Abstract
Adsorption is acknowledged as effective for the removal of pollutants from drinking water and wastewater. Biochar, as a widely available material, holds promises for pollutant adsorption. So far, biochar has been found to be effective for multiple purposes, including carbon sequestration, nutrient storage, and water-holding capacity. However, its limited porosity restricts its use in water treatment. Activation of biochars, when performed at a high temperature (i.e., 900 °C) and in the presence of certain chemicals (H3PO4, KOH) and/or gases (CO2, steam), improves the development of porosity through the selective gasification of carbon atoms. Physicochemical activation process is appropriate for the production of highly porous materials. As well, the morphological and chemical structure of feedstock together with pyro-gasification operating conditions for the biochar production can greatly impact the porosity of the final materials. The effectiveness of activated biochar as adsorbent depends on porosity and on some functional groups connected to its structure, both of these are developed during activation. This study provides a comprehensive synthesis of the effect of several activated biochars when applied to the treatment of organic and inorganic contaminants in water. Results show that high aromaticity and porosity are essential for the sorption of organic contaminants, while the presence of oxygen-containing functional groups and optimum pH are crucial for the sorption of inorganic contaminants, especially metals. Finally, although activated biochar is a promising option for the treatment of contaminants in water, further research is required to evaluate its performance with real effluents containing contaminants of emerging concern.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to extensive anthropogenic activities, including industrial operations (such as mining), agricultural processes, and disposal of industrial wastewater materials, the concentration of contaminants in water is progressively increasing. A large number of anthropogenic and natural substances are present in water, including pharmaceuticals (prescriptions, over-the-counter drugs, and veterinary drugs), personal care products (fragrances, cosmetics, and sunscreens), steroid hormones, radioactive elements, metals (e.g., Pb, Hg, and As), industrial chemicals (hydrocarbons and solvents), and pesticides. Most of these compounds have been recently defined as contaminants of emerging concern (CEC) because they are subject to critical action by regulatory agencies. CEC refer to naturally occurring, manufactured, or manmade chemicals or materials that have been recently discovered, or persist in the environment for some time, posing toxicity concerns for living organisms (Sauvé and Desrosiers 2014). Potential leaching of organic and inorganic CEC from poorly managed residual waste sites (e.g., landfills, septic tanks) is also a concern. The main challenge is that due to their very low concentrations, most wastewater treatment plants are not specifically designed to eliminate such contaminants, although they can have a chronic or long-term harmful impact on the environment. Given their persistence and continuous input, the CEC pass through treatment processes without being affected. Consequently, they end up in the aquatic environment and become dangerous to wildlife and probably are of concern with regard to drinking water (Sauvé and Desrosiers 2014; Petrie et al. 2015; Fairbairn et al. 2016).
Several regulatory bodies (e.g., United States Environmental Protection Agency, Health Canada, World Health Organization, etc.) monitor the levels of contaminants in wastewater treatment plants, drinking water, and effluent industries. Therefore, the discharge criteria should be below a level at which there is no known or expected risk to health. For example, according to Health Canada, metals such as copper and iron can be present in drinking water at maximum concentrations of 1.0 and 0.3 mg/L, respectively (Health Canada 2017). To maintain such low levels of contaminants, several methods are employed, such as chemical precipitation (Li et al. 1999), ion exchange (Chiavola et al. 2012; Li et al. 2015), reverse osmosis (Greenlee et al. 2009; Vaneeckhaute et al. 2012), electrochemical treatment (Martínez-Huitle and Ferro 2006; Moriwaki et al. 2017), membrane processes (ultra-, micro-, and nano-filtration) (Katsou et al. 2012; Sözen et al. 2016), coagulation-floculation (Verma et al. 2012), flottation (Rubio et al. 2002), ozonation (Wang et al. 2017), etc. Likewise, the combination of methods such as electro-Fenton process and chemical precipitation (Ghosh et al. 2011), membrane bio-reactor with reverse osmosis (Dialynas and Diamadopoulos 2009), or flotation with membrane filtration showed very high efficiency for metals removal (Sudilovskiy et al. 2008). All these methods have advantages but they also have disadvantages, including the chemicals and energy required, the increase in residual salinity of treated water, the generation of toxic sludge, high cost, and incomplete contaminants removal (Gaspard and Ncibi 2013).
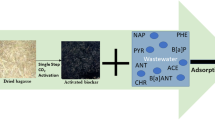
One effective and widely used method for removing toxic contaminants from aqueous solutions is adsorption process. It is the most versatile process to remove contaminants due to its simplicity of design and ease of operation; it also provides high removal capacity (Babel and Kurniawan 2003; Anastopoulos and Kyzas 2015; Hasan and Jhung 2015). The growing demand for adsorbent materials for environmentally protective processes (mining and metallurgy) calls for more research on the production of activated carbons from inexpensive materials (González-García 2018). The thermochemical conversion of biomass feedstock (e.g., crop residues, manure, wood, wastewater sludge) offers an efficient means to produce gaseous (biogas), liquid (bio-oil), and solid fuels (Fig. 1) compared to fossil fuel-derived materials. In various places, biomass waste thermochemical conversion is an economically feasible alternative to deal with millions of tons of waste generated by manufacturing activities in different industrial sectors (Dufour 2016; Pandey et al. 2015). The solid fuels produced are also called biochar, defined by the International Biochar Initiative (IBI) as a solid material obtained from the thermochemical conversion of biomass in a zero- or low-oxygen environment (IBI 2012). Indeed, there is growing interest in research communities, biorefineries, and related industries in converting biochar into activated biochar due to (i) its low-cost availability, (ii) potential economic feasibility in large-scale production, (iii) the increased profitability of existing thermochemical conversion processes for the production of diversified products (biochar, bio-oil, or syngas, and activated biochar), and (iv) its effectiveness in several applications such as the treatment (sorption) of drinking water and wastewater, energy storage, as electrodes in batteries and supercapacitors, and as catalyst support (Fig. 1) (Tan et al. 2017).
