Abstract
In this work ultrafiltration (UF) was coupled with suitable minerals and dried activated sludge for the pre-treatment of several industrial wastewater streams. The aim was to decrease heavy metal concentrations to low levels so that wastewater can be safely discharged into municipal sewers or biological wastewater treatment can take place without biomass inhibition problems. Industrial wastewater originating from metal plating, chemical and textile industries was employed. The experiments were conducted in a reactor where the UF membrane module was immersed. UF reduced the amount of heavy metals, but the performance was variable with removal efficiencies ranging from 20 to 99.7 %, depending on the metal type and on the wastewater initial characteristics. The prevailing wastewater characteristics were the pH, the presence of certain anions, the suspended solids concentration and the presence of competing cations. The addition of activated sludge and/or minerals could further increase heavy metal removal through the process of sorption. UF assisted by minerals could achieve variable colour and COD removal ranging from 22 to 94 % and 58 to > 99.9 % respectively. Minerals resulted in membrane fouling mitigation, while sludge adversely impacted on fouling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wastewater from several industrial activities including metal plating, chemical and dyeing can contain significant amounts of heavy metals. The negative impact of these wastewater streams upon the environment gives rise to increasingly strict regulations. The US EPA (2005) has set standards for industrial wastewater discharge into the municipal sewers, depending on the industrial sector. Recently, the Greek State has set limit values for the industrial effluents that are discharged in the Asopos river basin for the priority substances and other pollutants, as well as for other substances (KYA 20488/2010).
Direct biological treatment is usually problematic since high levels of heavy metals can inhibit the growth of microorganisms (Hammes et al. 2003; Patidar and Tare 2004; Gikas 2007, 2008). A loss in activated sludge viability, changes in sludge community structure, loss of floc structure (Harrison et al. 2007; Lock et al. 2007) and/or decrease in treatment efficiency may occur due to the accumulation of heavy metals inside the biological reactor (Kelly et al. 2004).
The quantity and composition of wastewater originating from metal plating industry depends on various factors including the industrial processes that are carried out, the size and shape of treated surfaces and the applied rinsing system (Bodzek et al. 1999). In addition to metal ions, counter anions, surfactants, dyes, brighteners, organic and sometimes inorganic agents are also present in metal plating effluents. Wastewater from the chemical industry contains a variety of pollutants, depending on the raw materials used in the production process, and exhibits high variability both in terms of quantity and composition. Wastewater of textile dyeing and finishing industries contains various pollutants such as heavy metals, colourants, reaction products, dye impurities, auxiliaries and surfactants. These effluents can be toxic to microorganisms and exhibit slow degradation kinetics (Baban et al. 2010). Due to the potential toxicity of the aforementioned industrial wastewater streams, direct biological treatment is not usually feasible, and suitable pre-treatment is required.
Several treatment processes are available for the removal and/or recovery of heavy metals from industrial wastewater in order to improve the treated effluent quality. These processes include adsorption, chemical precipitation, coagulation–flocculation, liquid–liquid extraction, flotation, ion exchange and membrane filtration. Ultrafiltration (UF) has been applied for the treatment of metal-contaminated wastewater. UF membranes act as the barrier to pollutants and dictate which substances are removed and which penetrate into the final effluent. UF membrane modules are able to retain suspended solids (SS) and the majority of colloidal matter. Thus, metal forms attached to SS are effectively removed by the UF membranes. Barakat and Schmidt (2010) applied a polymer-enhanced UF process to remove Cu(II), Ni(II) and Cr(III) from synthetic wastewater achieving rejection of 98.5 %, 76.4 % and 97.1 %, respectively, at pH 7. Borbély and Nagy (2009) also examined a polymer-enhanced UF process in order to remove nickel and zinc from wastewater accomplishing rejection of 93.2 % and 99.9 %, respectively, at pH greater than 8. Juang and Shiau (2000) investigated the removal of Cu(II) and Zn(II) from synthetic wastewater using chitosan-enhanced membrane filtration, achieving almost 100 % and 95 % rejection, respectively, at pH ranging from 8.5 to 9.5. The results indicated that chitosan enhanced metal removal by six to ten times compared to the use of membrane alone. Malamis et al. (2010) found that UF membranes combined with activated sludge resulted in Cu(II) removal ranging from 59 to 78 %, while the addition of bentonite further increased copper removal up to 99 %. When UF membranes were employed to filter sludge with mixed liquor suspended solids (MLSS) above 4.5 g/l at pH higher than 7, the Cr(III) removal was greater than 95 % (Malamis et al. 2009). Katsou et al. (2010) investigated the removal of nickel from wastewater by employing UF together with activated sludge and vermiculite and achieved 80 % metal removal at pH 6. Molinari et al. (2006) employed a polymer-assisted UF process to remove copper from aqueous solutions producing a final effluent having very low copper concentration. Petrov and Nenov (2004) removed copper, lead, nickel and zinc by 97.1 %, 95.4 %, 92.1 % and 72.3 %, respectively, from synthetic wastewater by applying complexation and membrane separation. Yang and Tsai (2006) evaluated the performance of a simultaneous electrocoagulation and electrofiltration module achieving 92.9 % Cu(II) removal from industrial wastewater. Zamboulis et al. (2004) combined sorption using synthetic zeolite and flotation for the removal of copper and zinc accomplishing in certain cases almost complete metal removal. Blöcher et al. (2003) examined a combined system of flotation, membrane separation and adsorption to remove nickel, copper and zinc from industrial wastewater from electronic industries, producing a final effluent with metal concentrations lower than 0.05 mg/l. Mavrov et al. (2003) found that the use of adsorption, microfiltration (MF) and flotation resulted in removal efficiencies higher than 97 % for zinc and copper and 84 % for nickel. Lazaridis et al. (2004) employed a two-stage process of sorption and dispersed-air flotation with MF for zinc removal from aqueous solutions, which was found to be very effective, resulting in almost complete Zn(II) removal. A polymer-enhanced UF process was employed to remove copper from wastewater and lead from high calcium content feed water, achieving removal efficiencies higher than 97 % and 99 %, respectively (Llanos et al. 2008; Canizares et al. 2007). Rivas et al. (2005) adopted a polyelectrolyte-assisted UF process for heavy metal removal obtaining very high removal at neutral pH. Camarillo et al. (2010) examined a batch polymer-enhanced UF process at bench-scale and achieved 99.5 % removal of copper from synthetic solutions. Channarong et al. (2010) investigated the removal of Ni(II) and Zn(II) from aqueous solutions using a micellar-enhanced UF and an activated carbon fibre hybrid process resulting in removal of 99.3 % and 99.9 %, respectively, while Landaburu-Aguirre et al. (2010) used micellar-enhanced UF accomplishing 98 % and 99 % retention of Zn(II) and Cd(II), respectively. Mbareck et al. (2009) achieved almost complete rejection of Pb(II), Cd(II) and Cr(III) from water by employing polysulfone/polyacrylic acid UF membranes.
