Abstract
In the transition from a fossil to a bio-based economy, it has become an important challenge to maximally recuperate and recycle valuable nutrients coming from manure and digestate processing. Membrane filtration is a suitable technology to separate valuable nutrients in easily transportable concentrates which could potentially be re-used as green fertilizers, in the meantime producing high quality water. However, traditional membrane filtration systems often suffer technical problems in waste stream treatment. The aim of this study was to evaluate the performance of vibratory shear enhanced processing (VSEP) in the removal of macronutrients (N, P, K, Na, Ca, Mg) from the liquid fraction of digestates, reducing their concentrations down to dischargeable/re-usable water. In addition, the re-use potential of VSEP-concentrates as sustainable substitutes for fossil-based mineral fertilizers was evaluated. Removal efficiencies for N and P by two VSEP filtration steps were high, though not sufficient to continuously reach the Flemish legislation criteria for discharge into surface waters (15 mg N l−1 and 2 mg P l−1). Additional purification can occur in a subsequent lagoon, yet further optimization of the VSEP filtration system is advised. Furthermore, concentrates produced by one membrane filtration step showed potential as N–K fertilizer with an economic value of €6.3 ± 1.1 t−1 fresh weight (FW). Further research is, however, required to evaluate the impact on crop production and soil quality by application of these new potential green fertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The European 2001/77/EG guideline states that by 2020 13% of the generated electricity in Belgium should be based on renewable resources. Strikingly, the current renewable contribution comprises only 4.7% in relative renewable share of the overall national energy production (Mira-T 2010). In this respect, the Flemish Energy Agency (2010) estimates that based on the production potential of various renewable technologies (solar, wind, hydro, biomass and others), 72% of the renewable objectives in Flanders need to be derived from bio-energy (FEA 2010). Hereby the production of biogas through anaerobic digestion of energy crops, organic residues and animal wastes has been evaluated as one of the most energy-efficient and environmentally beneficial technologies for bio-energy production (Fehrenbach et al. 2008).
In spite of its high potential, it was not until 2007 that, following adaptations in the Manure Decree, anaerobic digestion effectively launched as a budding market in Belgium. However, an important issue complicating development of bio-digestion in Flanders and other high-nutrient regions is that produced digestate may not, or only sparingly, be returned to arable land as a fertilizer in its crude unprocessed form (Lemmens et al. 2007). The underlying reason for this technical prerequisite is that, due to the intensive industrial animal production, the northern part of Belgium (Flanders) is confronted with an overproduction of animal manure in comparison to the available arable land to spread it on. As a consequence, overfertilization has led to eutrophication of water bodies. This resulted in the condemnation of Belgium in respect with the EU Nitrate Directive (91/676/EEC), forcing local administrators and government to enforce more stringent regulations regarding manure and digestates.
Initial steps of digestate processing generally involve the use of separation and/or dewatering technologies, using emulsion or powder based polymers for flocculation (Hjorth et al. 2010). The resulting thick fractions are commonly pasteurized and stabilized turning them into exportable organic soil conditioners, rich in phosphorous. The liquid fraction still contains most of the digestate’s potassium and inorganic nitrogen. Excess nitrogen can be processed by stripping or treatment in nitrification–denitrification bioreactors. However, nitrification–denitrification ultimately converts valuable nitrogen into nitrogen gas (N2), which is eliminated from the local agricultural cycle.
Mineral nitrogen nutrient production requires significant amounts of energy. Up to 37.4 GJ is needed for the production of 1 t of ammonia (Fertilizers Europe 2009). Prices for mineral fertilizers are increasing, whereas nutrient resources, such as phosphorous and potassium, are depleting (Öborn et al. 2005; Ruddock et al. 2003; Smit et al. 2009; Vilalba et al. 2008). It has therefore become an important challenge to recycle valuable nutrients in waste streams in a sustainable and environmentally friendly manner. In this context membrane filtration technologies are of increasing interest. Membrane filtration potentially may be used to separate nutrients from the liquid digestate in easily transportable and usable concentrates that can be applied when and where needed, according to plant requirements for optimum growth and contamination vulnerability of the agricultural site (Kertesz et al. 2010; Masse et al. 2007). These concentrates may thus be re-used as valuable inorganic fertilizers with high nutrient availability, providing a sustainable substitute for fossil-based mineral fertilizers. Moreover, selective reversed osmosis (RO) membranes (1 nm pore size) can also produce water of relatively high quality that could be discharged or re-used (Gagliardo et al. 1998; Roeper et al. 2007).
