et al. 2010; Srivastava et al. 2019).
In the present investigation, the secretion of extracellular protein was decreased with increasing concentration of CR by both fungi (Table 1). The decreasing concentration of protein may be due to the stress condition in fungi with increasing concentration of dye (Adnan et al. 2014). The production of H2O2 indirectly interlinked with the enzymatic activity which is involved in the decolorization process of CR. H2O2 content (1.304 ± 0.173, 1.512 ± 0.130 and 2.049 ± 0.057 nM g−1 FW by GS28 and 1.160 ± 0.026, 1.599 ± 0.038 and 2.064 ± 0.059 nM g−1 FW by CR500) increased with increasing concentration of CR (100,500 and 1000 mg L−1) treated with both fungi (Table 1). Production of enzymes (laccase and MnP) which is involved in the decolorization process was too high or low due to fluctuation in the concentration of H2O2 in the PDB medium (Kumar et al. 2009; Srivastava et al. 2019). It plays a unique role in the redical generation process which involves in the ligno-cellulose degradation process (Singh and Dwivedi 2020). A high quantity of H2O2 required as a co-factor for peroxidase action and however, sometimes it can also inhibit the enzymatic activities and a low quantity of H2O2 support to dye decolorization by the enzyme (Pandi et al. 2018).
To encounter the role of the functional group of fungal (A. terreus and A. flavus) mycelia surface in decolorization of CR, FTIR analysis was done. The results of the FTIR investigation of CR treated and without treated biomass of fungi (control) are shown in supplementary Fig.S3 and the detected peaks and their assigned functional group are listed in Table 2. FTIR result demonstrated the association of surface-active site (functional groups) in the biosorption of CR on the fungal cell surface. The major peaks were found at wave number 2270.7 cm─1, 1459.5 cm─1, 1235.2 cm─1 before CR biosorption in mycelia biomass corresponds to the C≡C stretching of unsaturated fatty acid, C–H stretching and C–N stretching respectively (Kumar and Dwivedi 2019) which completely disappeared from CR treated mycelia biomass of A. terreus. Azo dye biotransformation into several metabolites was confirmed by the disappearance of above mentioned major peaks (Singh and Dwivedi 2020). The peaks 3009.9 cm−1 (=C–H stretch), 2654.2 cm─1 (C–H stretching modes), 602.6 cm─1 (SO2 deformation) were shifted to 2924.2 cm─1, 2853.7 cm─1 and 636.9 cm─1. Some minor shifts in the bands were also observed at 1745.8 cm─1, 1650.5 cm─1, 1081.6 cm─1 and 1033.7 cm─1 which were shifted to 1742.3 cm─1, 1647.6 cm─1, 1077.1 cm─1 and 1038.2 cm─1 respectively for CR loaded biomass of A. terreus. No new peak was observed on CR treated mycelia of A. terreus biomass. However, few major IR peaks 2362.2 cm─1 (P–H stretching), 1408.9 cm─1 (C–N stretching of Amide III band), 1149.8 cm─1 (C=S stretching), 1077.4 cm─1 (C–N stretching vibration of amine groups) were not detected on CR treated mycelia of CR500 while high-intensity peak was detected on CR untreated biomass. Two new peaks (at 1449.0 cm─1 and 1377.0 cm─1) also occurred in CR treated biomass of CR500 which correspond to CH3 and CH stretching. These peaks may belong to the adsorb CR molecules. New peaks formation and vanishing of major IR peaks may be acknowledged the mineralization of parent (dye molecules) compound (Wang et al. 2017). The obtained results revealed that A. flavus biomass was more effective for surface sorption of CR as compare to A. terreus because A. flavus biomass treated new peaks that affect the parent compound of CR. The disappearance of the peaks also might indicate the C-N bond breaking and deformation of ammonium ion (NH4+) (Bankole et al. 2018).
To examine the degradation pattern and metabolites produced during the degradation pathway of CR by A. flavus and A. terreus were investigated by GC–MS analysis. The dye degraded metabolites were analyzed by GC–MS investigation based on m/z values. The chromatogram of GC–MS analysis has demonstrated the existence of several peaks, fragmentation patterns in the degraded metabolites (Fig. S4). The intermediate products benzidine and 3,4- diaminonapthelene-1-sulfonic acid were found in the degradation pathway of CR by A. terreus and similarly, several intermediate products were found in the degradation of CR by A. flavus (Fig. 2b, c). The end products were phenol and benzenedicarboxylic acid (Rt-41.467) by A. terreus and phenol and nitrobenzene by A. flavus. In the degradation of CR dye, the first step is the cleavage of the azo bond by releasing enzymes such as laccase and MnP. CR has a maximum functional group by which the biodegradation process is so tough and several intermediates may be released(He et al. 2018). Benzidine and Napthylamine both are extremely toxic for the organism by which they further degraded in harmless products which are beneficial for aquatic life (Zhang et al. 2020). Two types of the pathway (Symmetrical and asymmetrical) are involved in the cleavage of azo bond based on peroxidase degradation of sulfonated azo dyes (Bosco et al. 2016). Amino groups are not found at any step in asymmetrical cleavage while in symmetrical cleavage amino groups exists at one or multiple-step (He et al. 2018). In the present investigation, asymmetrical cleavage of CR happened to exists with the treatment of A. flavus however, symmetrical cleavage occurred with the treatment of A. terreus. Several researchers have reported the CR degradation pathway by GC–MS (Bankole et al. 2018).