Fatin-Rouge et al. (2006) found that membrane filtration combined with alginate effectively removed Pb(II), Cu(II) and Zn(II), while the performance was not satisfactory for Ni(II) removal. Saffaj et al. (2004) accomplished rejection efficiencies of 93 %, 93 % and 96 % for Pb(II), Cd(II) and Cr(III), respectively, by employing ceramic UF membranes deposited on support made of clay. Zou et al. (2009) removed 94 % of Pb(II), 92 % of Zn(II), > 99 % of Cr(III), 94 % of Cd(II) and 93 % of Cu(II), using a zeolite membrane. Bessbousse et al. (2008) accomplished removal efficiencies of 96 %, 99 % and 99.5 % for lead, copper and cadmium, respectively, using a complexing membrane.
The present work investigated the use of the UF process for the pre-treatment of wastewater from metal plating, chemical and textile industries to decrease heavy metals and colour to low levels so that biological wastewater treatment can effectively take place or the treated wastewater can be safely discharged into municipal sewers. This way the industries can install UF at their premises with no other treatment requirements. UF was combined with natural minerals and/or activated sludge to enhance the capability of the process to remove pollutants.
2 Experimental
2.1 Industrial Wastewater Sample Collection and Preparation
Industrial wastewater samples were collected from (a) five metal plating (EP1–EP5), (b) three chemical (CH1–CH3) and (c) three textile (TEX1–TEX3) industries located in the wider region of Oinofita–Schimatari of Viotia in Greece. Several industries are located in this zone, which faces immense environmental problems due to the discharge of poorly treated industrial wastewater in the Asopos river. The wastewater samples had received little or no treatment. The samples were collected in plastic 10-l bottles preserved at 4 °C before analysis and were analyzed within 1–2 days.
The heavy metal concentration (i.e. Zn, Cr, Cu, Ni, Pb and Cd) of the wastewater samples was usually kept constant at 50 mg/l or 200 mg/l for each metal by adding suitable amount of these metals in the samples. This way it is possible to compare the removal efficiency of the UF system for the treatment of various industrial wastewater streams. The metal salts that were added were the following: Zn(NO3)2·6H2O, Cu(NO3)2·3H2O, Pb(NO3)2, Ni(NO3)2·6H2O, Cr(NO3)3·9H2O and Cd(NO3)2·H2O and were supplied by Merck and Sigma-Aldrich.
2.2 Sorbents and Membranes
Zeolite (Z), bentonite (B) and vermiculite (V) were the minerals employed as adsorbents. These minerals exhibit high ion exchange and adsorption capacity. Vermiculite was supplied by Mathios Refractories S.A., while natural zeolite (clinoptilolite) and bentonite were supplied by S&B Industrial Minerals S.A. The minerals were washed and dried at 80 °C for at least 24 h and were placed in desiccators prior to their use. They were used in powder form (<0.18 mm) without chemical modification. This grain size was selected in order to have a large specific surface area and thus more active sites available for the adsorption process. Furthermore, powder minerals can remain in suspension more easily with adequate aeration than the granular minerals. The mineral dosage was kept constant at 10 g/l, since previous studies have shown that this was an adequate concentration for the effective removal of heavy metals (Malamis et al. 2010; Katsou et al. 2010). Scanning electron microscopy (SEM) was applied to examine the structure of all sorbents (i.e. zeolite, bentonite, vermiculite and dried activated sludge).
The sludge sorbent employed was activated sludge (S) collected from the aeration tank of a membrane bioreactor (MBR) treating municipal wastewater. The sludge was dried for several days at 60 °C, was sieved through a 0.18-mm sieve and was subsequently used as a sorbent for the removal of heavy metals at a constant concentration of 5.2 g/l. Fourier transform infrared (FTIR) analysis was used to determine the functional groups of dried activated sludge which are responsible for metal binding. Sludge pH, total solids (TS), total suspended solids (TSS) and volatile suspended solids (VSS) were determined. In addition, the activated sludge was analyzed with respect to heavy metals to ensure that their concentration was negligible compared to the heavy metal content of wastewater.
The amount of metals sorbed on minerals and on dried activated sludge q (milligrams per gram) at time t is given by the equation:
where C 0 and C t (milligrams per litre) are the liquid phase metal concentrations initially and at time t, respectively, m (grams) is the sorbent mass, and V (litres) is the volume of the solution.
The crystalline structure of the unused, clean membrane and of the used membrane was established by X-ray diffraction (XRD). XRD was also employed to characterize the metal precipitates that were formed in wastewater. Membrane fibres and precipitates were dried in an oven at 105 °C and were placed in a desiccator. The precipitate samples were additionally crushed to fine powder in an agate mortar before the diffraction data collection in order to avoid preferential orientation of the crystallites.