Activation is considered the most common method for tailoring the pore structure of biochar. Different pores sizes are created or developed by this approach: ultramicropores (< 0.7 nm), micropores (between 0 and 2 nm), and mesopores (between 2 and 50 nm). An appropriate pore size distribution is crucial in order to adsorb molecules of different sizes. There are three types of activation: (1) physical (in the presence of gases: CO2 or vapor water), (2) chemical (biochars impregnated with solutions of acids, salts, or bases), and (3) physicochemical (biochars impregnated with solutions of acids, salts, or bases in the presence of gases: CO2 or vapor water) at high temperatures (in an inert atmosphere), for the generation of a selective gasification of carbon atoms and consequently, the development of porosity (Marsh and Rodríguez-Reinoso 2006). In physical activation, the mechanism involved is as follows: the physical agents, CO2 or steam, remove the carbon atoms from the structure of the biochar according to Boudouard reaction Eq. 1 and Eq. 2, respectively (Calo and Perkins 1987). On the contrary, in chemical activation, different reactions occur simultaneously, depending on the chemical used. In general, chemicals such as acids (e.g., H3PO4) can act as a dehydration agent, whereas bases (e.g., KOH) can act as oxidant agents (Ozoemena and Chen 2016). Acids (e.g., H3PO4) can also act as catalysts, favoring activation, in the thermal treatment of biochar, whereas alkalis (e.g., KOH) can decompose in the form of metallic compounds (i.e., K, boiling-point elevation: 759 °C) (Eqs. 3˗6). In addition, the metallic compounds can be intercalated in the carbon lattice by thermal treatment, while gases (CO2 and CO) could form and act as physical agents to the latter activation (Eqs. 4˗6), thus favoring porosity development (Guo et al. 2002; Lozano-Castelló et al. 2007; Armandi et al. 2010).
The limited surface area and porosity of biochars restrain their potential to be applied in adsorptive processes. Removal of atrazine (the active substance in a pesticide) by KOH-activated biochar made from corn straw yielded a maximum sorption capacity 47 times higher compared to biochar due to its higher surface area and higher aromaticity (Tan et al. 2016). Broiler litter biochar and activated broiler litter biochar were applied by Lima et al. (2010) as adsorbents for the removal of metals (Cu2+, Cd2+, Ni2+) separately dispersed in water. In all cases, activated biochar performed better in the removal of metals, compared to biochars: 80% against 75% (Cu2+), 85% against 22% (Cd2+), and 96% against 10% (Ni2+). Thus, porosity plays a major role in the performance of carbonaceous adsorbents. Indeed, activated biochars have higher surface area and porosity compared to biochars as well as fewer functional groups connected to their carbonaceous structure. Thus, the main mechanism involved in contaminant removal is related to physical sorption (pores diffusion). However, depending on the chemical structures, characteristics of activated biochar, and the type of contaminants, additional mechanisms for adsorbent-contaminant interactions may also be involved during contaminant removal. In the case of inorganic contaminants, several interactions (physical sorption, ion exchange, metals electrostatic attraction, and metal precipitation) may be involved. Oppositely, in the case of organic pollutants, the main mechanisms involved are partitioning or adsorption and electrostatic interactions (Ahmad et al. 2014; Liu et al. 2015; Tan et al. 2017).
In the following sections, the contaminants removed by activated biochars are discussed separately (inorganics versus organics). A comprehensive list of such contaminants, together with the type of wastewater used (e.g., synthetic vs. real effluent), the biochar feedstock, the activation method used, as well as the corresponding surface area of the activated biochar, is compiled in Table 1. This table also includes the adsorption capacity for contaminants by activated biochar (mg/g), their experimental conditions, and a summary of the relevant results for each study.
2 Activated Biochar for Water Treatment
2.1 Organic Contaminants
Among the organic contaminants listed in Table 1 are methylene blue, phenol, and dyestuff, which have been extensively studied as contaminants removed through adsorption by the use of activated carbons (Dąbrowski et al. 2005; Demirbas 2009; Rafatullah et al. 2010). Other organic contaminants compiled in Table 1 are relatively new structures that have been recently studied, since they are part of the emerging contaminants concern such as herbicides (Uchimiya et al. 2010) and pharmaceutical compounds (Jung et al. 2013; Mondal et al. 2016b). In liquid-phase systems, the adsorption capacity of activated carbons for aromatic compounds depends on a number of factors: (i) the nature of the adsorbent (e.g., its porous structure, ash content, and functional groups), (ii) the nature of adsorbate (e.g., its pKa, polarity, functional groups, and molecular size), and finally (iii) the solution conditions (e.g., pH, concentration, ion strength) (Haghseresht et al. 2002).
The greatest concern in water treatment is mixes of contaminants. Probable competition can drastically reduce the adsorption capacity of adsorbents, even though contaminants are easily removed from uni-component solutions. Jung et al. (2015a) studied the removal of single and multiple contaminants present in solution such as diclofenac (DCF), naproxen (NPX), and ibuprofen (IBP) through NaOH-activated biochar made from loblolly pine chips. The presence of several contaminants in the same environment caused lower contaminant adsorption due to a combination of a series of mechanisms: lower binding energy, polarity, and π-energy (i.e., energy of molecular orbital of π electrons present in conjugated hydrocarbon systems), and electrostatic repulsion from the contaminants occupying adsorption sites.