In spite of all its benefits, traditional membrane technologies often experience technical problems for waste stream treatment, mainly caused by membrane fouling and clogging (Masse et al. 2007). Membrane fouling is characterized by a decline in flux, due to the deposition and accumulation of materials on the membrane surface or within the pore structure (Cheryan 1998). In its strictest sense, fouling causes an irreversible flux decline, which can only be restored by thermo-chemical cleaning, if it can be recovered at all. In short-term studies, clean water flux could always be recovered following intensive acidic and alkaline cleaning (Bilstad et al. 1992).
Atkinson (2005) and Johnson et al. (2004), from New Logic Research, reported on the use of vibrating shear enhanced processing (VSEP) for manure purification. The system uses vibrating (60–90 Hz) RO membranes to minimize flux reduction due to concentration polarization and membrane fouling (Kertesz et al. 2010). As such the VSEP technology has the potential to make it technically feasible to convert digestate into dischargeable water according to the Flemish legislation for discharge into surface waters (15 mg N l−1, 2 mg P l−1 and 125 mg COD l−1).
This paper concerns the fate of macronutrients (N, P, K, Na, Ca, Mg) in the treatment process of the liquid fraction of digestate, produced by co-digestion of animal manure, energy maize and residues from the food industry, using vibrating reversed osmosis to reduce their concentrations down to dischargeable/re-usable water. To this end, process streams have been characterized and mass balances throughout the treatment process were set up. First, the potential of VSEP technology to transform the liquid fraction of digestate into dischargeable/re-usable water is evaluated. Next, the prospects for re-using nutrient rich VSEP concentrates in a sustainable cradle-to-cradle concept are explored and evaluated.
2 Material and Methods
2.1 Site Description and Experimental Setup
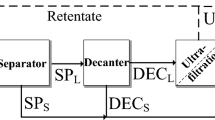
The test site is a pilot scale biogas plant (3,545 kWel) located in Diksmuide, Belgium. It concerns an anaerobic digester with an influent feed consisting of animal manure, energy maize and residues from the food industry with a total capacity of 12,000 t year−1 of fresh weight (FW). The digestate treatment process, operational since October 2007, is schematically represented in Fig. 1. The digestate (10% DW) is first separated in a liquid and thick fraction (19 ± 3% DW) using a rotating drum, after adding polymer solution. The resulting thick fraction is then guided to a screw press for further dewatering, followed by a dryer, in order to obtain an exportable end-product at 76 ± 1% DW. The liquid fraction is filtrated twice by a VSEP using RO membranes. Each filtration step results in a concentrate and permeate flow. The permeate produced by the second filtration should meet the Flemish legislation criteria for discharge into surface waters.
Total daily incoming feed volume to the VSEP for the first filtration is 50 m3 (Fig. 1). At an operational time of 12 h day−1, this results in a feed flow of 4.2 m3 h−1. The feed includes liquid fraction produced by the rotating drum (2.3 m3 h−1), recycled concentrate from the second membrane filtration step (0.50 m3 h−1), washing water from the rotating drum (0.50 m3 h−1) and cleaning water for the VSEP (0.80 m3 h−1). The membrane recovery rate is 80%, thus resulting in a permeate flow of 40 m3 day−1 and a concentrate flow of 10 m3 day−1 produced by the first filtration. The permeate (40 m3 day−1) is then forwarded towards the VSEP for the second filtration. At an operational time of 6 h day−1, this results in a feed flow of 6.7 m3 h−1. The second filtration, with membrane recovery rate of 85%, produces a permeate flow of 34 m3 day−1 and a concentrate flow of 6.0 m3 day−1.