2.3 Treatment Processes
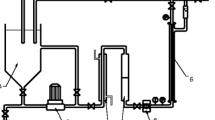
The treatment processes applied were: (a) UF of wastewater, (b) UF assisted by adsorption using low-cost minerals at a constant mineral (i.e. zeolite, bentonite, vermiculite) concentration of 10 g/l, (c) UF combined with dried activated sludge for sorption and (d) UF coupled with dried activated sludge and minerals. Wastewater from EP1–EP3 and CH1–CH3 was treated using processes (a) and (b) while wastewater from EP4–EP6 and TEX1–TEX3 was treated using processes (a)–(d). The experiments were conducted in a plexiglas reactor where the UF membrane module was immersed.
The membrane module consisted of hollow fibre UF membranes (ZeeWeed-1, GE Water and Process Technologies). The membranes were made of polyvinylidene fluoride (PVDF), having a filtration area of 0.047 m2, a nominal pore size of 0.04 μm and an absolute pore size of 0.1 μm. The filtration experiments were conducted for 60 min under constant transmembrane pressure of 30 kPa using a suitable vacuum pump. Constant fine bubble aeration of 8 l/min was provided to maintain the wastewater under suspension, and coarse bubble aeration of 6 l/min was provided to the membrane module to minimize fouling. The Ni, Zn, Cr, Cu, Cd, Pb, COD and colour content of the permeate was determined to assess the system performance. In the cases where mineral and/or sludge addition took place, the mixture was agitated for 2 h at 800 rpm. The pH of wastewater samples was not adjusted in order to evaluate the process performance without any modification of the initial industrial wastewater characteristics. After each filtration experiment, the membrane module was chemically cleaned as it was immersed into a solution of 2000 mg/l (as Cl2) NaOCl for 2 h and into 4000 mg/l of citric acid solution for 1 h.
Membrane permeability reduction was used to evaluate membrane fouling during the 60-min filtration experiment. During filtration, the decrease in permeate flux was determined by measuring the filtrate volume, and thus the membrane permeability was calculated. The permeability reduction (percent) due to the 60-min filtration experiment K drop was determined using the following formula:
where K 0 (litres per meter2-hour-bar) is the clean membrane permeability determined in water and K 60 (litres per meter2-hour-bar) is the membrane permeability at the termination of the filtration experiment. The membrane permeability was always corrected to the reference temperature of 20 °C (Fan et al. 2006).
2.4 Analytical Methods
The parameters of pH, total solids (TS), total suspended solids (TSS) and volatile suspended solids (VSS) were determined using standard methods of analysis (APHA, AWWA, WEF, 1998). Colour was measured photometrically according to ASTM D 1209 (ASTM, 2000). Lead, copper, zinc, nickel, cadmium, chromium, sodium, potassium, calcium and magnesium were determined using the fast sequential atomic absorption spectrometry (model AA240FS of Varian). Heavy metal measurements were conducted according to standard methods of analysis 3111 (APHA, AWWA, WEF, 1998). Dissolved organic carbon (DOC) was determined using the TOC analyzer TOC-VCSH of Shimadzu. DOC was determined in the filtrate collected after filtration through Whatman membranes with a pore size of 0.45 μm. Chemical oxygen demand (COD), total phosphorous (Ptotal), ortho-phosphates (PO4-P), sulphates (SO 2−4 ), ammonium nitrogen (NH4-N), nitrate nitrogen (NO3-N), total nitrogen (Ntotal) and chlorides (Cl−) were determined using the Merck Spectroquant Nova 60 photometer and Spectroquant Merck kits. All the aforementioned parameters were determined in the industrial wastewater samples.
FTIR analysis was used to determine the functional groups of dried activated sludge, which are responsible for metal binding. The Jasco 4200 spectrophotometer was employed. The FTIR spectra were recorded for wave numbers ranging from 700 to 5000 cm−1 with a resolution of 8 cm−1 using the ATR method with a PRO410-S. SEM was employed to examine the sorbents using an FEI Quanta 200 scanning electron microscope (resolution 6 nm). The instrument was operated in low vacuum mode at a chamber pressure of 80 Pa. The accelerating voltage varied from 20 to 25 kV, depending on the sample (i.e. mineral and dried activated sludge). No pre-treatment or coating was applied to the samples, which were secured to the specimen holder with adhesive conductive double-sided carbon tape.
XRD analysis was used to depict the structure of the clean and used membranes and of the metal precipitates. The samples were scanned with CuKα radiation (1.5406 Å) from 5° ≤ 2θ ≤ 60° at a scanning speed of 0.01°/0.5 s, using a Siemens D5000 powder X-ray diffraction unit, operating at 30 mA and 40 kV at room temperature.
3 Results and Discussion
The applied processes were examined for their ability to remove heavy metals, organics and colour. Furthermore, the fouling potential of UF membranes was investigated.
3.1 Industrial Wastewater Characteristics
Table 1 summarizes the activities of each industry as well as the origin of the produced wastewater, and Table 2 shows the characteristics of each type of wastewater. It is observed that even wastewater from the same industrial sector had significant differences depending on the production process, the production line and the raw materials that were used.
3.2 Sorbent Characteristics
Zeolite (clinoptilolite), bentonite and vermiculite were the aluminosilicate minerals that were employed for the adsorption process. The major mineral constituents were silica (SiO2) and alumina (Al2O3). The ratio of Si/Al was 6.67 for clinoptilolite, 3.10 for vermiculite and 3.38 for bentonite giving rise to significant cation exchange capacities of the minerals with decreasing Si/Al values. Zeolite was characterized by high calcium and potassium content and low sodium and magnesium content. Vermiculite had very high magnesium content, high potassium and low calcium and sodium content, while bentonite had higher sodium and calcium and lower potassium content. These mobile ions can be exchanged with heavy metals found in wastewater. The characterization of these minerals has been given in previous work (Katsou et al. 2010). Dried activated sludge was used for the sorption process, and its initial characteristics are given in Table 3. The sludge TS and TSS are the most important sludge properties, as they significantly impact on the sorption process.
SEM micrographs in Fig. 1 illustrate the morphology of minerals. Large number of pores, layers and sheets were evident ensuring high surface area for easy adsorption. The characteristic platy crystals with perfect cleavage in one direction of monoclinic clinoptilolite are shown on a micrograph (Fig. 1a) of the zeolite sample. As it is revealed from Fig. 1b, c, large layer crystals of vermiculite and bentonite crystal agglomerates were detected.