Furthermore, additional studies are needed for the treatment of real effluents that usually contain several classes of contaminants. The majority of scientific works cited in Table 1 examined single solutions prepared in the laboratory and studied mostly in batch sorption experiments. Ranitidine hydrochloride (RH), however, was adsorbed from fixed-bed columns (Mondal et al. 2016a). In this testing, the adsorbate is in continuous contact with the adsorbent, a method that has been widely used in industry for the removal of organic compounds by carbon adsorption (Unuabonah et al. 2010; Karunarathne and Amarasinghe 2013). This contaminant is a medicine normally used for gastric and intestinal problems, which is excreted through urine and feces and therefore reaches a sewage system and ends up in rivers because wastewater plants cannot completely remove it. Researchers also evaluated the influence of parameters such as bed depths, initial concentration of contaminant, and volumetric flow rates on the performance of the adsorbent. The main findings of sorption experiments with flow (column reactor type) were as follows: (i) At the highest bed-adsorbent height (3 cm), adsorption capacity was increased. A high amount of adsorbent offered more binding sites, thus increasing the adsorption and other interactions with RH molecules (Gupta et al. 1998; Al-Degs et al. 2009). (ii) At the highest flow rate (6 mL/min), adsorption capacity decreased due to adsorbate residence time insufficient to diffuse into the pores or interact with the functional groups present in the adsorbent (Charumathi and Das 2012; Sadaf and Bhatti 2014). (iii) At the highest initial concentration, the sorption of the column was enhanced due to the increased driving force for the mass transfer and RH loading rate (Han et al. 2007; Sancho et al. 2012).
In addition, the type of activation (physical, chemical, or physicochemical), diversity of feedstock, and pyro-gasification operating conditions applied for the preparation of biochars have great effect on the porosity of activated biochars (Table 1). Steam-activated broiler litter biochar synthesized after pyrolysis at 350 and 700 °C showed that for both materials, the same surface area of 335 m2/g was attained. The chemical composition of broiler litter (high ash content) is completely different from lignocellulosic precursors (low ash content). However, higher adsorption capacity of deisopropylatrazine (a herbicide) was reached by applying high pyrolysis temperature and subsequent activation (Uchimiya et al. 2010). Activated biochar has higher aromaticity, which is an important parameter related to enhanced removal of organic pollutants due to its higher hydrophobicity and π-π interactions (Boving and Zhang 2004; Moreno-Castilla 2004; Park et al. 2013). The same findings were observed for phenanthrene removal: faster initial sorption rate and higher equilibrium concentration for phenanthrene adsorption were reported when using activated biochar prepared at low pyrolysis temperature (300 °C; SBET = 1250 m2/g), whereas stronger binding with the sorbate was noted with the material pyrolyzed at 700 °C (SBET = 57 m2/g). The considerable decrease in surface area of the activated biochar pyrolyzed at 700 °C was due to the presence of condensed aromatic structures, which were more stable and had fewer oxygen-containing groups at the surface. Consequently, its carbon framework was resistant to thermal degradation (even when impregnated with harsh chemicals, e.g., NaOH) inhibiting the development of a porous structure. Although the activated biochar pyrolyzed at 700 °C presented higher aromaticity, the material having the highest surface area (pyrolyzed at 300 °C) exhibited higher initial sorption efficiency (156 mg/g) compared to a commercial activated carbon NORIT (130 mg/g) (Park et al. 2013).
In summary, surface area and aromaticity of activated biochars are the most important factors for enhancing the sorption capacity of organic pollutants in water. Physicochemical and chemical activation were found to be highly effective in producing these properties in activated biochars and, consequently, increased their efficiency in the removal of organic compounds. Materials pore size distribution [e.g., highly microporous (Ø < 2.0 nm) or even ultramicroporous (Ø < 0.7 nm)] play an important role on the sorption of differently sized organic molecules. For example, iodine was successfully adsorbed by activated biochars having smaller pores, compared to methylene blue, which due to its high-size molecule, was found by most studies to need to be adsorbed by materials with larger micropores and mesopores (Oh and Park 2002).
2.2 Inorganic Contaminants
Most of the compounds included in the inorganic contaminant group are present in mining effluents; they include both cations and anions Fe2+, Fe3+, Cu2+, Ni2+, Cd2+, Zn2+, As(III), and NH4+, as well as Na+ and PO43−. In fact, globally, the mining industry is responsible for the generation of the largest amount of waste material. The main problem is the exposure of chemically reactive minerals to air and water, leading to contaminated mine drainage, either acid (AMD) or neutral (CND) which generates pollution in surface water and groundwater (Westholm et al. 2014; Rakotonimaro et al. 2017; Calugaru et al. 2018). In inorganic contaminants removal, in addition to the porosity of the adsorbent materials, the presence of functional groups, such as carboxyl (–COOH), phenol (–OH), and amino groups (−NH2) on the surface of the adsorbents, may help in the removal of metals. In addition, the majority of studies listed in Table 1 reported that there is an optimal pH at which activated biochars most effectively adsorbed inorganic contaminants in water media.
The removal of Cu2+ was reported through the use of biochar and steam-activated biochar from the Miscanthus plant (Shim et al. 2015). Surface sorption occurs beginning at pH 5, but the pH level must be maintained below 7.5, since surface precipitation through Cu(OH)2 formation takes place at higher pH levels. The surface area of the activated biochar doubled compared to biochar (322 vs. 181 m2/g), while its polarity index [(O + N)/C] decreased. Cu2+ adsorption was better fitted to the Freundlich and Langmuir models by using biochar and activated biochar, respectively, but they presented almost the same adsorption capacity for Cu2+ in water (14 vs. 15 mg/g). The greater efficiency of biochar for Cu2+ could be because of the formation of metal complexes or chelates due to the presence of more oxygenated functional groups connected to it compared to activated biochar. Cu2+ may have been adsorbed through different mechanisms in both materials: intra-particle diffusion and π-cation interactions in activated biochar, and surface complexation with the metal in biochar.