For the assessment of the toxicity of dye degraded product, the Phytotoxicity test was conducted using used to evaluate the toxicity of fungal treated dye solution and pure dye solution using S. lycopersicum and T. aestivium seeds. The result of this work is given surety that uneven discharge of these substances may not give a negative impact on the aquatic system due to check their phytotoxicity test (Table 3). Similar work has been done in the previous report (Almida and Corso 2014; Singh et al. 2021) with the same fungi for degradation of Procion Red MX- 5B which gave a negative impact on the aquatic ecosystem due to their highly toxic degraded product as compared to pure dye. In the present study, in the control treatment and fungal treated dye solution the germination rate (90%, 80%, 75% for S. leucopersicum and 95, 75, 84% for T. aestivum), root length and shoot length was higher as compared with pure dye treatment (Table 3). According to Nouren and Bhatti (2015) GI values the toxicity of fungal degraded metabolites was less as compared to parent dye after the treatment with A. terreus and A. flavus.
Two fungi (A. terreus and A. flavus) were investigated in this study for decolorization (adsorption and degradation) of Congo red dye which was isolated from wastewater. Both fungi have efficient decolorization ability for CR up to the higher concentration (1000 mg L−1) under optimized condition (pH 8.0, 35°C, PDB + Glucose and Mn(II) for A. terreus and pH 6.0, 30°C, PDB + glucose and Mn(II) for A. flavus). Extracellular enzymes (laccase and MnP) are engaged in the decolorization via degradation of CR by both fungi. The role of adsorption of CR was confirmed by FTIR analysis and degradation of CR has been investigated by GC–MS analysis. The degraded product was benzenepropanic (Rt-26.147), 3, 4-diaminonapthelene-1-sulfonic acid and benzenedicarboxylic acid (Rt-26.660) by A. terreus, and benzenedicarboxylic acid (Rt-41.467) by A. flavus. In the phytotoxicity study, good germination rate and growth of the seedlings confirm those degraded dye products showed less toxicity as compared to crude dye (CR). Therefore, both fungi have high CR decolorization capability via degradation and adsorption mechanism and can be potentially applied in the removal of CR contaminated industrial effluent in a safe and environmentally pleasant way.
References
Adnan LA, Yusoff ARM, Hadibarata T, Khudhair AB (2014) Biodegradation of bis-azo dye reactive black 5 by white-rot fungus Trametes gibbosa sp. WRF 3 and its metabolite characterization. Water Air Soil Pollut 225(10):1–11
Almeida EJR, Corso CR (2014) Comparative study of toxicity of azo dye Procion Red MX-5B following biosorption and biodegradation treatments with the fungi Aspergillus niger and Aspergillus terreus. Chemosphere 112:317–322
Asses N, Ayed L, Hkiri N, Hamdi M (2018) Congo red decolorization and detoxification by Aspergillus niger: removal mechanisms and dye degradation pathway. Int J Biomed Res 7:1–9
Bankol OP, Adekunle AA, Govindwar SP (2018) Enhanced decolorization and biodegradation of Acid Red 88 dye by newly isolated fungus, Achaetomium strumarium. J Environ Chem Eng 6:1589–1600
Basak B, Bhunia B, Mukherjee S, Dey A (2013) Optimization of Physic-chemical parameters for phenol biodegradation by Candida tropicalis PHB5 using Taguchi Methodology. Desalin Water Treat 51:6846–6862
Bosco F, Mollea C, Ruggeri B (2016) Decolorization of congo red by Phanerochaete chrysosporium: the role of biosorption and biodegradation. J Environ Technol 38:2581–2588
Chakraborty S, Basak B, Dutta S, Bhunia B, Dey A (2013) Decolorization and biodegradation of congo red dye by a novel white rot fungus Alternaria alternata CMERI F6. Biores Technol 147:662–666
Cheng ZZ, Yang ZP, Hu R et al (2012) Decolorization of 12 kinds of dyes by the mycelium pellets of Trametes gallica under non-sterile condition. Mycosystema 31(6):878–889
Gao D, Du L, Yang J, Wu WM, Liang H (2010) A critical review of the application of white rot fungus to environmental pollution control. Crit Rev Biotechnol 30:70–77
Gautam A, Rawat S, Verma L, Singh J, Sikarwarb S et al (2018) Green synthesis of iron nanoparticle from extract of waste tea: an application for phenol red removal from aqueous solution. J Environ Nanotechnol Monit Manag 10:377–387
He XL, Song C, Li Y, Wang N, Xu L, Han X, Wei D (2018) efficient degradation of Azo dyes by a newly isolated fungus Trichoderma tomentosum under non-sterile conditions. Ecotoxicol Environ Saf 150(15):232–239