Because the VSEP permeate is warm (45°C) and biologically inactive, it cannot be discharged in surface waters as such. It is guided to a lagoon for cooling, biological reactivation and further water polishing. The lagoon consists of two compartments (width: 12 m, length: 21 m). The first compartment (depth: 2.5 m) is mechanically aerated in order to cool down the water and to provide oxygen for biological processes. In this compartment NH4-N is converted into NO3-N (nitrification). The second compartment (depth: 1 m) is half-filled with porous lava stones and has a low water flow velocity. This allows the rooting of different macrophyte species, such as the marsh marigold, which take up nutrients for growth. Also, in this compartment NO3-N is converted into N2 (denitrification), while organic matter is microbiologically degraded. The lagoon thus serves as a buffer zone where further biological purification, as well as natural purification by dilution with rainwater, of the VSEP permeate occurs.
In 2008, samples of the process streams were taken during two sampling campaigns (August–September), and physico-chemically analyzed. During each sampling event, two homogenized samples (10 l each) were taken of the different process streams on a different time of the day (=total of four samples per stream). The samples were collected in polyethylene sampling buckets and transported within 1 h from the test site to the laboratory, carried in cooler boxes filled with ice. In the laboratory, the four replicate samples were stored cool (1–5°C) and kept separate for replicate analysis. Each sample was analyzed twice in order to detect the precision of the analytical method. The following process flows were sampled (Fig. 1): raw digestate (1), thick (2) and liquid (3) fraction produced by the rotating drum, polymer solution (4), thick (5) and liquid (6) fraction produced by the screw press, permeate (7) and concentrate (8) produced by the first filtration step, permeate (9) and concentrate (10) produced by the second filtration step, and finally the exportable end-product (11). Moreover, the contents of nitrogen and phosphorous, as well as the chemical oxygen demand (COD) in the second compartment of the lagoon following membrane filtration were daily monitored at the test site during the experimental period (2 months).
2.2 Liquid Sample Analysis
Conductivity and pH were determined potentiometrically using a WTW F537 conductivity electrode (Wissenschaftlich Technischen Werkstäten, Weilcheim, Germany) and an Orion 520A pH meter (Orion Research, Boston, VS), respectively. Total nitrogen content was determined using a Kjeltec system 1002 distilling unit (Gerhardt Vapodest, Köningswinter, Germany) after digestion of the sample in a sulphuric–salicylic acid mixture. Finally, the captured ammonia in the distillate was titrated with 0.01 mol HCl l−1 in the presence of a methyl red bromocresol green mixed indicator (Van Ranst et al. 1999). Total phosphorous content was determined using the colorimetric method of Scheel (Van Ranst et al. 1999) after wet digestion of the liquid samples (2.5 g sample + 2 ml HNO3 + 1 ml H2O2). The absorbance at 700 nm of samples and standards was determined using a Jenway 6400 spectrophotometer (Barloworld Scientific T/As Jenway, Felsted, UK). Calcium and magnesium were analyzed using ICP-OES (Varian Vista MPX, Palo Alto, CA, USA) after wet digestion (see above). Sodium and potassium of the digested samples (see above) were analyzed using a flame photometer (Eppendorf ELEX6361, Hamburg, Germany). The COD was determined photometrically using Dr. Lange standardized cuvette tests (Dr. Bruno Lange GmbH, Düsseldorf, Germany).
2.3 Thick Sample Analysis
Dry weight content was determined as residual weight after 48 h drying at 100°C. Conductivity and pH were measured using a WTW F537 conductivity electrode (Wissenschaftlich Technischen Werkstäten, Weilcheim, Germany) and an Orion 520A pH meter (Orion Research, Boston, VS), respectively, after equilibration for 1 h in deionized water at a 5:1 liquid/dry sample ratio and subsequent filtering (white ribbon, MN 640 m, Macherey–Nagel, Düren, Germany). Total nitrogen was determined using the Kjeldahl procedure (Van Ranst et al. 1999). For the determination of phosphorous, dry samples were incinerated at 450°C during 4 h in a furnace (Nabertherm, Lilientahl, Germany). The phosphorous content was then determined by the colorimetric method of Scheel (Van Ranst et al. 1999) after digestion of the residual ash (1 g ash + 5 ml 3 mol HNO3 l−1 + 5 ml 6 mol HNO3 l−1). Calcium and magnesium of the digested samples (see above) were analyzed by means of ICP-OES (Varian Vista MPX). Sodium and potassium of the digested samples (see above) were determined using a flame photometer (Eppendorf ELEX6361, Hamburg, Germany).