The FTIR analysis of dried activated sludge showed that the predominant functional groups (carboxylic, amine, hydroxyl and phenolic groups) have for origin proteins, polysaccharides, organic acids and humic substances. In particular, the broad peak at 3284 cm−1 can be attributed to the O–H stretching vibrations. The peaks at 2919 and 2852 cm−1 are due to C–H stretching vibrations of CH, CH2 and CH3 groups. The peak at 1641 cm−1 is attributed to stretching vibration of −C=O and −N−H groups characteristic for peptic bond in proteins (amides I). Two peaks at 1556 and 1535 cm−1 were attributed to stretching vibration of −C−N and −N−H (amides II), respectively. The vibrations of −CH2 carboxylates are presented at 1423 cm−1 and at 1243 cm−1 −C−O vibrations of carboxylic acids. The intense 1043 cm−1 band is vibration of C−O−C and −OH of polysaccharides. Some bands observed in the “finger print” zone (<1000 cm−1) could be attributed to the phosphate and sulphur functional groups. These substances have also been identified by other researchers in activated sludge (Hong et al. 1995; Guibaud et al. 2003; Yee et al. 2004; Gulnaz et al. 2005; Nasir et al. 2007; Pagnanelli et al. 2009; Silva et al. 2012).
3.3 Heavy Metal Removal
The use of UF membranes was examined as a direct filtration technique for the pre-treatment of industrial wastewater. Heavy metal removal was variable even for wastewater of the same industrial sector, as it depended on initial wastewater characteristics (Fig. 2). For example, the nickel removal accomplished using UF varied from 26 to 79 % for metal plating wastewater, from 20 to 96 % for textile wastewater and from 22 to 95 % for chemical industry wastewater. The lead removal obtained for UF ranged from 39 to 90 %, 96 to 99 % and 36 to 99.7 % for metal plating, textile and chemical industry wastewater, respectively.
By examining the UF performance for the treatment of various industrial wastewater samples, the following were observed: direct UF of EP1 resulted in significant heavy metal removal (73–97 %) due to the rejection of metal precipitates (alkaline environment). For EP2, despite the low pH, metal removal was comparable to that of EP1 due to formation of particulate metal forms and metal complexes that were retained by the UF membranes. The above results showed that pH was not always the controlling parameter as opposed to aqueous solutions where low pH values (<3) always result in very low heavy metal removal. EP3 had a low pH value which resulted in limited metal removal when UF was applied. High Pb, Cd and Zn removal efficiencies were obtained through the UF of EP4. In this case, the permeate SO 2−4 and Cl− concentrations were 24 % and 33 % respectively lower than those of the untreated wastewater, showing that part of these anions had been retained. Also, the presence of organic substances in wastewater could favour the complexation with metals (Malandrino et al. 2006; Abollino et al. 2003). The formation of bulky metal complexes could enhance metal retention by the UF membranes.
In EP5 and EP6 the relatively low heavy metal removal is attributed to its highly acidic nature. UF of CH1 resulted in very high removal efficiencies for all metals (> 94 %) due to the extremely high TSS content that favoured the adsorption of metals on solids and due to its almost neutral pH. UF of CH3 produced a permeate with high metal concentrations mainly due to the acidic nature of the sample. UF of CH2 achieved lower metal removal than CH1 due to its lower pH and TSS concentration, but higher than CH3 due to its higher pH, COD and TSS content.
In TEX1 Ni and Zn removal was limited, while Cu and Pb were effectively removed as these metals mainly formed precipitates. Similar behaviour was observed in TEX2, due to the relatively high pH. The alkaline nature of TEX3 resulted in very high removal of all metals (> 95 %). The removal order of metals varied even for wastewater samples of the same industrial sector, depending on initial wastewater characteristics. For example, for EP2 the removal order was Zn > Cu > Ni > Pb, while for EP3 the sequence became Cu > Pb > Ni > Zn. These wastewater samples were characterized by acidic pH, but different COD, DOC and organic nitrogen concentrations. In most cases, the low removal efficiency of certain metals was accompanied by high removal of other metals that coexisted in wastewater. This showed that some metals are found at higher percentage in particulate and colloidal forms than other metals, depending on the pH and potentially the concentration of other substances present in wastewater. In wastewater samples with significant TSS concentration, adsorption of heavy metals on the solids is an important removal mechanism.
In summary, the pH, the TSS concentration as well as the presence of anions and other cations in wastewater can have a significant effect on the removal of the metals under examination. At an alkaline environment, significant metal precipitation can take place depending mainly on the metal type, which results in increased retention of metals by the UF membranes. At an acidic environment, heavy metal removal was lower mainly due to the higher solubility of metals. Another influential wastewater characteristic was organic matter, since certain organic ligands could form complexes with metal ions. The formation of complexes between heavy metals and organic substances has been documented in previous studies (Malandrino et al. 2006; Abollino et al. 2003) and affects metal retention. The presence of different anions at different concentrations also affects the removal of heavy metals by UF. The adoption of UF membranes is essential since they retain particulate metal forms that either precipitate or are in suspension in the solution, as well as bulky metal complexes. Apart from direct UF, wastewater samples EP1–EP3 and CH1–CH3 were also treated by employing the integrated mineral–UF system.
