Abstract
The treatment of mine drainage-impacted waters generates considerable amounts of sludge, which raises several concerns, such as storage and disposal, stability, and potential social and environmental impacts. To alleviate the storage and management costs, as well as to give the mine sludge a second life, recovery and reuse have recently become interesting options. In this review, different recovery and reuse options of sludge originating from active and passive treatment of mine drainage are identified and thoroughly discussed, based on available laboratory and field studies. The most valuable products presently recovered from the mine sludge are the iron oxy-hydroxides (ochre). Other by-products include metals, elemental sulfur, and calcium carbonate. Mine sludge reuse includes the removal of contaminants, such as As, P, dye, and rare earth elements. Mine sludge can also be reused as stabilizer for contaminated soil, as fertilizer in agriculture/horticulture, as substitute material in construction, as cover over tailings for acid mine drainage prevention and control, as material to sequester carbon dioxide, and in cement and pigment industries. The review also stresses out some of the current challenges and research needs. Finally, in order to move forward, studies are needed to better estimate the contribution of sludge recovery/reuse to the overall costs of mine water treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mining industry is an essential contributor to the world’s economy; however, its social and environmental impacts are not negligible. Mine operators must balance the need to optimize efficiency with the requirement to maintain clean operations. The management of mine wastes, which can be solid (e.g., tailings, sludge); liquid (e.g., water flowing through the different components of a mine site, water from ore processing); or gaseous (e.g., CO2, NOx, SO2), constitutes one of the major challenges facing mining operations.
Mine drainage-impacted waters are liquid wastes resulting from pyrite oxidation through chemical and biological processes (Neculita et al. 2007; Nordstrom et al. 2015). The two most problematic types include acid mine drainage (AMD) and contaminated neutral drainage (CND). Even though the pH is not the only relevant parameter to characterize these wastewaters, extremely contaminated AMD can have pH as low as −3.6, but it is usually between 2 and 6, with concentrations of dissolved metals and sulfate as high as 200 and 760 g/L, respectively (Nordstrom et al. 2000, 2015). In contrast, CND frequently has pH values between 6 and 9 (Nordstrom et al. 2015), but may still contain elevated concentrations of metals and metalloids (e.g., Cd, Cr, Co, Hg, Mo, Ni, Sb, As, Se, Zn) which are soluble at near-neutral pH (Stantec 2004).
The treatment of mine drainage produces secondary waste (sludge), which requires additional management strategies. The type of sludge addressed in this study is produced from the in situ treatment of AMD and CND. Although the data was very limited and inconsistent, Zinck and Griffith (2009, 2013) conducted an analysis of sludge produced from 108 mine sites located around the world. These mine sites were classified as follows: 46 % base metal, 23 % precious metal, 7 % coal, 5 % uranium, and 19 % other types of mines (e.g., Mo and Sb mines with AMD issues). The authors reported an average global production of mine sludge, per year and per site, of 9500 dry tons, with a range of 20–135,000 dry tons. The volume factor could be 2 to 70 times the mass of sludge (Zinck and Griffith 2013). However, very limited information on quantities of sludge is available because the sludge produced in water treatment plants was not systematically monitored (Zinck and Griffith 2013). The principal issues associated with the storage and disposal of sludge are (1) the high cost, (2) disposal capacity limits, and in some cases, (3) upkeep of constant chemical pretreatment which can be required to stabilize certain sludge even after closure (Zinck and Griffith 2006, 2013; Pedroni et al. 2006). Sludge management costs generally represent 5–20 % of the operational water treatment cost (Zinck and Griffith 2013). Dredging costs of sludge were about US$5–20/m3 and can be over $1 M per year per site (Zinck and Griffith 2013). The conditioning (i.e., dewatering, stabilization) and transport (i.e., hydraulic, haulage) can also lead to additional expenses, and may significantly increase the overall operational costs of a mine water treatment plant (Herrera et al. 2007; Marcello et al. 2008; Nodwell and Kratochvil 2012; Zinck and Griffith 2013). Additional concerns with the sludge may include the release and migration of metal/metalloids (e.g., Fe, Al, Mn, Cu, Zn, As) (Viadero et al. 2006; Beauchemin et al. 2010; Wang et al. 2013). With the goal of minimizing storage and management costs, as well as the spread of contaminants to the environment, mine operators rely on reclaiming sludge through recovery and reuse.
In this context, the objective of the paper is to thoroughly review and critically discuss the recovery and reuse of sludge derived from active and passive treatment of AMD and CND.
The presentation of the paper is based on the following structure: a brief review of basic principles of available technologies for the treatment (active/passive) of mine drainage-impacted water is mentioned first, comprising the sludge production. Then, the principal issues in sludge management are mentioned, including concerns about sludge stability, storage, and disposal. Afterwards, the main part of the discussion is divided into two distinctive subsections: the recovery of valuable by-products from the mine sludge and the different reuse options of sludge originating from active and passive treatment of mine drainage, based on available laboratory and field studies. Finally, the review brings out some of the current challenges and research needs.
Treatment of mine drainage and associated sludge
The treatment of mine drainage is commonly divided into either active and passive or chemical and biological. Each type has its principles, operating conditions, specific advantages, and drawbacks (Table S1, S2). Basically, these technologies are performed by neutralization and/or oxidation/aeration, often through addition of alkaline agents (e.g., NaOH, Na2CO3, CaO, NH4OH, MgO), as well as of oxidants (e.g., H2O2, KMnO4, O3) (Skousen et al. 2000; Johnson and Hallberg 2005; Taylor et al. 2005). Either active or passive, chemical treatment aims to rise the pH and alkalinity, and to remove metals and sulfate as hydroxides, carbonates, and sulfides (Johnson and Hallberg 2005; USEPA 2014). Likewise, biological treatment, whichever active or passive, relies on the microorganisms capable of producing alkalinity and sulfides to precipitate the metals, depending on E h (Johnson and Hallberg 2005; Neculita et al. 2007).
The treatment produces sludge with variable composition according to the type of treatment, the used alkaline agents, and the quality of the water to be treated (Table 1).
Active treatment sludge
The common active chemical treatment includes the sedimentation basin/pond, low-density sludge (LDS) and the high-density sludge (HDS) processes (Table S2). The LDS process produces sludge with low solid content (less than 5 %), whereas the HDS generates denser (>20 % solid) and chemically stable final residue due to recirculation of sludge in the system (sludge/lime mixture with a ratio of 15:1 to 35:1) (Aubé and Zinck 2003; Johnson and Hallberg 2005; Taylor et al. 2005). The chemical and physical characteristics of sludge from the active chemical treatment of AMD and CND may differ slightly, but, in general, the pH is high (8.2–10.8) which may confer them the advantage to be used as a substitute of neutralizing material (Zinck 2005; Zinck and Griffith 2013). They are mainly composed of Fe, Al, Mn, Ca, and Mg (Table 1) (Zinck et al. 1997; McDonald and Webb 2006; Viadero et al. 2006; Hedin 2012). Several species of metal/metalloid might be also present, contingently to the quality of the contaminated water (Beauchemin et al. 2010). Moreover, depending on the source of mine drainage, the composition varies, for example, sludge from the treatment of contaminated water from coal mines is less contaminated by toxic elements (e.g., As, Cd) than those from the polymetallic mines (Robertson and Shaw 1997; Zinck and Griffith 2006; Sibrell et al. 2009; Cui et al. 2013). Generally, the particle size of solids composing the sludge is between 2.89–42.5 μm (Zinck 2005; Viadero et al. 2006; Beauchemin et al. 2010). On the other side, the mineralogical composition consists basically of amorphous/crystallized Fe and Al oxides/hydroxides (e.g., ferrihydrite, goethite, hematite, magnetite) as well as gypsum, and calcite (Zinck et al. 1997; Zinck 2005; Viadero et al. 2006; Hedin 2012).
Active biological treatment use bioreactors (e.g., membrane bioreactor, fluidized-bed bioreactors, upflow anaerobic sludge blanket reactor, Bioteq, Biopaq, Rhodes BioSURE) which are designed to remove simultaneously sulfate from contaminated mine drainage besides acidity, metalloids/metals/heavy metals, since these requirements were found hardly achievable by conventional treatment (Bratty et al. 2006; Huisman and Weghuis 2011; Nodwell and Kratochvil 2012; Rose 2013). Despite their high cost, they do not practically produce sludge, but rather allow a selective recovery of precipitates (e.g., metal sulfides) useful for other applications, as well as of clean water that meets discharge limit (Lopez et al. 2009; Ňancucheo et al. 2012; Rose 2013). The bioelectrochemical technologies (microbial fuel/electrolysis cells (MFC/MEC)) are other types of active biological treatment, which besides to precipitate minerals, aim to recover as well energy (Logan 2008, 2010; Luo et al. 2014). Nevertheless, full-scale applications are not available yet (Bejan and Bunce 2015).
Because sludge characteristics depend on the type of treatment and the employed alkaline agents, a geochemical modeling (Phreeqc, Minteq.v4, statistical software R) was exploited to predict the sludge production (and its costs) from an active chemical treatment of mine drainage originating from abandoned sulfur, copper, and iron mines (Koide et al. 2012). The advantages of this method are not only its use to forecast the neutralizer agent requirements and the sludge generation, but it allows also to estimate whether additional storage is required and the water treatment costs (Koide et al. 2012). Furthermore, this approach might be useful for predicting the costs of mine reclamation by integrating the eventual generation of AMD/CND.
Passive treatment sludge
Passive treatment is preferred when mine water is slightly contaminated, especially with respect to the concentrations of Fe, Al, and dissolved oxygen (Hedin et al. 1994, 2013). Passive abiotic/chemical treatment includes sorption, limestone drains (anoxic limestone drain (ALD)), oxic limestone channel/drain/bed (OLC/OLD/OLB), diversion wells, cascade aeration, and settling pond (Table S2) (Gazea et al. 1995; Johnson and Hallberg 2005; Neculita et al. 2007).
Sludge from the passive abiotic treatment has a solid content higher than 25 % and is mainly composed of iron oxy-hydroxides (≈95 %), often in the form of goethite (FeOOH), which can be processed for eventual reuses (Taylor et al. 2005; Hedin 2003, 2012). Nonetheless, dewatering is still necessary and up to 52 % of solid content could be obtained (Hedin 2003). In addition, Al-containing solids can also be found, depending on water quality, but mostly perceived as contaminants (Hedin 2006, 2012). The new technique in passive treatment involving the use of dispersed alkaline substrate (DAS) generates different type of residues (granular wastes) rich either in Fe, Alor Zn (Pérez-López et al. 2011; Caraballo et al. 2011; Macías et al. 2012a). The elements found in Fe-rich sludge are Fe and S, precipitated as schwertmannite [Fe8O8(OH)6(SO4)·nH2O] and goethite (Caraballo et al. 2011). The wastes rich in Al contains S and Ca with the mineral phases in the form of hydrobasaluminite [Al4 (SO4) (OH)10.12–36(H2O)], gypsum and calcite (Caraballo et al. 2011). The Zn-rich wastes may include Cd, Co, and Ni as minor elements, and the mineral phases are hydrozincite (Zn5(CO3)2(OH)6) and loseyite [(Mn,Zn)7(CO3)2(OH)10] (Pérez-López et al. 2011; Macías et al. 2012a). Precipitation of minerals in the form of schwertmannite and hydrobasalumnite seems promising because important amounts of sulfate can be retained in the final waste (Pérez-López et al. 2011). Residues from the DAS treatment were found to be a potential source of metals, which recovery needs to be developed (Macías et al. 2012b).