The presence of several metals in the same environment may make the sorption of metal contaminants quite complex once each metal is removed at different pH ranges. The adsorption of three pollutants normally found in mining water, i.e., Fe2+, Cu2+, and As(V) within the pH range of 2–7, was reported by Banerjee et al. (2016). At lower pH (equal to 3), Fe2+ was highly adsorbed by the activated biochar, whereas at higher pH, its adsorption decreased due to the formation of ferrous species such as [Fe(H2O)6]2+ and [Fe(OH)(H2O)5]+, as well as the precipitation of Fe(OH)3, resulting in less adsorption of Fe2+ (Panday et al. 1985; Mohan and Pittman Jr. 2006; Hove et al. 2007; Banerjee et al. 2016). In relation to the contaminants As(V) and Cu2+, the maximum removals of 80 and 75%, respectively, were reached at pH 5 and 6, whereas low contaminants removal was found at low pH. In other cases, it was reported that Cu2+ was the most adsorbed metal ion in single solutions but the adsorption capacity of the activated biochar was reduced when other metals (Cd2+, Ni2+, Zn2+) were found in the same environment (Lima et al. 2010). A probable metals competition may have lowered the sorption of Cu2+.
The type of pyro-gasification process and operating conditions were also found to have an influence on the porosity of activated biochars and, consequently, on the removal of inorganic contaminants. The proper choice of pyrolysis temperature (500 vs. 700 °C) may influence the porosity of biochars (50 vs. 300 m2/g), and may have been responsible for the presence of carboxylic and phenolic groups at the surface of the material, improving the cation-exchange and complexation properties of the adsorbents for the removal of Fe3+ and Cu2+, in addition to physical sorption (Bouchelta et al. 2012). These findings were also confirmed by MgCl2.6H2O-activated biochars prepared after first-step pyrolysis at 600 °C, producing higher PO43− (102 mg/g) removal compared to a material made at 400 °C (17 mg/g) (Takaya et al. 2016). Different biochars made from slow and fast pyrolysis of the same feedstock also presented great differences in relation to physicochemical properties and consequently, in metal ion uptake (Lima et al. 2010).
The type of activation was also found to play a role in the development of porosity of activated biochars for the sorption of inorganic contaminants. For example, the removal of NH4+ from water through activated biochar prepared at different types of activation using steam, CO2, KOH, and NH3 was reported by Rambabu et al. (2015). By using NH4+ solution at 260 ppm, the sorption capacity was found to be 55, 18, 149, and 57 mg/g, respectively. Chemical activation was shown to be an effective approach for the development of porosity for the sorption of NH4+. However, the presence of minerals (high ash content), the presence of oxygenated and nitrogenated functional groups connected to the surface of activated biochar, and the chemical surface of modified materials [e.g., Na2S-modified biochar (Tan et al. 2016), ZnCl2-activated biochar (Xia et al. 2016)] were also found to be determinant for better heavy metals adsorption. In activated biochars, the overall metal interaction mechanisms with these surface functionalities are electrostatic interactions, ion exchange, surface complexation, and metal precipitation in addition to physical sorption.
3 Conclusions and Future Research Needs
The present study reviewed the pertinent literature dedicated to the application of activated biochars prepared from various feedstock and different pyro-gasification and activation operating conditions, for the sorption of organic and inorganic contaminants in water media. The main findings are outlined below:
-
i.
The developed porosity (surface area and pore volume) and higher aromaticity were important properties of activated biochars for the enhanced sorption of organic contaminants in water due to higher hydrophobicity and π-π interactions. For inorganic contaminants, activated biochar surface chemistry (e.g., the presence of oxygenated functional groups, minerals) is an important property for enhancement of the interactions with inorganics in water by various mechanisms: electrostatic interactions, ion exchange, and surface complexation.
-
ii.
The pH of the solution may also affect the sorption of organics and inorganics onto activated biochar. Specifically, metals at precisely pH range can lead to precipitation mostly in a form of hydroxides, which may solubilize afterwards depending on the pH conditions. Therefore, an optimization study on the effect of the pH on the adsorption process is recommended.
-
iii.
The pyro-gasification conditions, reactor design, as well as the quality of feedstock used for the preparation of biochars have considerable influence on the textural properties (porosity) of activated biochars as well on the environmental remediation applications. Optimization of operating conditions and physicochemical characterization of biochars are recommended for designing biochars for specific applications.
-
iv.
The majority of studies on the application of activated biochars for the removal of organic or inorganic contaminants in water were based on synthetic solutions in batch tests. Therefore, further studies should investigate column tests with real effluents characterized by a mixture of contaminants of emerging concern (CEC), and various heavy metals from mining industries, pharmaceuticals, and pesticides, since they pose an increasing concern to the environment, wildlife, and drinking water systems.
References
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., et al. (2014). Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere, 99, 19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071.
Al-Degs, Y. S., Khraisheh, M. A. M., Allen, S. J., & Ahmad, M. N. (2009). Adsorption characteristics of reactive dyes in columns of activated carbon. Journal of Hazardous Materials, 165(1–3), 944–949. https://doi.org/10.1016/j.jhazmat.2008.10.081.
Anastopoulos, I., & Kyzas, G. Z. (2015). Composts as biosorbents for decontamination of various pollutants: a review. Water, Air, & Soil Pollution, 226(3). https://doi.org/10.1007/s11270-015-2345-2.
Angın, D., Köse, T. E., & Selengil, U. (2013). Production and characterization of activated carbon prepared from safflower seed cake biochar and its ability to absorb reactive dyestuff. Applied Surface Science, 280, 705–710. https://doi.org/10.1016/j.apsusc.2013.05.046.
Armandi, M., Bonelli, B., Geobaldo, F., & Garrone, E. (2010). Nanoporous carbon materials obtained by sucrose carbonization in the presence of KOH. Microporous and Mesoporous Materials, 132(3), 414–420. https://doi.org/10.1016/j.micromeso.2010.03.021.
Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials, 97(1–3), 219–243. https://doi.org/10.1016/S0304-3894(02)00263-7.
Baçaoui, A., Yaacoubi, A., Dahbi, A., Bennouna, C., Ayele, J., & Mazet, M. (1998). Activated carbon production from Moroccan olive wastes—influence of some factors. Environmental Technology, 19(12), 1203–1212. https://doi.org/10.1080/09593331908616780.