The mineral and activated sludge concentrations in wastewater were always kept constant at 10 g/l and 5.2 g/l, respectively. The addition of 10 g/l mineral enhanced metal removal due to the ion exchange and adsorption of metal ions on minerals. Zeolite, bentonite and vermiculite have exchangeable cations in their structure (i.e. Na+, Ca2+, Mg2+, K+) which are exchanged with the heavy metals contained in wastewater, thus decreasing significantly the concentration of heavy metals in the liquid phase. For example, the addition of 10 g/l bentonite in EP2 increased Pb removal from 59 to 89 % and Cr removal from 78 to 97 %. Also, in CH3 the addition of 10 g/l vermiculite increased Ni removal from 22 to 82 %, Zn from 54 to 71 %, Cu from 61 to 93 %, Pb from 36 to > 99.9 % and Cr from 82 to > 99.9 %. In most cases, the mineral selectivity order was vermiculite > bentonite > zeolite for Ni and Cd, bentonite > vermiculite > zeolite for Zn and Cr and bentonite > zeolite > vermiculite for Cu and Pb. Significant amount of metals was removed by UF, indicating that the initial wastewater characteristics resulted in the formation of particulate metal forms. This also depended on the type of metal. The presence of competing cations also affects the process performance, particularly if the mineral is less selective to the metal under investigation compared to other cations. For example, in the case of EP2, the addition of sorbents was not very effective for the removal of lead, nickel and copper. This is probably attributed to the very high ammonium concentration. Ammonium acts as a competing cation reducing the sorbent performance for heavy metal uptake.
In Fig. 3 the heavy metal concentration in the permeate is given for the treatment processes of mineral–UF as well as UF alone. Direct filtration significantly reduced the final effluent metal concentration, while minerals further enhanced the metal removal, resulting in an effluent that in certain cases could attain for specific metals the discharge limits of industrial wastewater in the Asopos river basin. For example, the treatment of wastewater EP1 and CH1 with bentonite–UF and vermiculite–UF resulted in low heavy metal concentrations in the final effluent. In certain samples pH adjustment to alkaline values is required to ensure low metal concentrations in the permeate. The pH value of wastewater can be adjusted to > 8, resulting in higher precipitation and in the production of a final effluent with very low metal concentrations.
Wastewater samples EP4–EP6 and TEX1–TEX3 were treated using the treatment schemes of (a) UF, (b) minerals–UF, (c) dried activated sludge–UF and (d) dried activated sludge–minerals–UF. The addition of activated sludge as sorbent at a concentration of 5.2 g/l enhanced metal removal due to biosorption. The heavy metal concentration for the permeate is given in Fig. 4 for the aforementioned treatment processes. The treatment scheme of sludge–mineral–UF resulted in the highest metal removal. The adoption of sludge–bentonite–UF and sludge–vermiculite–UF process for the treatment of wastewater sample TEX3 resulted in a final effluent with low Ni, Zn, Cu and Pb concentrations that could meet the US EPA standards for discharge of industrial wastewater into municipal sewers, despite the high initial metal concentration of 200 mg/l for each metal.
Table 4 shows the heavy metal limit values which have been specified by the Greek State for the discharge of industrial wastewater in the Asopos river basin. Comparing these limit values with the heavy metal concentrations in the permeate, the following are concluded: (a) The nickel limit cannot be attained by the examined treatment schemes. (b) The cadmium limit was only attained in the cases where its initial concentration was lower than 3 mg/l and vermiculite alone and in combination with activated sludge were used. (c) The use of bentonite alone and in combination with activated sludge resulted in the attainment of the limit value for lead for most of the wastewater samples (i.e. EP1, EP4, EP5, EP6, CH1, CH3, TEX2, TEX3). (d) The chromium limit could be attained by all the examined treatment schemes for CH1 and CH3; however, no treatment scheme could obtain the required limit for EP1, EP2, EP5 and CH2. (e) Bentonite alone and in combination with activated sludge and in some cases the combined use of zeolite and sludge resulted in a treated effluent that satisfied the copper limit for EP1, EP5, EP6, CH1 and TEX3. However, for some industrial wastewater samples the applied treatment schemes were not effective. (f) The limit value specified for zinc is met by the use of several treatment schemes using minerals alone or in combination with sludge. In the cases where the limits are not respected, an increase of pH value and/or higher adsorbent concentration are recommended. However, it must be noted that in this work high initial metal concentrations were usually applied (i.e. 50 and 200 mg/l).
Figure 5 shows the sorption capacity of minerals and activated sludge for the selected wastewater stream of EP5. Dried activated sludge (5.2 g/l) exhibited the highest sorption capacity and is recommended as a suitable sorbent material, while minerals (10 g/l) exhibited variable adsorption capacities depending on the type of metal adsorbed. Mineral adsorption capacity was different (usually lower) when sludge was added as sorbent. It must also be mentioned that sorption capacity of each mineral or of sludge varied depending on the metal concentration that was initially available in wastewater for the sorption process. Vermiculite was found to be very effective for nickel removal and bentonite for chromium removal. The type of mineral impacted on the removal process, while its performance also depended on the type of heavy metal.
The minerals and sludge remaining after the treatment process contained high heavy metal concentrations. This is a disadvantage that can limit the full scale application of this sorbent-assisted UF system for the removal of heavy metals from industrial wastewater. Sorbent recycling is necessary to make the sorption process more cost effective. Sorbent regeneration will enable the metal recovery and the reuse of the sorbent for the removal of heavy metals from wastewater. Sorbent regeneration could be performed by employing various desorbing solutions (i.e. KCl, NaCl, NH4Cl, HNO3, HCl). The results of several studies reported in literature are promising, since high regeneration efficiencies have been obtained in many cases for the regeneration of minerals using acids or salt solutions as desorbing agents (Anirudhan and Suchithra 2010; Argun 2008; Turan et al. 2005; Otero et al. 2009).
3.4 Membrane Characterization
Figure 6a shows the XRD obtained for a new membrane and Fig. 6b the XRD of a membrane used several times for filtering industrial wastewater. Intensities and positions of Bragg peaks of crystalline phases were identified by comparing with those listed in the Joint Committee on Powder Diffraction Standards (JCPDS) data files for monoclinic PVDF No. 42–1650 and for base-centred orthorhombic PVDF No. 42–1649. According to the literature, the X-ray reflections are in accordance with those of α- and β-phase PVDF (Gregorio and Cestari 1994; Park et al. 2011; Satapathy et al. 2011). The peaks at 2θ = 17.9°, 18.4°, 20.1° and 26.7° refer to (100), (020), (110) and (021) reflections (blue colour) in α-phase crystal. Presence of peaks at 20.7° (200) and 20.8° (110) in the XRD pattern confirm the existence of β-phase in PVDF membrane (red colour) along with α-phase. Also, a broad peak near 41° is in accordance with those of the combined (201, 111) reflections of β-PVDF spherulites.