Passive biological treatment includes the wetlands/compost bioreactors (PCB), successive alkalinity production systems/vertical flow ponds (SAPS/VFPs), iron oxidizing bioreactors (IOBs), sulfate-reducing bioreactors (SRBs), and permeable reactive barriers (PRBs) (Table S2) (Johnson and Hallberg 2005; Neculita et al. 2007; USEPA 2014). Precipitates from these systems are retained either on plant surfaces (leaves, shoots); downstream (in the rhizomes, roots, and lateral roots); or in the spent reactive mixture itself (Johnson and Hallberg 2005). They are commonly composed of Fe, Al, Zn, Mn, and PO4 3−, and the main mineral phases are in the form of oxides/hydroxides (principally iron oxides), carbonates (e.g., calcite, siderite, rhodocrocite) and sulfides (e.g., makinawite, greigite) (Johnson and Hallberg 2005; Neculita et al. 2008; Genty et al. 2010). However, detailed characterization of the spent mixture, especially from SRBs, needs to be completed in order to determine their final destination (appropriate storage or reuse).
Sludge management issues
Two principal issues related to management of mine water treatment sludge are identified: the stability (chemical and physical) and the storage (disposal). Transportation to storage site or to smelter might become also a special issue inasmuch as it might raise impending social concerns and increase costs, but this is not addressed in this review.
Sludge stability
A stable sludge is harmless to the environment and can be disposed of by using the suitable long-term storage approaches and facilities. The stability depends on the degree of resistance to an aggressive environment that might significantly change the sludge chemical properties.
Stability assessment methods
Several methods are used to evaluate the chemical stability of mine sludge but the frequently used are leaching tests (Table S3). They are also employed to classify sludge (inert, hazardous, or non-hazardous), to assess its acceptability into storage (and select the storage approach) and to determine its potential reuse. The most common leaching tests are toxicity characteristic leaching procedure (TCLP), synthetic precipitation leaching procedure (SPLP), strong acid leach test (SALT) (Fiset et al. 2003; McDonald et al. 2006; Macías et al. 2012b), and continuous weathering cells (CANMET 1996; Genty et al. 2012). The SALT was found to be the most appropriate test to simulate site conditions because it uses sulfuric acid (commonly encountered on mine sites), and the serial acid extractions show the instability of the constituting elements, with respect to pH (McDonald et al. 2006). Depending on the sludge chemical composition, other tests might be required as well to evaluate the leachability of elements that can be released only at near-neutral pH (Table S3). The suggested methods include the use of neutralization potential (NP) that can give information on the possible proportion of released metals, according to the pH. The higher the NP is, the longer chemically stable the sludge is (McDonald et al. 2006). Other physical characteristics are also proposed, such as the zeta potential (ZP) that gives information on the particles mobility and the lower is its value, the lower is the sludge stability (Dempsey and Jeon 2001). Finally, the total volatile solid (TVS) is suggested to be used as a stability evaluation of sludge from passive biological treatment (e.g., passive biochemical reactors (PBRs)). Since the TVS content is an indicator of the amount of organic matter, it could be anticipated that the higher the content of volatile material in the sludge is, the less it is stable, as organic matter (and microorganisms) continue to degrade in the environment (Sanin et al. 2011).
Preliminary leaching tests are important because the conclusion drawn from the results regarding the fate of the sludge (to be stored or reused) allows determining the focus on the additional tests to be completed. For example, if sludge is to be stored, pretreatment/dewatering needs to be applied and the characteristics of interest might be physical. On the contrary, if sludge is to be utilized as a fertilizer, concerns on elemental content (e.g., Cu, Zn) are indispensable. Since the characteristics of active and passive sludge differ, their stability also differs.
Stability of active treatment sludge
Due to its low solid content, active treatment sludge is voluminous, difficult to dewater and has a low stability (Dempsey and Jeon 2001; Viadero et al. 2006; McDonald and Webb 2006). However, aged sludge may exhibit greater stability than fresh sludge, due to an increased solid content (25 % or higher) and recrystallization of calcite (Zinck et al. 1997).
The results of SALT test on HDS sludge showed that once the NP was consumed, elements started to be leached out (Fe, Al, Cu, and Zn at pH ∼3, ∼4.5, ∼5.5, and 6.5, respectively) (McDonald and Webb 2006). This means that HDS sludge should be at least kept at pH of around 7 to maintain its chemical stability.
Stability of passive treatment sludge
Some studies reported that sludge stability depends on its content in amorphous phases (Zinck 2005). This may explain the higher stability of sludge from OLC/OLD/OLB and aerobic wetland as this type of sludge is mainly composed of more or less amorphous iron-oxides/hydroxides and ferrihydrite that can be used to scavenge other metals in its structure (Zinck 2005). However, the ZP values (between +10 and −10 mV) still indicated a poor stability (Dempsey and Jeon 2001).
The stability of metal precipitates and their potential mobility in the residues from PBRs have been evaluated with a sequential extraction procedure, mineralogical analysis, and by thermodynamic equilibrium modeling (Genty 2012; Genty et al. 2012). Results showed that Al, Cr, and Pb were mainly present under relatively stable forms (residual fraction), whereas Cd was found in the soluble and exchangeable fraction, indicating a possible remobilization under specific pH-Eh conditions (Genty et al. 2012). Moreover, metals (Fe, Mn, Ni, and Zn) were bound to the reducible or Fe–Mn oxide/hydroxide fractions, but were also present in the oxidizable or bound to organic matter fractions (Genty et al. 2012). Geochemical modeling predicted that Fe precipitation occurred in the form of goethite, as most of the Fe was confined in the reducible phase, or in Fe–Mn oxide/hydroxide fractions (Genty 2012). These results suggest that metals were relatively stable under oxidizing conditions.
In summary, all sludge types (active/passive, chemical/biological) would become unstable if exposed to acidic conditions (McDonald and Webb 2006; Zinck and Griffith 2006; Genty et al. 2012), thus appropriate disposal needs to be carefully selected.
Sludge storage and disposal
Sludge intended for disposal often needs conditioning, which includes dewatering (Table S4), pretreatment (Table S5), and/or chemical stabilization/solidification (S/S) techniques (Table S6) to better manage storage space, as well as to chemically stabilize/fix the contaminants. Drying is the most commonly used dewatering technique (Table S4). Drying at low temperature was reported to be advantageous because unwanted effects on sludge chemical composition can be avoided (Cui et al. 2013). Natural drying (open air) would be beneficial, but not recommended when potential generation of toxic volatile compounds is expected. Most dewatering processes (Table S4) are usually accompanied by pretreatments, the most frequently used being thickening, filtration, and freeze/thaw (in cold climates) (Tables S5). The pretreatment aims to increase the percentage solid and, in certain case, to ease the dewatering (USEPA 1973; Fiset et al. 2003; Zinck et al. 1997). They could also be used alone, especially for sludge containing high Fe-hydroxide since up to 60 % of densification can be reached (Fiset et al. 2003). The S/S methods (Table S6) are used to prevent contaminants dissolution and transport, and to convert the waste into an inert material (Robertson and Shaw 1997; Fiset et al. 2003; Benzaazoua et al. 2004; Zinck and Griffith 2006). S/S techniques can be costly; for example, vitrification can cost up to US$300/ton sludge (Table S6).
After the conditioning, sludge can be disposed of in an appropriate setting, either in ponds, as covers over tailings, in mine pits, in underground mines (backfill), mixed with tailings, disposed in tailings ponds, under water covers or in landfills (Table S7). Disposal in ponds or lagoons, with tailings or other wastes are the most adopted methods, while dumping in landfill is not a preferable option and even not legal in some European countries (Benzaazoua et al. 2006; Song et al. 2014). Space availability was reported not of much concern in some cases (e.g., in Canada), but constant chemical treatment might become problematic (Zinck and Griffith 2006).
Leaching tests (TCLP and EN 12457-2) performed on DAS treatment residues allow to classify them as inert (Zn-rich), non-hazardous (Al-rich), and hazardous (Fe-rich) and to suggest their disposal in a dry environment (Macías et al. 2012b). The recommended storage type of Al- and Zn-rich residues would be in a covered surface impoundments or waste piles; whereas Fe-rich residues could be co-disposed with municipal wastes (Macías et al. 2012b). As for sludge from PBRs, storage in neutral water would be appropriate. However, further survey on the stability of metals in the spent reactive mixture is needed (Genty et al. 2012).
Sludge disposal cost was reported to be around US$23–75/ton depending on solids content (between 3 and 70 % solids) and the distance from origin to the final place of delivery (Zinck and Griffith 2013). This represents around 10 % of the global water treatment cost (about US$10 k to US$>300 k per year) (Zinck and Griffith 2013). Disposal costs are, therefore, among the major hurdles in sludge management and recovery or reuse could alleviate the expenses.
Recovery and reuse of sludge
The term “recovery” is defined here as “an extraction of useful substances from sludge”, such as metals and iron oxides (ochre) (Table 2), whereas the term “reuse” is defined as “using sludge again after processing, treatment, or adding value to sludge”. Recovery of by-products from mine sludge could potentially reduce mine water treatment costs (around 25 %) but needed to be studied case by case according to different feasibility factors (e.g., technical, economical, market) (Hedin 2008; Smith et al. 2013).
The characteristics of mine drainage treatment sludge, with the pH 8.2–10.8 and the content in Al and Fe oxides considered as highly adsorbent (high surface area and amphoteric quality), make it suitable to sequester and/or remove contaminants (Zinck 2005; Zinck and Griffith 2006; Sibrell 2007; Sibrell et al. 2010). As a result, mine sludge is exploited to recover by-products and is often reused in wastewater treatment, in soil remediation, in agriculture and horticulture, in construction, and in the prevention of contaminated mine drainage (Table 3).
Recovery of by-products from sludge
Among the recovered products from the mine drainage sludge are the following: metals, iron hydroxides (ochre), elemental sulfur, and others (including magnesium and barium hydroxides), some of which are thoroughly discussed herein.
Metal recovery
Sludge may contain a non-negligible amount of recoverable products, such as oxides/hydroxides and metals, having potential economic value (Table 2). Metal recovery is rarely adopted due to operational costs and has only been applied as an alternative option, when disposal costs are prohibitive (Zinck 2005; Zinck and Griffith 2013). Revenues from metal recovery are not always promising because of its close link to the metal market supply and demand, which may vary considerably (Smith et al. 2013). This could explain the very few studies reporting metal recovery from mine sludge (Table 2). Meanwhile, Ni could be recovered from mine sludge (with 86 % water) consisting of 6.2 % Ni, 16.8 % Fe, 20.3 % Mg, and 0.2 % Ca, by adsorption onto activated silica after sulfuric acid leaching (Table 2). A contact time of 1 h was sufficient to recover 98–99 % of Ni and, at the end of the process, the activated silica could be recycled. The same method was also applied for Zn recovery (El-Ammouri et al. 2000). Regardless of the simplicity of the procedure, it can still generate Fe-rich residues. In addition, if the sludge contains other metals (e.g., Cu) with similar solubility, the recovery onto silica might not be selective enough. Fe that can be used as coagulant was also recovered by the resolubilization of iron hydroxides into ferric sulfate solution, with H2SO4 addition (Keefer and Sack 1983). However, considerable concentration of sulfate could be found in the final effluent. Thus, this method might not be suitable for Fe recovery from mine sludge rich in sulfate. Cu is either recovered from cyanide sludge by multi-leaching process using H2SO4 and sulfate solution (Elamari et al. 2005; Lambert et al. 2014) and acid leaching combined with solvent extraction/selective precipitation/activated silica (USEPA 2001; Jandova et al. 2002) or by bacterial leaching (Shen et al. 2003; Johnson 2013).