Banerjee, S., Mukherjee, S., LaminKa-ot, A., Joshi, S. R., Mandal, T., & Halder, G. (2016). Biosorptive uptake of Fe2+, Cu2+ and As5+ by activated biochar derived from Colocasia esculenta: isotherm, kinetics, thermodynamics, and cost estimation. Journal of Advanced Research, 7(5), 597–610. https://doi.org/10.1016/j.jare.2016.06.002.
Bouchelta, C., Medjram, M. S., Zoubida, M., Chekkat, F. A., Ramdane, N., & Bellat, J.-P. (2012). Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. Journal of Analytical and Applied Pyrolysis, 94, 215–222. https://doi.org/10.1016/j.jaap.2011.12.014.
Boving, T. B., & Zhang, W. (2004). Removal of aqueous-phase polynuclear aromatic hydrocarbons using aspen wood fibers. Chemosphere, 54(7), 831–839. https://doi.org/10.1016/j.chemosphere.2003.07.007.
Calo, J. M., & Perkins, M. T. (1987). A heterogeneous surface model for the “steady-state” kinetics of the boudouard reaction. Carbon, 25(3), 395–407. https://doi.org/10.1016/0008-6223(87)90011-X.
Calugaru, I. L., Neculita, C. M., Genty, T., & Zagury, G. J. (2018). Metals and metalloids treatment in contaminated neutral effluents using modified materials. Journal of Environmental Management, 212, 142–159. https://doi.org/10.1016/j.jenvman.2018.02.002.
Charumathi, D., & Das, N. (2012). Packed bed column studies for the removal of synthetic dyes from textile wastewater using immobilised dead C. tropicalis. Desalination, 285, 22–30. https://doi.org/10.1016/j.desal.2011.09.023.
Chiavola, A., D’Amato, E., & Baciocchi, R. (2012). Ion exchange treatment of groundwater contaminated by arsenic in the presence of sulphate. Breakthrough experiments and modeling. Water, Air, & Soil Pollution, 223(5), 2373–2386. https://doi.org/10.1007/s11270-011-1031-2.
Dąbrowski, A., Podkościelny, P., Hubicki, Z., & Barczak, M. (2005). Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere, 58(8), 1049–1070. https://doi.org/10.1016/j.chemosphere.2004.09.067.
Demirbas, A. (2009). Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. Journal of Hazardous Materials, 167, 1–3), 1–9. https://doi.org/10.1016/j.jhazmat.2008.12.114.
Dialynas, E., & Diamadopoulos, E. (2009). Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater. Desalination, 238(1–3), 302–311. https://doi.org/10.1016/j.desal.2008.01.046.
Ding, Z., Hu, X., Wan, Y., Wang, S., & Gao, B. (2016). Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. Journal of Industrial and Engineering Chemistry, 33, 239–245. https://doi.org/10.1016/j.jiec.2015.10.007.
Dufour, A. (2016). Thermochemical conversion of biomass for energy and chemical production. Hoboken: Wiley.
El-Hendawy, A.-N. A., Samra, S. E., & Girgis, B. S. (2001). Adsorption characteristics of activated carbons obtained from corncobs. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 180(3), 209–221. https://doi.org/10.1016/S0927-7757(00)00682-8.
Fairbairn, D. J., Arnold, W. A., Barber, B. L., Kaufenberg, E. F., Koskinen, W. C., Novak, P. J., et al. (2016). Contaminants of emerging concern: mass balance and comparison of wastewater effluent and upstream sources in a mixed-use watershed. Environmental Science & Technology, 50(1), 36–45. https://doi.org/10.1021/acs.est.5b03109.
Gaspard, S., & Ncibi, M. C. (2013). Biomass for sustainable applications: pollution remediation and energy. Cambridge: Royal Society of Chemistry.
Ghosh, P., Samanta, A. N., & Ray, S. (2011). Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination, 266(1–3), 213–217. https://doi.org/10.1016/j.desal.2010.08.029.
Girgis, B. S., Soliman, A. M., & Fathy, N. A. (2011). Development of micro-mesoporous carbons from several seed hulls under varying conditions of activation. Microporous and Mesoporous Materials, 142(2–3), 518–525. https://doi.org/10.1016/j.micromeso.2010.12.044.
González-García, P. (2018). Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renewable and Sustainable Energy Reviews, 82(1), 1393–1414. https://doi.org/10.1016/j.rser.2017.04.117.
Greenlee, L. F., Lawler, D. F., Freeman, B. D., Marrot, B., & Moulin, P. (2009). Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Research, 43(9), 2317–2348. https://doi.org/10.1016/j.watres.2009.03.010.
Guo, Y., Yang, S., Yu, K., Zhao, J., Wang, Z., & Xu, H. (2002). The preparation and mechanism studies of rice husk based porous carbon. Materials Chemistry and Physics, 74(3), 320–323. https://doi.org/10.1016/S0254-0584(01)00473-4.
Gupta, V. K., Srivastava, S. K., Mohan, D., & Sharma, S. (1998). Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Management, 17(8), 517–522. https://doi.org/10.1016/S0956-053X(97)10062-9.
Haghseresht, F., Nouri, S., Finnerty, J. J., & Lu, G. Q. (2002). Effects of surface chemistry on aromatic compound adsorption from dilute aqueous solutions by activated carbon. The Journal of Physical Chemistry B, 106(42), 10935–10943. https://doi.org/10.1021/jp025522a.
Hameed, B. H., & Rahman, A. A. (2008). Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. Journal of Hazardous Materials, 160(2–3), 576–581. https://doi.org/10.1016/j.jhazmat.2008.03.028.
Hamid, S. B. A., Chowdhury, Z. Z., & Zain, S. M. (2014). Base catalytic approach: a promising technique for the activation of biochar for equilibrium sorption studies of copper, Cu(II) ions in single solute system. Materials, 7(4), 2815–2832. https://doi.org/10.3390/ma7042815.