Iark D, dos Reis Buzzo AJ, Garcia JAA (2019) Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour Technol 289:121655
Kannan A, Upreti RK (2008) Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J Hazard Mater 153:609–615
Kumar V, Dwivedi SK (2019) Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere 237:124567
Kumar K, Dastidar MG, Sreekrishnan TR (2009) Effect of process parameters on aerobic decolourization of reactive azo dye using mixed culture. World Acad Sci Eng Technol 58:952–955
Kumar V, Singh S, Singh G, Dwivedi SK (2019) Exploring the cadmium tolerance and removal capability of a filamentous fungus Fusarium solani. Geomicrobiol J 36(9):782–791
Levin L, Melignani E, Ramos AM (2010) Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresour Technol 101:4554–4563
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biologic Chem 193:265–275
Mahmud AA, Upadhyay SK, Srivastava AK, Bhojiya AA (2021) Biofertilizers: a Nexus between soil fertility and crop productivity under abiotic stress. Curr Res Environ Sustain 3:100063
Mnif I, Maktouf S, Fendri R, Kriaa M et al (2016) Improvement of methyl orange dye biotreatment by a novel isolated strain, Aeromonas veronii GRI, SPB1biosurfactant addition. Environ Sci Pollut Res 23:1742–1754
Nouren S, Bhatti HN (2015) Mechanistic study of degradation of basic violet 3 by Citrus limon peroxidase and phytotoxicity assessment of its degradation products. Biochem Eng J 95:9–19
Pandi A, Kamini NR, Saravanan P, Gowthaman MK (2018) A sustainable approach for degredation of leather dyes by a new fungal laccase. J Clean Prod 211:590–597
Sen SK, Raut S, Bandyopadhyay P, Raut S (2016) Fungal decolouration and degradation of azo dyes: a review. Fungal Biol Rev 30(3):112–133
Singh G, Dwivedi SK (2020) Decolorization and degradation of Direct Blue-1 (Azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environ Technol and Innovat 18:100751
Singh G, Upadhyay SK, Singh MP (2015) Dye-decolorization by native bacterial isolates, isolated from sludge of carpet industries Bhadohi-India. J Environ Sci Technol 2(6):81–85
Singh G, Kumar V, Dwivedi SK (2021) Comparative Investigation of Congo Red and Direct Blue-1 Adsorption on Mycosynthesized Iron Nanoparticle. J Clust Sci 2021:1–17
Srinu A, Vijaya LD, Murali S, Prasad DVR (2017) Decolorization of anthraquinone dyes by Aspergillus strains and also optimization of lignolytic enzymes. Int J Rec Sci Res 8(7):18547–18553
Srivastava AK, Upadhyay SK, Vishwakarma SK (2019) Mycodecolorization activity of Pleurotus Citrinopileatus for chemically different textile dye under varied aromatic amino acids and trace elements G. J Environ Sci Technol 6(4):14–17
Syafiuddin A, Fulazzaky MA (2021) Decolorization kinetics and mass transfer mechanisms of Remazol Brilliant Blue R dye mediated by different fungi. Biotechnol Rep 29:e00573
Tan L, Xu B, Hao J, Wang J, Shao Y, Mu G (2019) Biodegradation and detoxification of azo dyes by a newly isolated halotolerant yeast Candida tropicalis SYF-1. Environ Eng Sci 36(9):999–1010
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some anti oxidant system in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vitor V, Corso CR (2008) Decolorization of textile dye by Candida albicans isolated from industrial effluents. J Ind Microbiol and Biotechnol 35:1353–1357
Wang N, Chu Y, Wu F, Zhao Z, Xu X (2017) Decolorization and degradation of congo red by a newly isolated White rot fungus, Ceriporia lacerate, from decayed mulberry branches. Int J Biodetor Biodegrad 117:236–244
Zhang H, Zhang X, Geng A (2020) Expression of a novel manganese peroxidase from Cerrena unicolor BBP6 in Pichia pastoris and its application in dye decolorization and PAH degradation. Biochem Eng J 153:107402
Acknowledgements
The authors are grateful to Head, Department of Environmental Science, BBAU, Lucknow, U.P., India for facilitating all required laboratory facilities during experimental work. The help provided by USIC, B.B.A. University, Lucknow for FTIR analysis and AIRF of JNU, New Delhi for GC-MS analysis are greatly acknowledged. One of us (Garima Singh) would also like to thank University Grants Commission (UGC), Government of India for providing UGC-NON NET University fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This manuscript does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, G., Dwivedi, S.K. Biosorptive and Biodegradative Mechanistic Approach for the Decolorization of Congo Red Dye by Aspergillus Species. Bull Environ Contam Toxicol 108, 457–467 (2022). https://doi.org/10.1007/s00128-021-03380-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03380-8