Iron oxides-hydroxides recovery
In Europe, large quantities of sludge are often qualified as not reusable and are regularly deposited in landfills, whereas in the USA, the exploitation of ochre-rich sludge from passive treatment has been carried out since the 2000s for commercial purposes (Hedin 2003, 2012; Mayes et al. 2009). For example, in the USA, approximately 1000 tons of ochre could be obtained from about 2000 tons of sludge with 25–30 % solids, which could replace the natural iron oxides (mined), as well as their importation from abroad (Hedin 2003).
Iron oxides/hydroxides (ochre) from mine sludge, long considered as useless waste material, now have a competitive purity (∼77 %, without treatment, and 98–99 % with high-temperature treatment) to those obtained from natural or synthetic sources (99 %) (Hedin 2003; Zinck and Griffith 2006). The purity depends mainly on the quality of the treated water and the type of treatment. The purest ochre, having pigmentary characteristics, was recovered from passive treatment of acidic waters whereas semi-pure, with Si, Al, and Ca as secondary components, was recuperated from active treatment of Fe-rich alkaline water (Hedin 2006, 2012).
The difficulties encountered during sludge collection (raw wet) for ochre recovery are the transport outside the mine site, equipment supply, material handling, loading capacities, possible erosion, and wind disturbance (Hedin 2003; Heal et al. 2004). Other limitations related to ochre refining are the removal of impurities (e.g., Si, Ca, organic matter) and the pretreatment (e.g., calcination, blending) to meet the requirements of the pigment industries (e.g., size, percentage solids) (Hedin 2003, 2012).
Other products recovery
Recovery of products from sludge containing magnesium hydroxide/barium sulfate collected at the second stage of the Tshwane University of Technology—Magnesium-Barium-Oxide Process (for neutralization and desalination of AMD) has been investigated. In batch testing, sludge carbonation allowed the recovery of magnesium and barium hydroxides and of elemental sulfur (Rukuni et al. 2012). Other products from gypsum containing waste were also studied. Processes involving thermal reduction and aqueous carbonation were employed to recover elemental sulfur and low-grade quality calcium carbonate (<90 % mass as CaCO3) that could be used as a substitute of alkaline agent in AMD neutralization (De Beer et al. 2014).
Sludge reuse
Among the physical characteristics that play an important role in the reuse of sludge is the surface area, which influences the retention of contaminants (Dempsey and Jeon 2001). The surface area of recovered iron oxides from active treatment of mine drainage ranges from 23 to 114 m2/g, whereas that of sludge originating from the passive treatment ranges from 119 to 215 m2/g, which makes the latter a better sorbent (Hedin 2012). The higher is the surface area of a material, the higher is its sorption capacity. The capacity of a material to adsorb either oxyanions or cations depends also on its surface charge. The latter can change according to pH, resulting from protonation or deprotonation of the surface functions. Accordingly, the material can have the ability to participate in acid-based and ion exchange reactions.
Sludge composed of iron oxides and/or iron sulfate (oxy) hydroxides can sequester, essentially through adsorption and co-precipitation, oxyanions such as arsenate, selenite, and phosphate, as well as cations such as Pb, Cd, Cu, and Zn (Kirby et al. 1999; Cornell and Schwertmann 2003; Janneck et al. 2010; Hedin 2012; Sibrell and Tucker 2012).
Thus, several evaluations have been undertaken to reuse raw (not pretreated) or conditioned (dried to obtain ochre/pellets) sludge to remove contaminants in wastewater and contaminated soil, as fertilizer in agriculture, as substitute material in construction, as material in the prevention and control of AMD, in sequestration of carbon dioxide, and in other several fields such as in cement and pigment industries.
Sludge reuse for contaminants removal in wastewater
The reuse of mine sludge to remove contaminants, such as As, P, dye, heavy metals, and rare earth elements in contaminated waters are thoroughly discussed in the following.
Phosphorus removal using AMD sludge
Several studies using AMD sludge (AMDS) to remove P from wastewater sewage, including dirty water from animal production facilities (Heal et al. 2004; Wei et al. 2008; Fenton et al. 2009; Sibrell and Tucker 2012), agriculture and aquaculture effluents (Young et al. 2008; Sibrell et al. 2009; Sibrell and Tucker 2012), and municipal wastewater (Sapsford et al. 2015) have been conducted (Table 3). P is an important domestic wastewater contaminant because of its role on eutrophication, which leads to oxygen depletion and aquatic toxicity (Smol 2008; Sibrell et al. 2009). As aquatic systems are considered to be the most sensitive to P, the water regulation threshold content was set to 0.1–0.2 mg/L in Canada (MDDELCC 2013) or below 50 μg/L in European countries such as Germany and the UK (Genz et al. 2004; OFWAT 2005).
Removal mechanisms of P include a combination of adsorption onto Fe or Al oxide flocs (Eqs. 1 or 2), and a direct precipitation of Al or Fe phosphate (Eqs. 3 or 4) (Sibrell 2007):
The removal efficiency can be contradictory, particularly when influenced by pH. Some studies showed that whatever is the pH value, no significant change was found, whereas others showed better efficiency at higher pH (Heal et al. 2004; Chitrakar et al. 2006). The point of zero charge (PZC), an essential characteristic of the surface of solid materials is the pH value related to zero electrical charge density of the sorbent surface. It allows to evaluate qualitatively the surface charge of a material. Hence, at pH below PZC, the surface is positively charged (attracting oxyanions), whereas the opposite is valid at pH above PZC (attracting cations). Therefore, at higher pH, the negatively charged surfaces of oxides lead to P repulsion (as P surface is also negatively charged). The PZC of Fe/Al oxides/hydroxides is 8–9 (Cornell and Schwertmann 2003; Sparks 2003) and at pH >9 (between 9 and 11), P removal by iron oxides was higher, especially when combined with gypsum (Bastin et al. 1999). At pH 13, efficiency of P removal was reported to be up to 100 % (Kang et al. 2003). However, removal of 99 % P (in a municipal wastewater with a P concentration of 3.5 mg/L) and 97 % Zn (in a DNC with a Zn concentration of 16 mg/L) were found at a final pH 7–8, likely due to sorption/co-precipitation (Sapsford et al. 2015). Thus, P removal might not be dependent only on pH and the buffering capacity of the sludge but also on the availability of sorption sites, surface area, and complexation.
Ochre pellets obtained from AMDS (after drying, sieving, and coating with commonly 30 % Portland cement) tested on wastewater allowed removing 60 % P (Sibrell 2007). Ochre from AMD treatment was also mixed with anaerobic digested sewage sludge as a component of biochars (OCAD) for P removal from wastewater with initial concentration of 0.02 g/L (Shepherd et al. 2016). Results showed that the OCAD biochars had higher P removal rates (around 1.26 × 10−3–1.24 × 10−2 mg P/g) compared to biochars composed of only anaerobic digested sewage sludge (0.986 × 10−3–1.06 × 10−3 mg P/g) or even to other types of biochars such as zeolite (0.13 × 10−2 mg P/g) and activated carbon (0.884 × 10−2 mg P/g). However, with higher initial P concentrations in the wastewater (0.8 g/L), ochre alone (14.2 mg P/g) could remove more P than OCAD biochars (9.35 mg P/g) (Shepherd et al. 2016). The reuse of P-saturated OCAD biochars as fertilizer was found less effective, while further research on the design is needed (Shepherd et al. 2016). Dried solids from AMDS, without addition of binder (or cement), composed mainly of Fe and Al oxy-hydroxides called “ferroxysorb” showed 60–90 % P removal from agricultural wastewaters within less than 5 min (Sibrell et al. 2009). Floc from AMDS could also yield 10–20 mg P/g floc during P removal from wastewater having an initial concentration of 1.0 mg P/L (Adler and Sibrell 2003). Raw AMDS with 4.31 % solids (pH ≈ 8.1), just sieved through a 100-mesh screen, removed up to 98 % P from secondary effluent of municipal wastewaters with 1.80 mg P/L (at pH ≈ 5–8.5), in batch and continuous stirred tank reactors (Wei et al. 2008). Other iron oxide-based media prepared from AMDS was also used to adsorb P in wastewater (0.21–50 mg P/L) and presented about a sorption capacity of 10,000 mg P/kg sludge at equilibrium P concentration of 1 mg/L (Sibrell and Tucker 2012). The use of these pellets/AMDS shows the highest efficiency for wastewaters with a low P content.
Large-scale P removal from wastewaters (0.4–4.8 mg P/L) has been performed for 3 years using pelletized and granular hydroxide ferric oxide (Dobbie et al. 2009). The efficiency of the two different kinds of ochre (granular and pellets) did not show significant difference; thus, the use of granular ochre, which requires less preparation, is suggested. However, when two flow configurations were tested (vertical and horizontal inflow), P removal with vertical flow is less efficient (50 vs 80 % for horizontal flow) under optimal flow conditions (0.21 L/s) due to an early decrease of permeability. Other field tests support as well the promising future applications of ochre pellets to remove P from wastewaters as it was shown that the longevity of ochre was 3.1 years before it became saturated, which is 10 times greater than other adsorbent materials such as slag (107d) (Aubé and Zinck 1999; Dobbie et al. 2005). In addition, long-term P removal with iron oxides (ochre) indicated a removal capacity greater than other substrates (e.g., shale) (Heal et al. 2004). Likewise, the use of Fe/Al oxides/hydroxides seems to be advantageous since other P removal products (e.g., gel-based lime or lime) were found expensive and sometimes required in large quantities (Sibrell 2007). The P-saturated ochre in its turn can be reused as a slow-release fertilizer without causing adverse environmental threats (Heal et al. 2004; Dobbie et al. 2005, 2009). In addition, this spent ochre can still be recycled to remove As from mine wastewater or from soils since iron (III) phosphate (amorphous and crystalline) was found to be an interesting sorbent (Lenoble et al. 2005). Nonetheless, in order to be implemented in contaminated soil cleanup, further investigation is required in terms of desorption mechanisms, and interactions according to different type of soils.
Sludge was proved efficient in P sorption, but the performance varies according to its characteristics and the type of water treatment from which it derived (Table 1) (Sibrell et al. 2009). For example, pretreated water (with hydrogen peroxide and polymer) produced sludge with coarser grain size, granular texture ochres (iron oxide), and high saturated hydraulic conductivity that was more suitable for P removal (faster P kinetic sorption). On the contrary, unamended sludge had finer grains, lower saturated hydraulic conductivity and was more appropriate for dosing wastewater (Heal et al. 2004). Potential metal release from ochre deserves closer investigation as batch testing, with the use of heavy-metal rich ochre, showed a release of Fe, Zn, Pb, As, and Cu (Fenton et al. 2009).