Han, R., Wang, Y., Yu, W., Zou, W., Shi, J., & Liu, H. (2007). Biosorption of methylene blue from aqueous solution by rice husk in a fixed-bed column. Journal of Hazardous Materials, 141(3), 713–718. https://doi.org/10.1016/j.jhazmat.2006.07.031.
Hasan, Z., & Jhung, S. H. (2015). Removal of hazardous organics from water using metal-organic frameworks (MOFs): plausible mechanisms for selective adsorptions. Journal of Hazardous Materials, 283, 329–339. https://doi.org/10.1016/j.jhazmat.2014.09.046.
Health Canada. (2017). Guidelines for Canadian drinking water quality. Summary Table (pp. 9, 12). Ottawa, Canada. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/water-eau/sum_guide-res_recom/sum_guide-res_recom-eng.pdf.
Hove, M., van Hille, R. P., & Lewis, A. E. (2007). Iron solids formed from oxidation precipitation of ferrous sulfate solutions. AICHE Journal, 53(10), 2569–2577. https://doi.org/10.1002/aic.11264.
IBI. (2012). International Biochar Initiative. http://www.biochar-international.org/. Accessed 21 Jan 2018.
Iriarte-Velasco, U., Sierra, I., Zudaire, L., & Ayastuy, J. L. (2016). Preparation of a porous biochar from the acid activation of pork bones. Food and Bioproducts Processing, 98, 341–353. https://doi.org/10.1016/j.fbp.2016.03.003.
Jang, H. M., Yoo, S., Choi, Y.-K., Park, S., & Kan, E. (2018). Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresource Technology, 259, 24–31. https://doi.org/10.1016/j.biortech.2018.03.013.
Jung, S.-H., & Kim, J.-S. (2014). Production of biochars by intermediate pyrolysis and activated carbons from oak by three activation methods using CO2. Journal of Analytical and Applied Pyrolysis, 107, 116–122. https://doi.org/10.1016/j.jaap.2014.02.011.
Jung, C., Park, J., Lim, K. H., Park, S., Heo, J., Her, N., et al. (2013). Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. Journal of Hazardous Materials, 263(Part 2), 702–710. https://doi.org/10.1016/j.jhazmat.2013.10.033.
Jung, C., Boateng, L. K., Flora, J. R. V., Oh, J., Braswell, M. C., Son, A., & Yoon, Y. (2015a). Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: experimental and molecular modeling study. Chemical Engineering Journal, 264, 1–9. https://doi.org/10.1016/j.cej.2014.11.076.
Jung, C., Phal, N., Oh, J., Chu, K. H., Jang, M., & Yoon, Y. (2015b). Removal of humic and tannic acids by adsorption–coagulation combined systems with activated biochar. Journal of Hazardous Materials, 300, 808–814. https://doi.org/10.1016/j.jhazmat.2015.08.025.
Karunarathne, H. D. S. S., & Amarasinghe, B. M. W. P. K. (2013). Fixed bed adsorption column studies for the removal of aqueous phenol from activated carbon prepared from sugarcane bagasse. 10th Eco-Energy and Materials Science and Engineering Symposium, 34, 83–90. https://doi.org/10.1016/j.egypro.2013.06.736.
Katsou, E., Malamis, S., Kosanovic, T., Souma, K., & Haralambous, K. J. (2012). Application of adsorption and ultrafiltration processes for the pre-treatment of several industrial wastewater streams. Water, Air, & Soil Pollution, 223(9), 5519–5534. https://doi.org/10.1007/s11270-012-1255-9.
Li, X. Z., Zhao, Q. L., & Hao, X. D. (1999). Ammonium removal from landfill leachate by chemical precipitation. Waste Management, 19(6), 409–415. https://doi.org/10.1016/S0956-053X(99)00148-8.
Li, W., Zhang, L., Peng, J., Li, N., & Zhu, X. (2008). Preparation of high surface area activated carbons from tobacco stems with K2CO3 activation using microwave radiation. Industrial Crops and Products, 27(3), 341–347. https://doi.org/10.1016/j.indcrop.2007.11.011.
Li, W.-B., Song, Y.-B., Xu, H.-K., Chen, L.-Y., Dai, W.-H., & Dong, M. (2015). Ion-exchange method in the collection of nitrate from freshwater ecosystems for nitrogen and oxygen isotope analysis: a review. Environmental Science and Pollution Research, 22(13), 9575–9588. https://doi.org/10.1007/s11356-015-4522-7.
Lima, I. M., Boateng, A. A., & Klasson, K. T. (2010). Physicochemical and adsorptive properties of fast-pyrolysis bio-chars and their steam activated counterparts. Journal of Chemical Technology & Biotechnology, 85, 1515–1521. https://doi.org/10.1002/jctb.2461.
Lima, I. M., Boykin, D. L., Thomas Klasson, K., & Uchimiya, M. (2014). Influence of post-treatment strategies on the properties of activated chars from broiler manure. Chemosphere, 95, 96–104. https://doi.org/10.1016/j.chemosphere.2013.08.027.
Liu, W.-J., Jiang, H., & Yu, H.-Q. (2015). Development of biochar-based functional materials: toward a sustainable platform carbon material. Chemical Reviews, 115(22), 12251–12285. https://doi.org/10.1021/acs.chemrev.5b00195.
Lozano-Castelló, D., Calo, J. M., Cazorla-Amorós, D., & Linares-Solano, A. (2007). Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon, 45(13), 2529–2536. https://doi.org/10.1016/j.carbon.2007.08.021.
Malik, P. K. (2003). Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of acid yellow 36. Dyes and Pigments, 56(3), 239–249. https://doi.org/10.1016/S0143-7208(02)00159-6.
Mandal, S., Sarkar, B., Igalavithana, A. D., Ok, Y. S., Yang, X., Lombi, E., & Bolan, N. (2017). Mechanistic insights of 2,4-D sorption onto biochar: Influence of feedstock materials and biochar properties. Bioresource Technology, 246, 160–167. https://doi.org/10.1016/j.biortech.2017.07.073.