3 Results
3.1 Physico-chemical Characterization of Process Flows
Average macronutrient contents (±standard deviations of the replicates) in the different process flows were analyzed (Table 1). It is clear that the permeate produced by one filtration step did not meet the Flemish discharge legislation criteria of 15 mg N l−1 and 2 mg P l−1. Nitrogen and phosphorous contents in the VSEP permeate produced by the second filtration were low, although average concentrations were not below the discharge criteria. However, in the subsequent lagoon the average concentrations for nitrogen and phosphorous based on daily monitoring during the experimental period were 12 ± 6 mg N l−1 and 1.6 ± 1.0 mg P l−1, respectively, and thus met the discharge criteria. Furthermore, it was observed that the COD in the VSEP permeates can reach high peaks related to the addition of citric acid (C6H8O7) during acidic cleaning events. Nevertheless, the COD in the lagoon (26 ± 10 mg COD l−1) was constantly below the Flemish discharge level of 125 mg COD l−1 due to microbial breakdown of the organic matter and dilution with rainwater. Finally, it was observed that average concentrations of calcium, magnesium and sodium in the permeate produced by the second filtration step were very low, in agreement with the low salt content (0.56 g salt kg−1 FW or 0.88 mS cm−1) and total hardness (0.19 ± 0.12 D°H) of this process flow.
3.2 Mass Balances
Figures 2, 3 and 4 exhibit the mass balances of the process for nitrogen and phosphorous, potassium and sodium, and calcium and magnesium, respectively. The volumetric flow rates (m3 h−1) can be found in Fig. 1. It should be remarked that the flow rate of some streams (3, 7 and 10) change during the process. As a first step in the process, the incoming mass flow to the rotating drum is determined mainly by the raw digestate produced by the anaerobic digester. Also the liquid fraction produced by the subsequent screw press is recycled to the rotating drum. Polyelectrolyte was used to improve the separation efficiency. The resulting thick fraction is further dewatered by the screw press, and dried to an exportable end-product. The separated liquid mass flow contained more nitrogen, potassium and sodium than the corresponding thick flow. Reversely, the thick mass flow was richer in phosphorous, calcium and magnesium. The liquid fraction produced by the rotating drum enters the VSEP filtration system. As expected, most of the macronutrients after the first filtration step ended up in the concentrate. The permeate produced by the first VSEP filtration step is submitted to a second filtration. The concentrate produced by this second filtration is recycled to the VSEP for the first filtration step. During the sampling period, the permeate produced by the second filtration did not continuously meet the Flemish legislation criteria for discharge into surface waters. It was guided to a lagoon for further biological and natural purification, as well as for cooling.
4 Discussion
4.1 Mass Balance Equilibrium
Total incoming and outgoing mass flows to each particular system component are approximately equal (Figs. 2, 3 and 4), showing that the mass balances are roughly in equilibrium. Small deviations can be caused by the accuracy and precision of the used physico-chemical laboratory protocols. Larger deviations can also be caused by biological activity or physico-chemical reactions. This can occur, for example when nitrogen escapes from the system as nitrogen gas or ammonia, or forms ammonium sulfate by reaction with sulfuric acid or H2S. Nitrogen losses to air are an important issue in manure and digestate processing. Based on the observed data, it is estimated that the average nitrogen losses in the rotating drum, the screw press and the dryer are 0.85%, 0.44%, and 5.0%, respectively, resulting in a total nitrogen loss of 6.3% over these process steps. The released ammonia is captured in an acidic air scrubber, thereby producing ammonium sulfate as a waste stream. This product could potentially be re-used as green fertilizer in agriculture in order to close the nitrogen cycle. On the contrary, it was observed that for the VSEP system total outgoing mass flows can be larger than total incoming flows. This is related to the fact that sludge from previous filtration steps is retained on the membrane surface and can end up in concentrates produced by subsequent filtrations. Finally, the use of washing water can also cause mass balance deviations, for example when the rotating drum is cleaned with permeate produced by the 2nd filtration step.