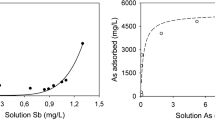
Arsenic (As) removal using AMDS
Arsenic is a highly toxic element, with the two forms commonly found in water being arsenic (III) and arsenic (V), as arsenite (AsO3 −) and arsenate (AsO4 3−), respectively (Mohan and Pittman 2007). Iron oxy-hydroxides were found to be stable compounds for As scavenging at low pH (Picquet 1995, Coussy et al. 2010). Therefore, adsorption of As on iron oxy-hydroxides (Dixit and Hering 2003; Banerjee et al. 2005) and on zero-valent iron (ZVI) (Bang et al. 2005) has been investigated. The two following reactions (Eqs. 5 & 6) were suggested by Banerjee et al. (2005):
The immobilization of As in water (and in soil) by using AMDS containing iron and iron oxides (PZC at pH = 7.6) was tested (Ko et al. 2013, 2015). Results showed that soluble arsenite and arsenate were removed from solution under various pH conditions obeying to the Langmuir isotherm. This removal was respectively 98.8 and 99.0 % for arsenite and arsenate at pH ≈ 7.0. Arsenate adsorption was reported faster (1 min) comparing to arsenite (20 min) at reaching equilibrium but the maximum adsorption capacity of AMDS was higher for arsenite than for arsenate (58.5 and 19.7 mg/g, respectively). The problem associated with As removal by Fe oxides/hydroxides is the disposal of Fe–As-rich waste. Storage under anaerobic conditions were used or suggested (Silva et al. 2007; Beauchemin et al. 2010). However, avoidance of more dangerous secondary waste products is necessary because in a reducing environment, dissolution of Fe oxides/hydroxides might occur, resulting in the release of As as reduced species, As (III), which is much more toxic and more mobile than the oxidized form As (V) (Schnoor 1996; Dobran and Zagury 2006).
Dye removal using AMDS
Dyes/colors are resistant to degradation (chemically stable) and to discoloration (Cornell and Schwertmann 2003). Dyes removal from wastewater by using AMDS with metal oxide/hydroxides has been evaluated because of the ineffectiveness of traditional wastewater treatment. Adsorption of dye was found better at pH 8–9 with a maximum adsorbent capacity of 48–62 mg dye per gram sludge (Netpradit et al. 2003; Zinck 2005). Dye adsorption capacity was over 90 % (389.1 mg dye/g sludge), at pH <8.5, when wet sludge (3.91 % solids) was used, but which declined at pH ≥8.5 (Wei and Viadero 2007). Therefore, by pH control, dye adsorption (pH<8.5) or desorption (pH >9) could be performed (Wei and Viadero 2007). Recovered iron oxides from AMDS, used as catalyst in the oxidation of dyes (through a Fenton-like mechanism) or adsorption from aqueous solution, showed comparable quality to those produced using analytical-grade reagents (Flores et al. 2012).
Heavy metals removal in AMD and CND using coal mine drainage sludge
Raw sludge or iron (oxy) hydroxides or ferrihydrite (from active/passive chemical treatment) have been reused to remove heavy metals and/or to neutralize acidity from AMD/CND (Zinck and Griffith 2006; Cui et al. 2012). The use of coal mine drainage sludge (CMDS) for Zn removal from AMD at a pilot scale showed an efficiency of 97.2–99.8 % (final concentration of Zn (II) <1 mg/L which was initially 44 mg/L) (Cui et al. 2012). The removal of Zn (II) was particularly efficient because the FeOH–SO4 2− in the sludge has an electrostatic attraction to Zn (II). Other pilot-scale test using pelletized hydrous ferric oxide recovered from a CMDS has been also undertaken in order to remove Zn from CND (Mayes et al. 2009). The efficiency of the treatment was 32 %, at 49-min residence time. The low performance was explained by the non-renewal of sludge during the testing, leading to grain armoring (Mayes et al. 2009). Replacing the sludge would improve the efficiency, but the disposal and fate of the final residue are still challenging. In addition, an increase of contaminants in the final effluent might occur. Thus, this method needs to be carefully monitored.
Future research encompasses the needs for the assessment of using alternative binders (e.g., Ca-silicate-based cement) and the longevity of hydroxide ferric oxide pellets (Mayes et al. 2009). Additional studies are also required on the performance parameters, such as efficiency and maximum adsorption capacity, to optimize the system design and estimate the treatment costs.
Rare earth removal in wastewater using CMDS
A novel multi-stage treatment to remove radioactive elements and heavy metals from rare earth elements (REE) wastewater has been undertaken. The process consists into using in its second unit synthetized polyurethane (PU) impregnated by CMDS (PUCMDS) for Th and U removal (Cui et al. 2016). Results showed that 95.8–99.4 % of U and Th were removed at different pH (3.5, 5, and 6.5) by ion exchange followed by complexation. Th and U reacted with HCO3 − originating from the dissolution of CaCO3 in CMDS (78.8 wt% goethite, 21.2 wt% calcite), giving Th(OH)CO3 −/Th(CO3)5 6− and UO2(CO3)2 2−/UO2(CO3)2 2− species before being complexed in their turn with ≡FeOH2 +–SO4 2− (Eqs. 7 and 8).
At pH <3.5, Th was removed at lower rate as hydroxide [Th (OH)4], whereas U was removed as carbonate (UO2CO3). However, at pH 8.6, U was found to be in a soluble phase [UO2(CO3)3 4−] and, hence, removed at a lower rate. The sequential process was very effective, but still generated sludge that needs to be handled. Moreover, the use of PUCMDS could be very costly as its operational cost alone was 50 times higher than the other units (Cui et al. 2016).
Stabilization of contaminated soil using mine sludge
Mine sludge is appropriate for sorbing contaminants and has been used as amendments to stabilize contaminated soil (Ko et al. 2013, 2015; Tsang et al. 2013; Lee et al. 2013; Tsang and Yip 2014; Moon et al. 2016). A quantity of mine sludge between 0.5–10 wt% was added to contaminated soils to immobilize As (Ko et al. 2013, 2015; Tsang et al. 2013; Tsang and Yip 2014). No significant difference in terms of contaminant concentrations in the soil pore water was found with addition of 5 and 10 wt%; thus, smaller amounts were suggested to be used to avoid potential detrimental impacts (Tsang and Yip 2014). Addition of 3 and 5 wt% had similar efficiency to reduce As leaching (Ko et al. 2013, 2015). Consequently, 3 wt% is the recommended optimal ratio (Ko et al. 2015). The problem related to this type of decontamination is the association of As with heavy metals (e.g., Cu, Pb). AMDS can immobilize As but not Cu, when both are present in the contaminated soil (Tsang et al. 2013). Similarly, CMDS could stabilize As but barely Cu, especially water-soluble Cu (Lee et al. 2013). Therefore, determining the metal speciation is essential, prior to strategy selection in simultaneous immobilization. Following these findings, an attempt to use a mixture of AMDS or CMDS with other wastes in order to simultaneously stabilize heavy metals and As was performed. A mixture of CMDS (3 wt%) and calcined oyster shell (5 wt%) was then tested. Results showed that despite the relative high solubility of divalent metals (e.g., Cu) under alkaline conditions, the immobilization of As, Pb, and Cu yielded over 90 % of efficiency (Lee et al. 2013). Another combination of CMDS with waste oyster shells (10 wt% CMDS–10 wt% waste oyster shells) also showed a good retention of As (>93 %), of Cu and Pb (>99 %) (Moon et al. 2016). Nevertheless, the calcined wastes mixed with mine sludge might raise Ca content, inducing a potential increase of hardness in the surrounding water in case of leaching. In addition, the increase of soil alkalinity and/or dissolved organic carbon could entail higher risk of As mobilization (Dobran and Zagury 2006; Lee et al. 2013). Leaching tests, such as TCLP and SPLP must therefore be performed to assess the stability of the different elements (Tsang and Yip 2014). However, the TCLP might be too aggressive to reproduce field conditions, while the SPLP would not reveal much, particularly on Pb and Cu leachability (Lee et al. 2013; Tsang and Yip 2014). In addition, these leaching tests might be incomplete since the alkali-metals (e.g., Pb, Cu) can be released at very high/low pH value depending on their concentration. Thus, complementary methods, such as continuous column flushing, plant uptake and accumulation, as well as sequential extraction were suggested (Tsang and Yip 2014).
Ochre has been also used as soil amendment for P sequestration (Fenton et al. 2012). Results showed that 30 g ochre/kg soil was required to decrease dissolved reactive P for an initial concentration less than 10 mg/L. However, it was found unsuccessful due to release of heavy metals from reduction of iron oxides in ochre/soil.
In summary, mine sludge combined with other wastes (organic or calcined) can be used as an amendment for contaminated soil, but the optimization of the ratio sludge/wastes to immobilize altogether As and heavy metals, and to prevent other undesirable environmental impacts, have yet to be improved. The use of sludge with organic wastes might be advantageous to stabilize contaminated acidic soils and avoid Al and Fe mobilization because of their toxicity for living organisms, if present in high concentrations (up to 45 wt% for Al and 35 wt% for Fe) (Pilon-Smits et al. 2009; Tsang et al. 2013; Jucoski et al. 2013). Nonetheless, the mixture with organic waste should be employed cautiously as the dissolution of organic matter can entail the lixiviation of negatively charged contaminants (often As) (Tsang et al. 2013).
Sludge reuse as fertilizer in agriculture and horticulture
Sludge with high alkalinity and low concentrations of trace metals can be used to raise soil pH and as fertilizer (Dobbie et al. 2005; Zinck and Griffith 2006). The CMDS has slight liming effect, with a gradual release of available N and P, making it a source of carbon and other essential elements for plant growth (Zinck and Griffith 2006). However, the concentration of heavy metals and/or other contaminants in the sludge may limit its reuse in agriculture (Elliott and Dempsey 1991). Thus, regulations on soil amendment have to be acknowledged prior to sludge utilization. Moreover, an assessment of oral bioavailability following incidental ingestion of amended soils to be used for horticultural purposes should be also performed (Zagury et al. 2016). If no approved procedures exist for the application in agriculture, guidelines for land application of biosolids (e.g., as fertilizers) could be used as a reference. These guidelines could help in apprehending the main concerns about using mine by-products in agriculture (e.g., microorganisms, potentially toxic contaminants for plants, animals, and human health).
Sludge reuse as substitute materials in construction
The inorganic components of sludge make them suitable for use in building materials, particularly in cement manufacturing (after drying) (Simonyi et al. 1977). Coal and gold-mine drainage sludge may contain high concentrations of Al and can be used to produce aluminous cement (Lubarski et al. 1996; Zinck and Griffith 2006). In concrete application (after pulverization and dewatering), sludge can replace up to 30 % of Portland cement in blended cement (Tay and Show 1991). In this case, sludge can be expected to lower the binder use. Addition of 5–20 % of sludge was found to improve compressive properties of concrete (Zinck and Griffith 2006).
The production of bricks was also investigated. The compressive strength and the quality of bricks depend on the proportion of the added sludge and the curing temperature. Bricks cured over long times at higher temperature were found to have a greater compressive strength (Simonyi et al. 1977). According to Weng et al. (2003), 10 % sludge (24 % moisture) and a firing temperature of 880–960 °C is enough to produce good-quality bricks (shrinkage <8 %). Bricks made of 15 % sludge (containing As and Fe) mixed with clay, cured at high temperature (1000 °C), had greater compressive strength compared to normal clay bricks, and presented less release of As (Rouf and Hossain 2003), but the use of less than 6 wt% was still recommended (Hassan et al. 2014). A quantity of more than 4 wt% As-sludge as an additive in bricks does not produce a good quality of ornamental bricks, and was not suitable in manufacturing mortar (Mahzuz et al. 2009). Hence, As-containing sludge is not recommended for brick making, but sludge containing both As and Fe, at a lower proportion, is more desirable. Nonetheless, further investigation is needed for the prudent use of As-sludge because of high toxicity of As.