The used UF membrane did not have any significant structural changes compared to the new one. Comparing the pattern of the blank to that of the used membrane, it can be observed that the intensities of all diffraction peaks decrease, probably due to the membrane surface covering by amorphous or crystallized compounds after filtration. Due to the overlapping of the diffraction peaks of the membrane material and the various foulants and precipitates on the membrane surface, their presence on the membrane surface could not be completely included or excluded. Also, the mass percentage of these compounds is very low to identify them by XRD. X-ray diffractogram of precipitates is shown in Fig. 6c. Few crystalline compounds could be presented, namely hydroxides of copper, lead, chromium and nickel.
3.5 Organic Matter and Colour Removal
UF resulted in significant COD removal ranging from 45 to 93 %, depending on wastewater characteristics. High COD decrease was observed in wastewater samples where most of the organic matter was in particulate form (e.g. CH1), while lower COD removal was obtained in samples characterized by high soluble organic matter (e.g. EP4), since the latter can penetrate the membranes. UF treatment resulted in higher COD removal for wastewater from chemical and textile industries than for metal plating wastewater. Minerals enhanced organic removal as COD removal ranged from 58 to 95 % for vermiculite, from 60 to > 99 % for bentonite and from 60 to 94 % for zeolite (Fig. 7a). The adsorption of organic matter on natural minerals has been investigated in recent literature with promising results (Santi et al. 2008). In several cases the examined treatment processes were not sufficient for the effective removal of organic matter, and thus they can be applied only as a pre-treatment step.
The examined processes were also investigated with respect to colour removal (Fig. 7b). UF resulted in colour removal ranging from 18 to 57 % for metal plating, from 51 to 92 % for the chemical industry and from 15 to 55 % for textile wastewater. Mineral addition resulted in higher colour removal which ranged from 32 to 85 % for metal plating, 54 to 94 % for the chemical industry and 22 to 72 % for textile wastewater. In most cases zeolite and bentonite exhibited higher colour removal than vermiculite.
3.6 Membrane Fouling
UF membrane fouling was assessed through the calculation of permeability reduction with time occurring due to the 60-min filtration experiment. This time interval is considered sufficient to evaluate membrane fouling and to directly compare the membrane performance for the filtration of different types of industrial wastewater. Longer filtration experiments (e.g. 2–3 h) would not change significantly the obtained results since in all cases, after the first 20–30 min the rate of membrane permeability reduction was relatively constant. Figure 8 shows the permeability reduction (K drop) for the examined cases. It was observed that fouling depended on wastewater characteristics. Direct UF of metal plating wastewater resulted in permeability reduction ranging from 5 to 16 % with the exception of EP4 where the reduction was 64 % probably owing to the high dissolved organic content that accelerated fouling. The majority of organic matter of EP4 was in soluble form and penetrated the UF membranes, while the TSS concentration was low. Therefore, the organic fouling observed is more likely to occur at the interior of the membranes rather than on the surface. When industrial wastewater samples from the chemical industries were filtered, K drop ranged from 6 to 20 % with the exception of CH1 where the K drop was 55 % due to the extremely high TSS that hindered filtration (TSS > 40 g/l) and created clogging problems. In textile wastewater samples, significant fouling occurred (38–53 %) partly due to the much higher initial heavy metal concentrations which increased inorganic fouling. Mineral addition mitigated membrane fouling with zeolite exhibiting the best performance. Minerals favoured the adsorption of organic compounds on their surface. The organic compounds which were present in industrial wastewater would otherwise remain in suspension, and could be deposited on the membrane surface or interior or penetrate into the final effluent. As a result, the addition of minerals in wastewater reduced the amount of organic substances that fouled the membranes. On the contrary, the use of sludge as additive deteriorated the membrane performance probably due to the presence of extracellular polymeric substances (EPS) that are considered as major fouling substances (Malamis and Andreadakis 2009). The combined use of minerals and sludge in wastewater lowered to some extent the beneficial effect of minerals with respect to fouling mitigation.
3.7 Application of Treatment Scheme
UF membranes can effectively retain particulate metal forms and partly colloidal metal forms. The addition of sludge and/or minerals resulted in enhanced metal removal through sorption. The results of this work showed that such combined processes can remove heavy metals, resulting in certain cases in a treated effluent characterized by low heavy metal levels that can be treated biologically or discharged into municipal sewers. Industries facing space limitations may choose to adopt such pre-treatment schemes.
4 Conclusions
This work showed that UF combined with minerals and/or sludge is a viable pre-treatment option for the decrease of heavy metals, colour and organic matter from industrial wastewater. The performance of these combined processes with respect to heavy metal removal was variable, as it depended on the type of sorbent employed and on the wastewater characteristics. The addition of activated sludge and/or minerals enhanced the system performance with respect to metal removal. The most important wastewater characteristics affecting the removal of metals were the pH, the organic content, the TSS concentration and the presence of competing cations. COD removal by UF ranged from 45 to 93 %, while mineral addition further increased the removal efficiency to 58– > 99 %. The UF process resulted in colour removal ranging from 18 to 92 %, while mineral addition increased the efficiency to 22–94 %. Finally, it was found that mineral addition mitigated membrane fouling, while sludge adversely impacted on fouling.
References
Abollino, O., Aceto, M., Malandrino, M., Sarzanini, C., & Mentasti, E. (2003). Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Research, 37, 1619–1627.
Anirudhan, T. S., & Suchithra, P. S. (2010). Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: kinetics and equilibrium modelling. Chemical Engineering Journal, 156, 146–156.
APHA, AWWA, WEF. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington, DC: American Public Health Association, American Water Works Association, Water Environment Federation.