Marsh, H., & Rodríguez-Reinoso, F. (2006). Activated carbon (1st ed.). Amsterdam: Elsevier.
Martínez-Huitle, C. A., & Ferro, S. (2006). Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chemical Society Reviews, 35(12), 1324–1340. https://doi.org/10.1039/B517632H.
Mohan, D., & Pittman Jr., C. U. (2006). Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. Journal of Hazardous Materials, 137(2), 762–811. https://doi.org/10.1016/j.jhazmat.2006.06.060.
Mondal, S., Aikat, K., & Halder, G. (2016a). Ranitidine hydrochloride sorption onto superheated steam activated biochar derived from mung bean husk in fixed bed column. Journal of Environmental Chemical Engineering, 4(1), 488–497. https://doi.org/10.1016/j.jece.2015.12.005.
Mondal, S., Bobde, K., Aikat, K., & Halder, G. (2016b). Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. Journal of Environmental Management, 182, 581–594. https://doi.org/10.1016/j.jenvman.2016.08.018.
Moreno-Castilla, C. (2004). Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon, 42(1), 83–94. https://doi.org/10.1016/j.carbon.2003.09.022.
Moriwaki, H., Yamada, K., & Usami, H. (2017). Electrochemical extraction of gold from wastes as nanoparticles stabilized by phospholipids. Waste Management, 60, 591–595. https://doi.org/10.1016/j.wasman.2016.07.010.
Oh, G. H., & Park, C. R. (2002). Preparation and characteristics of rice-straw-based porous carbons with high adsorption capacity. Fuel, 81(3), 327–336. https://doi.org/10.1016/S0016-2361(01)00171-5.
Ozoemena, K. I., & Chen, S. (Eds.). (2016). Nanomaterials in advanced batteries and supercapacitors. Switzerland: Springer.
Panday, K. K., Prasad, G., & Singh, V. N. (1985). Copper(II) removal from aqueous solutions by fly ash. Water Research, 19(7), 869–873. https://doi.org/10.1016/0043-1354(85)90145-9.
Pandey, A., Bhaskar, T., Stöcker, M., & Sukumaran, R. (Eds.). (2015). Recent Advances in Thermochemical Conversion of Biomass (1st ed.). Amsterdam: Elsevier.
Park, J., Hung, I., Gan, Z., Rojas, O. J., Lim, K. H., & Park, S. (2013). Activated carbon from biochar: Influence of its physicochemical properties on the sorption characteristics of phenanthrene. Bioresource Technology, 149, 383–389. https://doi.org/10.1016/j.biortech.2013.09.085.
Park, C. M., Han, J., Chu, K. H., Al-Hamadani, Y. A. J., Her, N., Heo, J., & Yoon, Y. (2017). Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: experiment and modeling. Journal of Industrial and Engineering Chemistry, 48, 186–193. https://doi.org/10.1016/j.jiec.2016.12.038.
Peng, H., Gao, P., Chu, G., Pan, B., Peng, J., & Xing, B. (2017). Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environmental Pollution, 229, 846–853. https://doi.org/10.1016/j.envpol.2017.07.004.
Petrie, B., Barden, R., & Kasprzyk-Hordern, B. (2015). A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Occurrence, fate, removal and assessment of emerging contaminants in water in the water cycle (from wastewater to drinking water), 72, 3–27. https://doi.org/10.1016/j.watres.2014.08.053.
Rafatullah, M., Sulaiman, O., Hashim, R., & Ahmad, A. (2010). Adsorption of methylene blue on low-cost adsorbents: a review. Journal of Hazardous Materials, 177(1–3), 70–80. https://doi.org/10.1016/j.jhazmat.2009.12.047.
Rajapaksha, A. U., Vithanage, M., Ahmad, M., Seo, D.-C., Cho, J.-S., Lee, S.-E., et al. (2015). Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. Journal of Hazardous Materials, 290, 43–50. https://doi.org/10.1016/j.jhazmat.2015.02.046.
Rakotonimaro, T. V., Neculita, C. M., Bussière, B., Benzaazoua, M., & Zagury, G. J. (2017). Recovery and reuse of sludge from active and passive treatment of mine drainage-impacted waters: a review. Environmental Science and Pollution Research, 24(1), 73–91. https://doi.org/10.1007/s11356-016-7733-7.
Rambabu, N., Rao, B. V. S. K., Surisetty, V. R., Das, U., & Dalai, A. K. (2015). Production, characterization, and evaluation of activated carbons from de-oiled canola meal for environmental applications. Industrial Crops and Products, 65, 572–581. https://doi.org/10.1016/j.indcrop.2014.09.046.
Rengaraj, S., Moon, S.-H., Sivabalan, R., Arabindoo, B., & Murugesan, V. (2002). Agricultural solid waste for the removal of organics: adsorption of phenol from water and wastewater by palm seed coat activated carbon. Waste Management, 22(5), 543–548. https://doi.org/10.1016/S0956-053X(01)00016-2.
Rostamian, R., Heidarpour, M., Mousavi, S. F., & Afyuni, M. (2015). Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. Journal of Agricultural Science and Technology, 17(4), 1057–1069.
Rubio, J., Souza, M. L., & Smith, R. W. (2002). Overview of flotation as a wastewater treatment technique. Minerals Engineering, 15(3), 139–155. https://doi.org/10.1016/S0892-6875(01)00216-3.
Sadaf, S., & Bhatti, H. N. (2014). Batch and fixed bed column studies for the removal of indosol yellow BG dye by peanut husk. Journal of the Taiwan Institute of Chemical Engineers, 45(2), 541–553. https://doi.org/10.1016/j.jtice.2013.05.004.
Saikia, R., Goswami, R., Bordoloi, N., Senapati, K. K., Pant, K. K., Kumar, M., & Kataki, R. (2017). Removal of arsenic and fluoride from aqueous solution by biomass based activated biochar: optimization through response surface methodology. Journal of Environmental Chemical Engineering, 5(6), 5528–5539. https://doi.org/10.1016/j.jece.2017.10.027.