Cemented paste backfill (CPB), consisting of a mixture of water, tailings material (70–85 % solids) and hydraulic binders, was used not only to stabilize waste materials but also to prevent AMD generation (Benzaazoua et al. 2004, 2006). Incorporation of sludge within CPB (at low %), alone or combined with hydraulic agents, such as ordinary Portland cement, fly ash, or blast-furnace slag, has been studied. Sludge rich in Fe (0.15 and 0.30 %) and 5–7 % of hydraulic agents (ordinary Portland cement and ordinary Portland cement/slag) was added to CPB and metal leachability was evaluated with SPLP tests (60/40 wt% sulfuric/nitric acid) (Benzaazoua et al. 2006). Results showed that sludge had no significant undesirable impact in terms of leachability of metals from the CPB, but ordinary Portland cement with a sludge rich in sulfate may constitute an issue, due to the CPB low resistance to sulfate attack, resulting in strength deterioration (Benzaazoua et al. 1999). Primary hydrated sulfates (e.g., gypsum), when resolubilized, are however, not damaging.
Sludge reuse in mine drainage prevention: cover over tailings
The use of wet or dry sludge as an oxygen barrier over tailings, to prevent AMD generation in mine restoration, was very recently evaluated (Zinck et al. 2010; Bouda et al. 2012; Zinck and Griffith 2013; Demers et al. 2015a, b). Used alone as a cover over tailings, sludge was not effective over a long-term period due to cracking and preferential channeling that favored oxidation (Zinck et al. 2010). The use of HDS treatment sludge also entails minimal metal mobility; however, in the long term, significant leaching of Fe, Zn, and sulfate can occur (Zinck et al. 2010). Addition of water or vegetative cover, over the sludge, might be an optimal way to enhance the efficiency of the technique. Sludge can be maintained saturated by limiting drainage of its pore water, and improve the distribution of alkalinity. Thereby, the buffering capacity of the maintained sludge can reduce 10 % of metal mobility (Zinck and Griffith 2013). This is technically difficult in arid and semi-arid areas where water might not be always available.
Laboratory and intermediate field-scale tests using sludge mixed with other materials at 10–25 % (sludge-tailings mixture or sludge-waste rock mixture) was also used to prevent AMD generation (Demers et al. 2015a, b). Results showed that due to the neutralizing capacity depletion, the mixture can be considered only as temporary option for oxygen barrier. Even though the mixtures did not limit oxygen transport, the metal loading in the effluent can decrease. Tailings mixed with 10 wt% of sludge and 2 wt% of cement could be a promising material to reduce sulfide oxidation (Demers et al. 2015b). Nonetheless, geotechnical and geochemical investigations, as well as long-term field cell validation are still needed (Bouda et al. 2012; Demers et al. 2015b).
A preliminary geotechnical evaluation of AMDS (10–25 %)-soil mixtures, used as a cover over waste rocks to prevent AMD generation, was also performed (Bouda et al. 2012; Mbonimpa et al. 2015). Addition of up to 25 % sludge was found to have comparable properties to other materials used as covers with capillary barrier effects (hydraulic conductivity 10−5 cm/s; air entry value 30 kPa). However, since higher amount of sludge than tested could be required, assessment on the effect addition of higher percentage of sludge in the mixture should be undertaken. Further studies are recommended, such as tests on mixtures composed of different type of soils, chemical stability, and potentially long-term environmental harms (Mbonimpa et al. 2015).
Sludge reuse as material for carbon dioxide sequestration
Atmospheric concentrations of carbon dioxide have been rising, from approximately 280 ± 20 parts per million (ppm) in the late eighteenth century to a current atmospheric average of approximately 390 ppm (Indermühle et al. 1999; Blunden and Arndt 2013). The Netherlands environmental assessment agency (NEAA 2013) reported that global CO2 emission increased over 1.4 % with a total amount of 34.5 billion tons in 2012. The main anthropogenic activities that contribute to the rise of atmospheric concentration of CO2 are fossil fuel combustion and deforestation (Melillo et al. 1996; IEA 2013). Geological, oceanic, and mineral sequestration of CO2 is under development (Voormeij and Simandl 2004). The CO2 sequestration can be carried out also by the incorporation of iron hydroxide sludge into acidic lakes. This is considered as a form of mineral carbonation because the iron hydroxides react with CO2 in order to produce solid carbonates (Eq. 9) (Merkel et al. 2005; Unger-Lindig et al. 2010).
The mechanism that drives the carbonation depends principally on the dissolution of CO2 in water and it has been successfully modeled according to Henry’s law of equilibrium between gas and liquid (at pressure <20 bars). In the study performed by Unger-Lindig et al. (2010), the carbonate anion CO3 2− reacted with hydroxide to give solid carbonate (Eq.10).
The results of batch testing showed that addition of low-density sludge to acidic water (from mining lake) increased the pH of the water and CO2 could be captured, mainly in the form of metal-bicarbonate complexes at a low rate. This was associated to the availability of oxy-hydroxides contained in the sludge. Advantageously, the main cations in the low-density sludge were Fe3+ (≈31 mass %), which promotes the formation of carbonates such as siderite (FeCO3), and Ca2+ (aproximately 5 mass %). At room temperature, the predominant species at pH 5–6 are CO2(aq)/H2CO3 and HCO3 −, and the buffering of acidic water would rather be stimulated but not the formation of carbonates due to the lack of CO3 2−. The temperature effect was not discussed, while CO2 dissolution is strongly dependent on it. The advantage of sludge incorporation in an acidic pit lake, for CO2 sequestration, is its capacity to be employed as a neutralizer because it still contains unreacted hydrated lime (or calcite if dried), reducing acidity of the lake by up to 30 % and stabilize the sludge itself (Kalin et al. 2006; Unger-Lindig et al. 2010; Schultze et al. 2013; Zinck and Griffith 2013). However, the possibility of contaminant release into the surrounding environment is still a risk, depending on the element concentrations and mineral solubility. This is also strongly influenced by the sludge NP to insure acid lake buffering and alkalinity rise. The limitations that might occur during CO2 sequestration by mine sludge are the variation of pressure, temperature, and availability of oxides/hydroxides in the sludge (Merkel et al. 2005).
Other sludge reuse approaches
Additional sludge reuse approaches are also documented, including a rock dust substitute for explosion control (Simonyi et al. 1977) and the production of a lightweight high-strength aggregate (Tay and Show 1997).
Ochre recovered from active treatment sludge has poor potential to be used as pigment, unless being pretreated (e.g., drying, calcination) (Hedin 2012). However, either derived from active or passive treatment sludge, ochre is generally used as yellow (goethite), orange-brown (ferrihydrite), and red (hematite) inorganic color pigments (Kirby et al. 1999; Cornell and Schwertmann 2003; Wei and Viadero 2007; Hedin 2003, 2012). The advantages of using ochre as pigments are its extreme stability, in addition to its non-bleeding, non-fading, high resistance to acids and alkalis properties, and hence, the possibilities to be exposed to outdoor conditions (Cornell and Schwertmann 2003). Mixed with oxides, sulfides, hydroxides, silicates, sulfates, or carbonates, ochre can be used in paints, resins, polymers, and for ceramic tile coloration. These mixtures are used to cover tiles (added to glazes) or to color the entire body (porcelain tiles) (Bondioli et al. 1998; Bernardin et al. 2006). In the ceramic industry, ochre offers the possibility to immobilize harmful metals (Marcello et al. 2008). High-quality ochre is desirable, but the use of heterogeneous ochre in the cultivation of biofuel crops was also suggested (Bailey et al. 2013).
Other reuse of sludge includes catalysts, abrasives, and polishing agents (Cornell and Schwertmann 2003). Potential industrial applications in electroplating, metal processing, steel hardening, photography, and synthetic rubber production were also identified, with no further details being provided (Zinck and Griffith 2006).
Conclusion
More stringent water quality regulations have resulted in an ongoing challenge in mine water treatment. The produced sludge, which usually has no commercial value, must be disposed in long-term in on-site/off-site areas with a management at high cost. Every potentially beneficial option for lowering sludge management costs should be reconsidered closely, including product recovery and reuse, or a densification of sludge to reduce storage space. Sludge from active/passive chemical treatment of coal mine drainage is the most exploited as a source of metals and iron oxides (ochre). Metal recovery from sludge is possible with various techniques but could be costly. Raw or treated sludge and ochre are reused in wastewater treatment, in soil remediation, agriculture and horticulture, civil engineering, mine drainage prevention, and CO2 sequestration. In wastewater treatment, sludge reuse mainly consists in removal of P, As, dye, and heavy metals. The P-laden ochre can be reused again as a fertilizer. The coal mine sludge was reused to treat highly contaminated AMD from polymetallic mines which may offer the possibility of recycling valuable metals, decreasing mine water treatment costs, and preventing environmental issues. Reusing sludge in the mine water treatment might be a promising method because of its capacity to immobilize toxic elements, but requires caution due to potential release of other contaminants. Prior to using secondary products from sludge in agriculture and horticulture, regulations pertaining to agricultural amendment must be respected as the content of heavy metals might limit its use. Attempts to reuse sludge as an oxygen barrier over tailings were found inefficient or partially efficient, but suggested to be combined with a water cover or vegetative cover. The sequestration of CO2 has also been achieved by using iron oxy-hydroxides from sludge as a cation source to produce metal carbonates. However, a better control of each reuse technique is needed, according to the field of use. The reuse of sludge from passive biochemical treatment might be difficult as precipitates are confined in the residual compost. Insufficient knowledge on practices and valuable products limits new uses of mine sludge and, hence, more information on classical and innovative management methods is still required. Finally, only the recovery and reuse of sludge from AMD and CND treatment was reviewed, but not other types of mine sludge that may contain various contaminants, such as red mud or the sludge from the treatment of cyanide contaminated effluents.