Argun, M. E. (2008). Use of clinoptilolite for the removal of nickel ions from water: kinetics and thermodynamics. Journal of Hazardous Materials, 150, 587–595.
ASTM. (2000). Standard test method for color of clear liquids (platinum–cobalt scale, D1209-00). West Conshohocken: ASTM.
Baban, A., Yediler, A., Avaz, S., & Hostede, S. (2010). Biological and oxidative treatment of cotton textile dye-bath effluents by fixed and fluidized bed reactors. Bioresource Technology, 101, 1147–1152.
Barakat, M. A., & Schmidt, E. (2010). Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination, 256, 90–93.
Bessbousse, H., Rhlalou, T., Verchère, J.-F., & Lebrun, L. (2008). Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. Journal of Membrane Science, 307, 249–259.
Blöcher, C., Dorda, J., Mavrov, V., Chmiel, H., Lazaridis, N. K., & Matis, K. A. (2003). Hybrid flotation–membrane filtration process for the removal of heavy metal ions from wastewater. Water Research, 37, 4018–4026.
Bodzek, M., Korus, I., & Loska, K. (1999). Application of the hybrid complexation–ultrafiltration process for removal of metal ions from galvanic wastewater. Desalination, 121, 117–121.
Borbély, G., & Nagy, E. (2009). Removal of zinc and nickel ions by complexation–membrane filtration process from industrial wastewater. Desalination, 240, 218–226.
Camarillo, R., Llanos, J., García-Fernández, L., Pérez, A., & Cañizares, P. (2010). Treatment of copper (II)-loaded aqueous nitrate solutions by polymer enhanced ultrafiltration and electrodeposition. Separation and Purification Technology, 70, 320–328.
Canizares, P., Pérez, A., Camarillo, R., Llanos, J., & López, M. L. (2007). Selective separation of Pb from hard water by a semi-continuous polymer-enhanced ultrafiltration process (PEUF). Desalination, 206, 602–613.
Channarong, B., Lee, S. H., Bade, R., & Shipin, O. V. (2010). Simultaneous removal of nickel and zinc from aqueous solution by micellar-enhanced ultrafiltration and activated carbon fiber hybrid process. Desalination, 262, 221–227.
Fan, F., Zhou, H., & Husain, H. (2006). Identification of wastewater sludge characteristics to predict critical flux for membrane bioreactor processes. Water Research, 40, 205–212.
Fatin-Rouge, N., Dupont, A., Vidonne, A., Dejeu, J., Fievet, P., & Foissy, A. (2006). Removal of some divalent cations from water by membrane-filtration assisted with alginate. Water Research, 40, 1303–1309.
Gikas, P. (2008). Single and combined effects of nickel (Ni(II)) and cobalt (Co(II)) ions on activated sludge and on other aerobic microorganisms: a review. Journal of Hazardous Materials, 159, 187–203.
Gikas, P. (2007). Kinetic responses of activated sludge to individual and joint nickel (Ni(II)) and cobalt (Co(II)): an isobolographic approach. Journal of Hazardous Materials, 143, 246–256.
Gregorio, R., Jr., & Cestari, M. (1994). Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene Fluoride). Journal of Polymer Science Part B: Polymer Physics, 32, 859–870.
Guibaud, G., Tixier, N., Bouju, A., & Baudu, M. (2003). Relation between extracellular polymers’ composition and its ability to complex Cd, Cu and Pb. Chemosphere, 52, 1701–1710.
Gulnaz, O., Saygideger, S., & Kusvuran, E. (2005). Study of Cu(II) biosorption by dried activated sludge: effect of physico-chemical environment and kinetics studies. Journal of Hazardous Materials, B120, 193–200.
Hammes, F., Boon, N., De Villiers, J., Verstraete, W., & Siciliano, S. D. (2003). Strain-specific ureolytic microbial calcium carbonate precipitation. Applied and Environmental Microbiology, 69, 4901–4909.
Harrison, J. J., Ceri, H., & Turner, R. J. (2007). Multimetal resistance and tolerance in microbial biofilms. Nature Reviews Microbiology, 5, 928–938.
Hong, S. G., Young, J. D., Chen, G. W., Chang, I. L., Hung, W. T., & Lee, D. J. (1995). Freeze/thaw treatment on waste activated sludge: a FTIR spectroscopic study. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology, 30, 1717–1726.
Juang, R. S., & Shiau, R. C. (2000). Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. Journal of Membrane Science, 165, 159–167.
Katsou, E., Malamis, S., Haralambous, K. J., & Loizidou, M. (2010). Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater. Journal of Membrane Science, 360, 234–249.
Kelly, C. J., Tumsoroj, N., & Lajoie, C. A. (2004). Assessing wastewater metal toxicity with bacterial bioluminescence in a bench-scale wastewater treatment system. Water Research, 38, 423–431.
Landaburu-Aguirre, J., Pongrácz, E., Perämäki, P., & Keiski, R. L. (2010). Micellar-enhanced ultrafiltration for the removal of cadmium and zinc: use of response surface methodology to improve understanding of process performance and optimisation. Journal of Hazardous Materials, 180, 524–534.
Lazaridis, N. K., Blöcher, C., Dorda, J., & Matis, K. A. (2004). A hybrid MF process based on flotation. Journal of Membrane Science, 228, 83–88.
Llanos, J., Perez, A., & Canizares, P. (2008). Copper recovery by polymer-enhanced ultrafiltration (PEUF) and electrochemical regeneration. Journal of Membrane Science, 323, 28–36.
Lock, K., Eeckhout, H. V., De Schamphelaere, K. A. C., Criel, P., & Janssen, C. R. (2007). Development of a biotic ligand model (BLM) predicting nickel toxicity to barley (Hordeum vulgare). Chemosphere, 66, 1346–1352.
Malamis, S., & Andreadakis, A. (2009). Fractionation of proteins and carbohydrates of extracellular polymeric substances in a membrane bioreactor system. Bioresource Technology, 100, 3350–3357.