Sancho, J. L. S., Rodríguez, A. R., Torrellas, S. Á., & Rodríguez, J. G. (2012). Removal of an emerging pharmaceutical compound by adsorption in fixed bed column. Desalination and Water Treatment, 45(1–3), 305–314. https://doi.org/10.1080/19443994.2012.692062.
Sauvé, S., & Desrosiers, M. (2014). A review of what is an emerging contaminant. Chemistry Central Journal, 8(1), 15. https://doi.org/10.1186/1752-153X-8-15.
Shim, T., Yoo, J., Ryu, C., Park, Y.-K., & Jung, J. (2015). Effect of steam activation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Bioresource Technology, 197, 85–90. https://doi.org/10.1016/j.biortech.2015.08.055.
Sözen, S., Teksoy Başaran, S., Akarsubaşı, A., Ergal, I., Insel, G., Karaca, C., & Orhon, D. (2016). Toward a novel membrane process for organic carbon removal—fate of slowly biodegradable substrate in super fast membrane bioreactor. Environmental Science and Pollution Research, 23(16), 16230–16240. https://doi.org/10.1007/s11356-016-6795-x.
Sudilovskiy, P. S., Kagramanov, G. G., & Kolesnikov, V. A. (2008). Use of RO and NF for treatment of copper containing wastewaters in combination with flotation. Desalination, 221(1–3), 192–201. https://doi.org/10.1016/j.desal.2007.01.076.
Sumalinog, D. A. G., Capareda, S. C., & de Luna, M. D. G. (2018). Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. Journal of Environmental Management, 210, 255–262. https://doi.org/10.1016/j.jenvman.2018.01.010.
Takaya, C. A., Fletcher, L. A., Singh, S., Okwuosa, U. C., & Ross, A. B. (2016). Recovery of phosphate with chemically modified biochars. Journal of Environmental Chemical Engineering, 4(1), 1156–1165. https://doi.org/10.1016/j.jece.2016.01.011.
Tan, G., Sun, W., Xu, Y., Wang, H., & Xu, N. (2016). Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresource Technology, 211, 727–735. https://doi.org/10.1016/j.biortech.2016.03.147.
Tan, X., Liu, S., Liu, Y., Gu, Y., Zeng, G., Hu, X., et al. (2017). Biochar as potential sustainable precursors for activated carbon production: multiple applications in environmental protection and energy storage. Bioresource Technology, 227, 359–372. https://doi.org/10.1016/j.biortech.2016.12.083.
Uchimiya, M., Wartelle, L. H., Lima, I. M., & Klasson, K. T. (2010). Sorption of deisopropylatrazine on broiler litter biochars. Journal of Agricultural and Food Chemistry, 58(23), 12350–12356. https://doi.org/10.1021/jf102152q.
Unuabonah, E. I., Olu-Owolabi, B. I., Fasuyi, E. I., & Adebowale, K. O. (2010). Modeling of fixed-bed column studies for the adsorption of cadmium onto novel polymer–clay composite adsorbent. Journal of Hazardous Materials, 179(1), 415–423. https://doi.org/10.1016/j.jhazmat.2010.03.020.
Vaneeckhaute, C., Meers, E., Michels, E., Christiaens, P., & Tack, F. M. G. (2012). Fate of macronutrients in water treatment of digestate using vibrating reversed osmosis. Water, Air, & Soil Pollution, 223(4), 1593–1603. https://doi.org/10.1007/s11270-011-0967-6.
Verma, A. K., Dash, R. R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93(1), 154–168. https://doi.org/10.1016/j.jenvman.2011.09.012.
Wang, F., Gao, B., Yue, Q., Bu, F., & Shen, X. (2017). Effects of ozonation, powdered activated carbon adsorption, and coagulation on the removal of disinfection by-product precursors in reservoir water. Environmental Science and Pollution Research, 24(21), 17945–17954. https://doi.org/10.1007/s11356-017-9451-1.
Westholm, L. J., Repo, E., & Sillanpää, M. (2014). Filter materials for metal removal from mine drainage—A review. Environmental Science and Pollution Research, 21(15), 9109–9128. https://doi.org/10.1007/s11356-014-2903-y.
Wu, F.-C., & Tseng, R.-L. (2006). Preparation of highly porous carbon from fir wood by KOH etching and CO2 gasification for adsorption of dyes and phenols from water. Journal of Colloid and Interface Science, 294(1), 21–30. https://doi.org/10.1016/j.jcis.2005.06.084.
Xia, D., Tan, F., Zhang, C., Jiang, X., Chen, Z., Li, H., et al. (2016). ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal. Applied Surface Science, 377, 361–369. https://doi.org/10.1016/j.apsusc.2016.03.109.
Acknowledgments
This study was supported by Québec’s Ministry of Economy, Science, and Innovation (Ministère de l’Économie, de la Science et de l’Innovation du Québec), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Research Chair Program, Abitibi-Témiscamingue College, and the Technology Center for Industrial Waste (Centre Technologique des Résidus Industriels) through its partner on this project, Airex Energy.
Funding
The first author, Dr. Flavia Lega Braghiroli, sincerely acknowledges financial support by the NSERC via a Banting Postdoctoral Fellowship (2017–2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Activated biochars are highly efficient for the removal of various contaminants in water
• The variation of feedstock and operating conditions of pyro-gasification can affect the porosity of activated biochars
• Physicochemical and chemical activation are appropriate techniques for production of activated biochars
• High aromaticity and surface area are important characteristics for the removal of organic contaminants
• The presence of surface functionalities in activated biochars is required for interactions between sorbent and inorganic contaminants
Rights and permissions
About this article
Cite this article
Braghiroli, F.L., Bouafif, H., Neculita, C.M. et al. Activated Biochar as an Effective Sorbent for Organic and Inorganic Contaminants in Water. Water Air Soil Pollut 229, 230 (2018). https://doi.org/10.1007/s11270-018-3889-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3889-8