Challenges and research needs
Important recovery and reuse options of mine sludge in different fields were identified and thoroughly discussed. Further research work is still needed and additional suggestions that could minimize sludge management costs are proposed, some of which are listed below:
-
(a)
Further monitoring of sludge in water treatment plant, including record of the produced quantities, for a better management;
-
(b)
Detailed characterization of sludge depending on the targeted fate (disposal, recovery, or reuse);
-
(c)
Additional leaching tests to assess chemical stability over a broad range of pH, such as the use of serial acid and base extractions, in order to estimate the different metals leached. Alkaline leaching using slaked lime (pH 12) or a multi-stage leaching test with NaOH is proposed as a complementary test to the conventional acidic leaching tests and to evaluate the stability of specific metals (Pb, Zn, Cd, and Cu). The “alkali-leaching tests” are recommended, especially when sludge is to be disposed with other alkaline wastes (e.g., fly ash) or during the spread of alkaline sludge on top of residues;
-
(d)
Optimal exploitation of existing mining sites for sludge disposal. According to the environmental, technical/technological and regulatory framework, as well as economic advantages, disposing sludge in underground mines or as backfill is the preferred storage approach, followed by disposal in ponds, tailings ponds, landfills, as tailings cover, and finally combined with residues. Disposing sludge as a backfill is undeniably beneficial because it is also considered as a valorization method and a stabilization technique. Since no precise recipe exists for the manufacture of paste backfill, the incorporation of sludge at a higher rate can be explored as sludge had been suggested to replace ordinary Portland cement up to 30 %; this must obviously take into account the nature of the sludge. Sludge as a cover over tailings is recommended if maintained in a reduced condition (e.g., permeable reactive barrier), where oxidation might be limited and the alkalinity would remain high. The potential use of sludge from active treatment (water content up to 90 %) as a cover over tailings is suggested rather than HDS that could be easily prone to desiccation. In turn, HDS could be tested in multilayer covers where the material is protected from evaporation;
-
(e)
Additional research work in the refining of by-products (particularly for ochre recovery) quality by improving the collection and treatment on-site to minimize contamination. This would also increase not only the quantity and the quality of the collected products but would also expand the field of use, as well as provide economic benefits. Additional studies need also to be conducted on the recovery of metals;
-
(f)
Assessment of the mechanisms of As, P, and dye removal by iron oxides from mine sludge should also be performed for a better understanding of the ratio of adsorption/desorption;
-
(g)
Sludge reuse in soil remediation needs further investigation, particularly with respect to physicochemical and biological mechanisms, interaction between sludge or P-fertilizer with different types of soils, plant uptake, and phytotoxicity. Evaluation in terms of stability, long-term and large-scale field efficiency is also recommended;
-
(h)
Sludge from passive chemical treatment could be used in non-food crops, such as an amendment of grasses, ornamental trees, and plants for landscape enhancement or as an additive for potting soils. The fate of residues from passive biochemical treatment is still a challenge; however, disposing the residues on-site could be an option since they have been shown to be stable even under aerobic conditions;
-
(i)
An optimistic annual CO2 sequestration rate with residues and sludge (not including steel mill sludge) has been estimated to be around 60,000 tons, but further evaluation is needed, particularly the sequestration of CO2 by mine sludge. Due to the complexity of CO2 and the carbon cycle (in an open system) in nature, mineral carbonation should be at least studied by involving temperature, CO2 pressure, and pH.
-
(j)
Cost estimation related to the reuse of sludge (conditioned or not) in different fields should be further addressed by additional studies to evaluate its economic advantage, particularly the contribution in reducing the storage and management costs.
References
Adler P, Sibrell P (2003) Sequestration of phosphorus by acid mine drainage floc. J Environ Qual 32:1122–1129
Aubé B (2004) Sludge disposal in mine workings at Cape Breton Development Corporation. In: Proc. of the Ontario Mine Environment Neutral Drainage (MEND) Workshop, May 26–27. MEND report W.017, Sudbury, ON, Canada, CD-ROM
Aubé BC, Zinck JM (1999) Comparison of AMD treatment processes and their impact on sludge characteristics. In: Goldsack D, Belzile N, Yearwood P, Hall G (eds) Proc. of the Sudbury ‘99 Mining and the Environment II, September 7–13, Sudbury, ON, Canada, pp 261–270
Aubé B, Zinck JM (2003) Lime treatment of acid mine drainage in Canada. In: Barbosa JP, Soares PSM, Dixon B, Tisch B (eds) Proc. Brazil-Canada Seminar on Mine Rehabilitation, December 1–3, Florianópolis, Santa Catarina, Brazil, pp 89–105
Bailey MT, Moorhouse AM, Byrom AJ, Kershaw S (2013) Applications of hydrous ferric oxide mine water treatment sludge—a review. In: Brown A, Figueroa L, Wolkerdorfer C (eds) Proc. of the International Mine Water Association (IMWA) Conference, August 5–9 pp 519–524
Banerjee K, Gary LM, Prevost M, Shokoufeh N, Jekel M, Gallagher PM, Blumenschein CD (2005) Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxid (GFH. Water Res 42:3371–3378
Bang S, Korfiatis GP, Meng X (2005) Removal of arsenic from water by zero-valent iron. J Hazard Mater 121:61–67
Bastin O, Janssens F, Dufey J, Peeters A (1999) Phosphorus removal by a synthetic iron oxide-gypsum compound. Ecol Eng 12:359–351
Beauchemin S, Fiset J-F, Poirier G, Ablett J (2010) Arsenic in an alkaline AMD treatment sludge: characterization and stability under prolonged anoxic conditions. Appl Geochem 25:1487–1499
Bejan D, Bunce NJ (2015) Acid mine drainage: electrochemical approaches to prevention and remediation of acidity and toxic metals. J Appl Electrochem 45:1239–1254
Benzaazoua M, Ouellet J, Servant S, Newman P, Verburg R (1999) Cementitious backfill with high sulfur content: physical, chemical, and mineralogical characterization. Cement Concrete Res 29:719–725
Benzaazoua M, Marion P, Picquet I, Bussière B (2004) The use of pastefill as a solidification and stabilization process for the control of acid mine drainage. Miner Eng 17:233–243
Benzaazoua M, Fiset J-F, Bussière B, Villeneuve M, Plante B (2006) Sludge recycling within cemented backfill: study of the mechanical and leachability properties. Miner Eng 19:420–432
Bernardin AM, Marcello RR, Peterson M, Galato S, Izidoro G, Saulo V, Riella HG (2006) Inorganic pigments obtained from coal mine drainage residues. In: Proc. of the 8th World Congress on Ceramic Tile Quality, February 12–15. Official Chamber of Commerce, Industry and Navigation, Castellón, Spain, 3, p 169–174
Blunden J, Arndt DS (2013) State of the climate in 2012. Bull Amer Meteor Soc 94:S1–S258
Bondioli F, Ferrari AM, Leonelli C, Manfredini T (1998) Syntheses of Fe2O3/silica red inorganic inclusion pigments for ceramic applications. Mater Res Bull 35:723–729
Bouda M, Mbonimpa M, Demers I, Benzaazoua M, Gagnon M (2012) Hydro-geotechnical characterization of AMD treatment sludges and sludge-based mixtures, in: Proc. of the GeoManitoba’12, September 30–October 3, Winnipeg, MB, Canada, 8p
Bratty M, Lawrence R, Kratochvil D, Marchant B (2006) Applications of biological H2S production from elemental sulfur in the treatment of heavy metal pollution including acid rock drainage. In: Barnhisel RI (ed) Proc. of the 7th international conference on acid rock drainage (ICARD), march 26–30. American Society of Mining and Reclamation (ASMR), St. Louis 11p
Canada Centre for Mineral and Energy Technology (CANMET) (1996) Investigation on the placement of lime neutralization sludge on acid generating waste rock. NB Coal Lim. MEND- CANMET Contract 2344G-1196, Minto, NB, Canada, 135p
Caraballo MA, Macías F, Castillo J, Quispe D, Nieto JM, Ayora C (2011) Hydrochemical performance and mineralogical evolution of a dispersed alkaline substrate (DAS) remediating the highly polluted acid mine drainage in the full scale passive treatment of Mina Esperanza (SW, Spain). Am Miner 96:1270–1277
Chitrakar R, Tezuka S, Sonoda A, Sakane K, Ooi K, Hirotsu T (2006) Phosphate adsorption on synthetic goethite and akaganeite. J Colloid Interf Sci 298:602–608
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences, and uses, second edn. Wiley-VCH GmbH&Co. KGaA, Weinheim 694p
Coussy S, Benzaazoua M, Bussière B, Peyronnard O, Blanc D, Moszkowicz P, Malchère A (2010) Stabilization/solidification of arsenic in cemented paste backfill: geochemical modeling as a mineralogical characterization tool. In: Proc. of the 1st International Stabilization/Solidification Technology Forum, June 15–17, Sydney, NS, Canada, p 161–170
Cui M, Jang M, Cho SH, Khim J, Cannon FS (2012) A continuous pilot-scale system using coal-mine drainage sludge to treat acid mine drainage contaminated with high concentrations of Pb, Zn, and other heavy metals. J Hazard Mater 215–216:122–128
Cui M, Jang M, Cannon FS, Na S, Khim J, Park JK (2013) Removal of dissolved Zn (II) using coal mine drainage sludge: implications for acidic wastewater treatment. J Environ Manag 116:101–112
Cui M, Jang M, Kang K, Kim D, Snyder SA, Khim J (2016) A novel sequential process for remediating rare-earth wastewater. Chemosphere 144:2081–2090
De Beer M, Maree JP, Liebenberg L, Doucet FJ (2014) Conversion of calcium sulphide to calcium carbonate during the process of recovery of elemental sulphur from gypsum waste. Waste Manag 11:2373–2381
Demers I, Bouda M, Mbonimpa M, Benzaazoua M, Bois D, Gagnon M (2015a) Valorisation of acid mine drainage treatment sludge as remediation component to control acid generation from mine wastes, part 1: material characterization and laboratory kinetic testing. Miner Eng 76:109–116
Demers I, Bouda M, Mbonimpa M, Benzaazoua M, Bois D, Gagnon M (2015b) Valorisation of acid mine drainage treatment sludge as remediation component to control acid generation from mine wastes, part 2: field experimentation. Miner Eng 76:117–125
Dempsey BA, Jeon B-H (2001) Characteristics of sludge produced from passive treatment of mine drainage. Geochem Explor Environ Anal 1:89–94
Dixit S, Hering JG (2003) Comparison of arsenic (v) and arsenic (iii) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Eng 37:4182–4189
Dobbie KE, Heal KV, Smith KA (2005) Assessing the performance of phosphorus-saturated ochre as a fertiliser and its environmental acceptability. Soil Use Manage 21:231–239
Dobbie KE, Heal KV, Aumônier J, Smith KA, Johnston A, Younger PL (2009) Evaluation of iron ochre from mine drainage treatment for removal of phosphorus from wastewater. Chemosphere 75:795–800
Dobran S, Zagury GJ (2006) Arsenic speciation and mobilization in CCA-contaminated soils: influence of organic matter content. Sci Total Environ 364:239–225