Malamis, S., Katsou, E., Chazilias, D., & Loizidou, M. (2009). Investigation of Cr(III) removal from wastewater with the use of MBR combined with low-cost additives. Journal of Membrane Science, 333, 12–19.
Malamis, S., Katsou, E., Stylianou, M., Haralambous, K. J., & Loizidou, M. (2010). Copper removal from sludge permeate with ultrafiltration membranes using zeolite, bentonite and vermiculite as adsorbents. Water Science and Technology, 61, 581–589.
Malandrino, M., Abollino, O., Giacomino, A., Aceto, M., & Mentasti, E. (2006). Adsorption of heavy metals on vermiculite: influence of pH and organic ligands. Journal of Colloid and Interface Science, 299, 537–546.
Mavrov, V., Erwe, T., Blocher, C., & Chmiel, H. (2003). Study of new integrated processes combining adsorption, membrane separation and flotation for heavy metal removal from wastewater. Desalination, 157, 97–104.
Mbareck, C., Nguyen, Q. T., Alaoui, O. T., & Barillier, D. (2009). Elaboration, characterization and application of polysulfone and polyacrylic acid blends as ultrafiltration membranes for removal of some heavy metals from water. Journal of Hazardous Materials, 171, 93–101.
Molinari, R., Argurio, P., & Poerio, T. (2006). Ultrafiltration of polymer–metal complexes for metal ion removal from wastewaters. Macromolecular Symposia, 235, 206–214.
Nasir, M. H., Nadeem, R., Akhtar, K., Hanif, M. A., & Khalid, A. M. (2007). Efficacy of modified distillation sludge of rose (Rosa centifolia) petals for lead(II) and zinc(II) removal from aqueous solutions. Journal of Hazardous Materials, 147, 1006–1014.
Otero, M., Rozada, F., Morán, A., Calvo, L. F., & García, A. I. (2009). Removal of heavy metals from aqueous solution by sewage sludge based sorbents: competitive effects. Desalination, 238, 46–57.
Pagnanelli, F., Mainelli, S., Bornoroni, L., Dionisi, D., & Toro, L. (2009). Mechanisms of heavy-metal removal by activated sludge. Chemosphere, 75, 1028–1034.
Park, Y. J., Kang, Y. S., & Park, C. (2011). Micropatterning of semicrystalline poly(vinylidene fluoride) (PVDF) solutions. European Polymer Journal, 41, 1002–1012.
Patidar, S. K., & Tare, V. (2004). Influence of nutrients on biomass evolution in an upflow anaerobic sludge blanket reactor degrading sulfate-laden organics. Water Environment Research, 76, 2620–2627.
Petrov, S., & Nenov, V. (2004). Removal and recovery of copper from wastewater by a complexation–ultrafiltration process. Desalination, 162, 201–209.
Rivas, B. L., Pereira, E., Cid, R., & Geckeler, K. E. (2005). Polyelectrolyte-assisted removal of metal ions with ultrafiltration. Journal of Applied Polymer Science, 95, 1091–1099.
Saffaj, N., Loukili, H., Younssi, S. A., Albizane, A., Bouhria, M., Persin, M., & Larbot, A. (2004). Filtration of solution containing heavy metals and dyes by means of ultrafiltration membranes deposited on support made of Moroccan clay. Desalination, 168, 301–306.
Santi, C. A., Cortes, S., D’Acqui, L. P., Sparvoli, E., & Pushparaj, B. (2008). Reduction of organic pollutants in olive mill wastewater by using different mineral substrates as adsorbents. Bioresource Technology, 99, 1945–1951.
Satapathy, S., Pawar, S., Gupta, P. K., & Varma, K. B. R. (2011). Effect of annealing on phase transition in poly(vinylidene fluoride) films prepared using polar solvent. Bulletin of Materials Science, 34, 727–733.
Silva, J. O., Filho, G. R., Meireles, C. S., Ribeiro, S. D., Vieira, J. G., Da Silva, C. V., & Cerqueira, D. A. (2012). Thermal analysis and FTIR studies of sewage sludge produced in treatment plants The case of sludge in the city of Uberlândia—MG, Brazil. Thermochimica Acta, 528, 72–75.
Turan, M., Mart, U., Yuksel, B., & Celik, M. S. (2005). Lead removal in fixed-bed columns by zeolite and sepiolite. Chemosphere, 60, 1487–1492.
US, EPA (2005). Streamlining the general pretreatment regulations for existing and new sources of pollution 40 CFR Parts 9, 122 and 403. Washington, DC: EPA.
Yang, G. C. C., & Tsai, C. M. (2006). Performance evaluation of Cu-CMP and oxide-CMP wastewaters. Journal of Membrane Science, 286, 36–44.
Yee, N., Benning, L. G., Phoenix, V. R., & Ferris, F. G. (2004). Characterization of metal-Cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environmental Science and Technology, 38, 775–782.
Zamboulis, D., Pataroudi, S. I., Zouboulis, A. I., & Matis, K. A. (2004). The application of sorptive flotation for the removal of metal ions. Desalination, 162, 159–168.
Zou, X., Zhu, G., Guo, H., Jing, X., Xu, D., & Qiu, S. (2009). Effective heavy metal removal through porous stainless-steel-net supported low siliceous zeolite ZSM-5 membrane. Microporous and Mesoporous Materials, 124, 70–75.
Κ.Υ.Α. 20488/2010 (ΦΕΚ 749/Β) «Καθορισμός Ποιοτικών Περιβαλλοντικών Προτύπων στον ποταμό Ασωπό και Οριακών Τιμών Εκπομπών υγρών βιομηχανικών αποβλήτων στη λεκάνη απορροής του Ασωπού.»
Acknowledgments
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heraclitus II. Investing in Knowledge Society through the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsou, E., Malamis, S., Kosanovic, T. et al. Application of Adsorption and Ultrafiltration Processes for the Pre-treatment of Several Industrial Wastewater Streams. Water Air Soil Pollut 223, 5519–5534 (2012). https://doi.org/10.1007/s11270-012-1255-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-012-1255-9