Elamari K, Benzaazoua M, Bussière B, Archambault M (2005) Copper recovery from sludges of the Laronde mine, Canada. In: Proc. of the Post-Mining Conference, November 16–17, Nancy, France, 15p
El-Ammouri E, Disten PA, Rao SR, Finch JA, Ngoviky K (2000) Treatment of acid mine drainage sludge by leaching and metal recovery using activated silica. In: Proc. of the 5th ICARD, May 21–24, Denver, CO, USA, 2, 8p
Elliott HA, Dempsey BA (1991) Agronomic effects of land application of water treatment sludge. J Am Water Works Ass 83:126–131
Fenton O, Healy M, Rodgers M (2009) Use of ochre from an abandoned metal mine in the south east of Ireland for phosphorus sequestration from dairy dirty water. J Environ Qual 38:1120–1125
Fenton O, Kirwan L, Ó hUallacháin D, Healy MG (2012) The effectiveness of using ochre as a soil amendment to sequester dissolved reactive phosphorous in runoff. Water Air Sol Pollut 223(3):1249–1261
Fiset JF, Zinck JM, Nkinamubanzi PC (2003) Chemical stabilization of metal hydroxide sludge. In: Proc. of the 10th International Conference on Tailings and Mine Waste, October 12–15, Vail, CO, USA, p 329–352
Fish CL, Hedin RS, Partezana J (1996) Chemical characterization of iron oxide precipitates from wetlands constructed to treat polluted mine drainage. In: Burger JA, Zipper CE (eds) Proc. of the 13th ASMR, Princeton, May 18–23, Daniels, WV, USA, p 541–549
Flores RG, Floriani Andersen SL, Komay Maia LK, José HJ, Muniz Moreira RFP (2012) Recovery of iron oxides from acid mine drainage and their application as adsorbent or catalyst. J Environ Manag 111:53–60
Gazea B, Adam K, Kontopoulos A (1995) A review of passive systems for the treatment of acid mine drainage. Miner Eng 9:23–42
Geller W, Schultze M, Kleinmann R, Wolkersdorfer C (2013) Acidic pit lakes—the legacy of coal and metal surface mines. Environ Sci Eng 525p
Genty T (2012) Comportement hydro-bio-géochimique de systèmes passifs de traitement du drainage minier acide fortement contaminé en fer. PhD Dissertation (in French), Applied Sciences, UQAT, Rouyn-Noranda, QC, Canada 271p
Genty T, Bussière B, Zagury GJ, Benzaazoua M (2010) Passive treatment of high-iron acid mine drainage using sulphate reducing bacteria: comparison between eight biofilter mixtures. In: Proc. of the 10th IMWA, April 21–24, Sydney, NS, Canada, p 229–232
Genty T, Neculita CM, Bussière B, Zagury GJ (2012) Environmental behaviour of sulphate-reducing passive bioreactor mixture. In: Proc. of the 9th International ICARD, May 21–25, Ottawa, Canada, 11p
Genz A, Kornmüller A, Jekel M (2004) Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminum oxide and granulated ferric hydroxide. Water Res 38:3523–3530
Hassan KM, Fukushi K, Turikuzzaman K, Moniruzzaman SM (2014) Effects of using arsenic-iron sludge wastes in brick making. Waste Manag 34:1072–1078
Heal KV, Smith KA, Younger PL, McHaffie H, Batty LC (2004) Removing phosphorus from sewage effluent and agricultural runoff using recovered ochre. In: Valsami-Jones E (ed) Phosphorus in environmental technologies: principles and applications, chapter 14. International Water Association (IWA), London, pp. 321–334
Hedin RS (2003) Recovery of marketable iron oxide from mine drainage in the USA. Land Contam Reclamat 11:93–97
Hedin RS (2006) Sustainable mine drainage treatment through the passive production of saleable iron oxide solids. In: Proc. of the 7th ICARD, March 26–30, St Louis, MO, USA, 10p
Hedin RS (2008) Iron removal by a passive system treating alkaline coal mine drainage. Mine Water Environ 27:200–209
Hedin RS (2012) Advances in the production of marketable products from mine water treatment systems. In: Proc. of the 9th ICARD, May 20–26, Ottawa, ON, Canada, 8p
Hedin RS, Watzlaf GR, Nairn RW (1994) Passive treatment of acid mine drainage with limestone. J Environ Qual 23:1358–1345
Hedin R, Weaver T, Wolfe N, Watzlaf G (2013) Effective passive treatment of coal mine drainage. In: Proc. of the 35th Annual National Association of Abandoned Mine Land Programs Conference, September 22–25, Daniels, WV, USA, 13p
Herrera P, Uchiyama H, Igarashi T, Asakura K, Ochi Y, Iyatomi N, Nagae S (2007) Treatment of acid mine drainage through a ferrite formation process in Central Hokkaido, Japan: evaluation of dissolved silica and aluminum interference in ferrite formation. Miner Eng 20:1255–1260
Huisman JL, Weghuis MO (2011) Biotechnology-based processes for arsenic removal. In: Proc. of the 9th International Conference on Clean Technologies for the Mining Industry, April 10–12, Santiago, Chile, 11p
Indermühle A, Stocker TF, Joos F, Fischer H, Smith HJ, Wahlen M, Deck B, Mastroianni D, Tschumi J, Blunier T, Meyer R, Stauffer B (1999) Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor dome Antarctica. Nature 398:121–126
International Energy Agency (IEA) (2013) CO2 emissions from fuel combustion: highlights, 2013 edn, Paris, France, 158p
Jandova J, Maixner J, Grygar T (2002) Reprocessing of zinc galvanic waste sludge by selective precipitation. Ceram Silik 46:52–55
Janneck E, Arnold I, Koch T, Meyer J, Burghard D, Ehinger S (2010) Microbial synthesis of schwertmannite from lignite mine water and its utilization for removal of arsenic from mine waters and for production of iron pigments. In: Wolkersdorfer C, Freund A (eds) Proc. of the 10th IMWA Symposium, September 5–9, Sydney, NS, Canada, p 131–134
Johnson DB (2013) Development and application of biotechnologies in the metal industry. Environ Sci Pollut Res 20:7768–7776
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 35:3–14
Jucoski GO, Cambraia J, Ribeiro C, Oliveira JA, De Paula SO, Oliva MA (2013) Impact of iron toxicity on oxidative metabolism in young Eugenia uniflora L. Plants. Acta Physiol Plant 35:1645–1657
Kalin M, Fyson A, Wheeler WN (2006) The chemistry of conventional and alternative systems for the neutralization of acid mine drainage. Sci Total Environ 366:395–408
Kang S, Choo K, Lim K (2003) Use of iron oxide particles as adsorbents to enhance phosphorus removal from secondary wastewater effluent. Separ Sci Technol 38:3853–3874
Keefer GB, Sack WA (1983) Sludge recycle and reuse in acid mine drainage treatment. Water Environ Feder 55:278–284
Kirby CS, Thomas HM, Southam G, Donald R (1999) Relative contributions of abiotic and biological factors in Fe (II) oxidation in mine drainage. Appl Geochem 14:511–530
Ko M-S, Kim JY, Lee J-S, Ko J-I, Kim K-W (2013) Arsenic immobilization in water and soil using acid mine drainage sludge. Appl Geochem 35:1–6
Ko M-S, Kim J-Y, Park H-S, Kim K-W (2015) Field assessment of arsenic immobilization in soil amended with iron rich acid mine drainage sludge. J Clean Prod 108:1073–1080
Koide R, Tokoro C, Murakami S, Adachi T, Takahashi A (2012) A model for prediction of neutralizer usage and sludge generation in the treatment of acid mine drainage from abandoned mines: case studies in Japan. Mine Water Environ 31:287–296
Lambert A, Drogui P, Daghrir R, Zaviska F, Benzaazoua M (2014) Removal of copper in leachate from mining residues using electrochemical technology. J Environ Manag 133:78–85
Lee KY, Moon DH, Lee SH, Kim KW, Cheong KH, Park JH, Ok YS, Chang YY (2013) Simultaneous stabilization of arsenic, lead, and copper in contaminated soil using mixed waste resources. Environ. Earth Sci 69:1813–1820
Lenoble V, Laclautre C, Delucaht V, Serpaud B, Bollinger J-C (2005) Arsenic removal by adsorption on iron (III) phosphate. J Hazard Mater B123:262–268
Logan BE (2008) Microbial fuel cells. John Wiley & Sons, Inc., Hoboken 216p
Logan BE (2010) Scaling up microbial fuel cells and other bioelectrochemical. Appl Microbiol Biotechnol 85:1665–1671
Lopez O, Sanguinetti D, Bratty M, Kratochvil D (2009) Green technologies for sulphate and metal removal in mining and metallurgical effluents. In: Wiertz J, Moran C (eds) Proc. of the 1st International Seminar on Environmental Issues in the Mining Industry (Enviromine), September 30–October 2, Santiago, Chile. 9p
Lubarski V, Levlin E, Koroleva E (1996) Endurance test of aluminous cement produced from water treatment sludge. Vatten 52:39–42
Luo H, Liu G, Zhang R, Bai Y, Fu S, Hou Y (2014) Heavy metal recovery combined with H2 production from artificial acid mine drainage using the microbial electrolysis cell. J Hazard Mater 270:153–159
Macías F, Caraballo MA, Rötting TS, Péréz- Lopez R, Nieto JM, Ayora C (2012a) From highly polluted Zn-rich acid mine drainage to nonmetallic waters: implementation of multi-step alkaline treatment system to remediate metal pollution. Sci Total Environ 435:323–350
Macías F, Caraballo MA, Nieto JM (2012b) Environmental assessment and management of metal-rich wastes generated in acid mine drainage passive remediation systems. J Hazard Mater 229-330:107–114
Mahzuz HMA, Alam R, Alam MN, Basak R, Islam MS (2009) Use of arsenic contaminated sludge in making ornamental bricks. Int J Environ Sci Tech 6:291–298
Marcello RR, Galato S, Peterson M, Riellac HG, Bernardin AM (2008) Inorganic pigments made from the recycling of coal mine drainage treatment sludge. J Environ Manag 88:1280–1284
Mayes WM, Potter HAB, Jarvis AP (2009) Novel approach to zinc removal from circum-neutral mine waters using pelletised recovered hydrous ferric oxide. J Hazard Mater 162:512–520
Mbonimpa M, Bouda M, Demers I, Benzaazoua M, Bois D, Gagnon M (2015) Preliminary geotechnical assessment of the potential use of mixtures of soil and acid mine drainage neutralization sludge as materials for the moisture retention layer of covers with capillary barrier effects. Can Geotech J 53(5):828–838
McDonald DM, Webb JA (2006) Chemical stability of acid rock drainage treatment sludge and implications for sludge management. Environ. Sci. Technol. 40(6),1984–1990
McDonald DM, Webb JA, Musgrave RJ (2006) The effect of neutralisation method and reagent on the rate of Cu and Zn release from acid rock drainage treatment sludge. In: Barnhisel RI (ed) Proc. of the 7th ICARD, March 26–30, St. Louis, MO, USA, p 1198–1218
Melillo JM, Houghton RA, Kicklighter DW, McGuire AD (1996) Tropical deforestation and the global carbon budget. Annu Rev Energ Env 21:293–310
Merkel BJ, Werner F, Wolkersdorfer C (2005) Carbon dioxide elimination by using acid mine lakes and calcium oxide suspensions (CDEAL). In: Geotechnologien Science Report, 6, p 4–12
Ministère du Développement durable, de l’environnement et lutte contre les changements climatiques (MDDELCC) (2013) Critère de qualité de l’eau de surface. Direction de suivi de l’état de l’environnement. Bibliothèque et archives nationales du Québec, QC, Canada. 510p
Mohan D, Pittman CU Jr (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53
Moon DH, Cheong KH, Koutsospyros A, Chang Y-Y, Hyun S, Ok YS, Park J-H (2016) Assessment of waste oyster shells and coal mine drainage sludge for the stabilization of As-, Pb-, and Cu-contaminated soil. Environ Sci Pollut Res 23:2362–2370
Ňancucheo I, Hedrich S, Johnson DB (2012) New microbiological strategies that enable the selective recovery and recycling of metals from acid mine drainage and mine process waters. Miner Mag 76(7):2683–2692
Neculita CM, Zagury GJ, Bussière B (2007) Passive treatment of AMD in the bioreactors using sulfate-reducing bacteria—critical review and research needs. J Environ Qual 36(1):1–16
Neculita CM, Zagury GJ, Bussière B (2008) Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: II. Metal removal mechanisms and potential mobility. Appl. Geochem. 23:3545–3560.
Netherlands Environmental Assessment Agency (NEAA) (2013) Trends in global CO2 emissions: 2013 Report. Planbureau voor de Leefomgeving (PBL), Inc., JRC Technical Note number: JRC83593. The Hague, Holland, 64p
Netpradit S, Thiravetyan P, Towprayoon S (2003) Application of ‘waste’ metal hydroxides for adsorption of azo reactive dyes. Water Res 37:763–772
Nodwell M, Kratochvil D (2012) Sulphide precipitation and ion exchange technologies to treat acid mine drainage. In: Proc. of the 9th ICARD, May 20–21, Ottawa, ON, Canada
Nordstrom DK, Alpers CN, Ptacek CJ, Blowes DW (2000) Negative pH and extremely acidic mine waters from Iron Mountain, California. Environ Sci Technol 34:254–258
Nordstrom K, Blowes DW, Ptacek CJ (2015) Hydrogeochemistry and microbiology of mine drainage: an update. Appl Geochem 57:3–16
Office of Water Services (OFWAT) (2005) Water framework directive economic analysis of water industry costs. Final Report, OFWAT/WFD/003A.82p
Pedroni L, Dromer JB, Aubertin M, Kennedy G (2006) Properties of treatment sludge during sedimentation and consolidation tests. In: Proc. of the 7th ICARD, March 26–30, St. Louis, MO, USA, p 1531–1544
Pérez-López R, Macías F, Caraballo MA, Nieto JM, Román-Ross G, Tucoulou R, Ayora C (2011) Mineralogy and geochemistry of Zn-rich mine-drainage precipitates from an MgO passive treatment system by synchrotron-based x-ray analysis. Environ Sci Technol 45:7826–7833
Picquet I (1995) Techniques de stabilisation physico-chimique à base de liant hydraulique appliquées aux résidus miniers sulfurés et arséniés. PhD Dissertation (in French), Institut National Polytechnique de Lorraine, Nancy, France, 289p
Pilon-Smits EAH, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12:267–274
Robertson AMG, Shaw SC (1997) Options for the stabilization of sludge from acid mine drainage water treatment plants. In: Proc. of the Wismut 97 Workshop on Water treatment and residue management-Conventional and Innovative solutions, September 24–26, Wismut, Chemnitz, Germany, 11p
Rose P (2013) Long-term sustainability in the management of acid mine drainage wastewaters—development of the Rhodes BioSURE process. Water SA 39:583–592
Rouf A, Hossain D (2003) Effects of using arsenic-iron sludge in brick making. In: Proceed. of the Bangladesh University of Engineering and Technology and The United Nations University (BUET-UNU) International Symposium on Fate of Arsenic in the Environment, February 5–6, Dhaka, Bangladesh, p 193–208
Rukuni TT, Maree JP, Zvinowanda CM (2012) Separation of magnesium hydroxide and barium sulphate from a barium sulphate—magnesium hydroxide mixed sludge by carbonation: the effect of temperature. J Civil Environ Eng 2(4) 5p
Sanin FD, Clarkson WW, Vesilind PA (2011) Sludge engineering: the treatment and disposal of wastewater sludges, first edn. Destech, Inc., Lancester 389p
Sapsford D, Santonastaso M, Thorn P, Kershaw S (2015) Conversion of coal mine drainage ochre to water treatment reagent: production, characterisation and application for P and Zn removal. J Environ Manag 160:7–15
Schnoor, JL (1996) Environmental modeling: fate and transport of pollutants in water, air, and soil. In: Schnoor JL, Zehnder A (eds). John Wiley & Sons, Inc., 682p
Shen SB, Tyagi RD, Blais JF, Surampalli RY (2003) Bacterial leaching of metals from tannery sludge by indigenous sulphur-oxidizing bacteria-effect of sludge solids concentration. J Environ Eng 129:513–519
Shepherd JG, Sohi SP, Heal KV (2016) Optimizing the recovery and re-use of phosphorous from wastewater effluent for sustainable fertilizer development. Water Res 94:155–165
Sibrell PL, Tucker TW (2012) Fixed bed sorption of phosphorus from wastewater using iron oxide-based media derived from acid mine drainage. Water Air Soil Pollut 223:5105–5117
Sibrell PL, Montgomery GA, Ritenour KL, Tuckeer TW (2009) Removal of phosphorus from agricultural wastewaters using adsorption media prepared from acid mine drainage sludge. Water Res 43:2240–2250
Sibrell PL, Cravotta CA, Lehman WG, Reichert W (2010) Utilization of AMD sludge from the anthracite region of Pennsylvania for removal of phosphorus from wastewater. In: Proc. of the 27th National Meeting of the ASMR, June 5–11, Pittsburgh, PA, USA, p 1085–1100
Silva J, Mello JWV, Gasparon M, Abrahão WAP, Jong T (2007) Arsenate adsorption onto aluminium and iron (hydro) oxides as alternative for water treatment. In: Cidu R, Frau F (eds) Proc. of the IMWA Symposium. May 27–31, Cagliari, Italy, 4p
Simonyi T, Akers D, Grady W (1977) The character and utilization of sludge from acid mine drainage treatment facilities. In: Technical Report of the Coal Research Bureau no.165, Morgantown, W.V, USA, 6p
Skousen JG, Sexstone A, Ziemkiewicz PF (2000) Acid mine drainage control and treatment. In: Barnhisel RI, Darmody RG, Daniels WL (eds) Reclamation of drastically disturbed lands. Agronomy 41, 1082p
Smith KS, Figueroa LA, Plumlee GS (2013) Can treatment and disposal costs be reduced through metal recovery? In: Proc. of the IMWA Mid-Conference Tour, August 5–9. Wolkerdorfer, Brown & Figueroa (Eds), Golden, CO, USA, p 729–734
Smol JP (2008) Pollution of lakes and rivers: a paleoenvironmental perspective, second edn. Blackwell, Oxford 396p
Song Y, Wang M, Liang J, Zhou L (2014) High-rate precipitation of iron as jarosite by using a combination process of electrolytic reduction and biological oxidation. Hydrometallurgy 143:23–27
Sparks DL (2003) Environmental soil chemistry, second edn. Academic Press, San Diego 352p
Stantec Consulting Ltd. (2004) Priority assessment of metal leaching in neutral drainage. In: Review of water quality issues in neutral pH drainage: examples and emerging priorities for the mining industry in Canada. MEND Initiative Report 10.1, Ref. 631–22996, July 2004. MEND, Ottawa, ON, Canada, 58p
Tay J, Show K (1991) Properties of cement made from sludge. J. Environ. Eng. 117:236–246
Tay J, Show K (1997) Resource recovery of sludge as a building and construction material—a future trend in sludge management. Water Sci Technol 36:259–266
Taylor J, Pape S, Murphy N (2005) A summary of passive and active treatment technologies for acid and metalliferous drainage (AMD). Fifth Australian workshop on acid drainage. 29–31 August, Fremantle, Western Australia, 49p
Tsang DCW, Yip ACK (2014) Comparing chemical-enhanced washing and waste-based stabilization approach for soil remediation. J Soil Sediment 14:936–947
Tsang DCW, Olds WE, Weber PA, Yip ACK (2013) Soil stabilization using AMD sludge, compost and lignite: TCLP leachability and continuous acid leaching. Chemosphere 93:2839–2847
Unger-Lindig Y, Merkel B, Schipek M (2010) Carbon dioxide treatment of low density sludge: a new remediation strategy for acidic mining lakes? Environ Earth Sci 60:1711–1722
United States Environmental Protection Agency (USEPA) (1973) Dewatering of mine drainage sludge. EPA R-2-73-169. Office of research and monitoring, WV, USA, 162p
USEPA (2001) Metals recycling from waste sludges by ammoniacal leaching followed by solvent extraction. EPA (# 68D01033). Small Business Innovation Research, Final report. https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/1215/report/F. Last access Apr 2016
USEPA (2014) Reference guide to treatment technologies for mining-influenced water. EPA 542-R-14-001, 94p. https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/1215/report/F. Last access Apr 2016
Viadero RC, Wei X, Buzby KM (2006) Characterization and dewatering evaluation of acid mine drainage sludge from ammonia neutralization. Environ Eng Sci 23:734–743
Voormeij DA, Simandl GJ (2004) Geological, ocean, and mineral CO2 sequestration options: a technical review. J Geosci Can 31:11–22
Wang YR, Tsang DCW, Olds WE, Weber PA (2013) Utilizing acid mine drainage sludge and coal fly ash for phosphate removal from dairy wastewater. Environ Technol 34(24):3177–3182
Wei X, Viadero RG Jr (2007) Adsorption and precoat filtration studies of synthetic dye removal by acid mine drainage sludge. J Environ Eng 135:635–640
Wei X, Viadero RC Jr, Bhojappa S (2008) Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants. Water Res 42:3275–3284
Weng CH, Lin DF, Chiang PC (2003) Utilization of sludge as brick materials. Adv Environ Res 7:679–685
Young CA, Taylor PR, Anderson CG, Choi Y (2008) Hydrometallurgy 2008. In: Proc. of the 6th International symposium, 1st ed. Society for Mining, Metallurgy and Exploration (SME), Inc., Littletown. 1177p
Zagury GJ, Rincon Bello JA, Guney M (2016) Valorization of a treated soil via amendments: fractionation and oral bioaccessibility of Cu, Pb, Ni and Zn. Environ Monit Assess 188:1–11
Zinck J (2005) Review of disposal, reprocessing and reuse options for acidic drainage treatment sludge. MEND Report 3.42.3, 68p. http://mend-nedem.org/wp-content/uploads/2013/01/3.42.3.pdf. Last access March, 2016
Zinck J, Griffith W (2006) Evaluation of sludge management options. In: Proc. of the 7th ICARD, Leadership: Gateway to the future, March 27–30, St Louis, MI, USA, 16p
Zinck J, Griffith W (2009) International ARD treatment and sludge management survey. In: Proc. of the 8th ICARD and Securing the future: Mining, metals & the environment in a sustainable society 2009, June 23–26, Skellefteå, Sweden, 10p. http://www.proceedings-stfandicard-2009.com/pdfer/Janice_Zinck_B1_T3_International-ARD-Treatment-and-Sludge-Management-Survey.pdf. Last access Mar 2016
Zinck J, Griffith W (2013) Review of acidic drainage treatment and sludge management operations, MEND Report 3.43.1. CANMET-MMSL, 101p. http://mend-nedem.org/wp-content/uploads/3.43.1_ReviewMineDrainageTreatmentSludge.pdf. Last access Mar 2016
Zinck JM, Wilson LJ, Chen TT, Griffith WM, Mikhail S, Turcotte AM (1997) Characterization and stability of acid mine drainage treatment sludges. MEND Report MMSL 96–079 (CR), MEND, 397p. http://mend-nedem.org/mend-report/characterization-and-stability-of-acid-mine-drainage-sludges/. Last access Mar 2016
Zinck J, Fiset JF, Griffith W (2010) Stability of treatment sludge in various disposal environments: a multi-year leaching study. In: Wolkersdorfer C, Freund A (eds) Proc. of the IMWA Symposium, September 5–9, Sydney, NS, Canada, p 527–530
Acknowledgment
The present study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), and the industrial partners of RIME-UQAT-Polytechnique (Agnico Eagle, Canadian Malartic Mine, Iamgold Corporation, Raglan Mine-Glencore, and Rio Tinto). The authors gratefully acknowledge the assistance of Professors John W. Molson and Vincent Cloutier, as well as of Dr. Robin Potvin during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 94.6 kb)
Rights and permissions
About this article
Cite this article
Rakotonimaro, T.V., Neculita, C.M., Bussière, B. et al. Recovery and reuse of sludge from active and passive treatment of mine drainage-impacted waters: a review. Environ Sci Pollut Res 24, 73–91 (2017). https://doi.org/10.1007/s11356-016-7733-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7733-7