4.2 Digestate Pretreatment
In general, digestate processing tends to be limited to an initial separation and/or dewatering step, producing a liquid and thick fraction with different macronutrient contents (Hjorth et al. 2010). In this particular case, the initial separation occurred using a rotating drum after adding polymer solution, followed by a screw press for further dewatering of the resulting thick fraction. As expected (Hjorth et al. 2010), most of the phosphorous (91%), calcium (96%) and magnesium (92%) was recovered in the thick fraction, which can be dried to an exportable, organic soil conditioner. In contrast, most of the nitrogen (57%), potassium (78%) and sodium (72%) ended up in the liquid fraction. The VSEP system aims to separate these valuable macronutrients into easily transportable concentrates, producing permeates low in nutrient contents that meet the Flemish legislation criteria for discharge into surface waters.
4.3 VSEP Performance in Water Treatment of Digestate
Monitoring results for the first VSEP filtration step show average removal efficiencies of 93% for nitrogen present in the total incoming feed and 59% for phosphorous, which are insufficient to achieve the Flemish discharge criteria (15 and 2 mg l−1, respectively). Yet, in this study nitrogen removal was higher than that reported by Johnson et al. (2004) for hog manure (79%), while phosphorous removal was less (86%). Forwarding the permeate to a second VSEP filtration step resulted in a total nitrogen and phosphorous removal of 95% and 69%, respectively, which is still not sufficient to meet the discharge criteria. Also, the COD in the produced permeates was often too high for discharge in surface waters due to intensive cleaning events with citric acid (C6H8O7). Further purification in the lagoon through microbiological nitrification–denitrification, nutrient accumulation, plant nutrient uptake (autotrophic photosynthesis) and dilution with rainwater, allowed to improve the water quality to the standards for dischargeable water. However, due to technical and mechanical problems, the VSEP performance was instable and legislation levels were also frequently exceeded in the lagoon. Moreover, during the nitrification–denitrification process in the lagoon, nitrogen gas (N2) is released in the atmosphere and eliminated from the local agricultural cycle. It is therefore advised to further optimize VSEP process parameters, such as vibration frequency and amplitude, filtration time, pH and temperature, as well as condition and pre-filtration of the feed (Frappart et al. 2008; Johnson et al. 2004; Petala and Zouboulis 2006). Johnson et al. (2004) and Masse et al. (2007) found that pH and temperature have significant effects on the ammonia–ammonium equilibrium and thus on the removal efficiency of nitrogen from manure wastewater by VSEP filtration systems. In this context, also the membrane type is of particular importance. During the anaerobic digestion most of the nitrogen is transformed into positively charged ammonium, which is better retained using negatively charged membranes.
There exist no discharge criteria for potassium, sodium, calcium and magnesium, though regarding future re-use perspectives these elements are of particular interest. Results show that both salt content (0.88 mS cm−1 or 0.56 g salt kg−1 FW) and total hardness (0.19 ± 0.12 D°H) in the produced permeates were low, making it a valuable source of high quality water that could potentially be re-used, for example as drinking water or process water. RO membranes have also been evaluated positively in the past for the elimination of viruses and bacteria from waste water streams (Gagliardo et al. 1998; Roeper et al. 2007; Tam et al. 2007). Regarding worldwide increasing scarcity of water resources and augmenting prices of tap water (€2 m−3; Flemish Water Supply Company VMW 2009, personal communication), it is an important challenge, economically as well as ecologically, to maximally recuperate this high quality water source in a sustainable cradle-to-cradle approach. In addition, water re-use could turn out in economic benefits for anaerobic digestion plants, thereby stimulating the further development of this bio-energy technology in Flanders.
Compared to other membrane filtration systems, previous studies have shown that the gel layer is much lower in the case of the VSEP, because of the high shear-enhanced forces on the membrane surface during the experiments (Bian et al. 2000; Culkin et al. 1998; Johnson et al. 2004; Wei and Mark 2008). Though there are currently several VSEP’s in operation for agricultural wastewater treatment (New Logic Research Inc. 2008), there are little data available on the energy consumption and treatment costs of this technology. Akoum et al. (2005) reported on the potential energy saving of the vibratory concept. When 61-cm-diameter membranes are used, a total of 151 m2 can be installed on a single shaft (VSEP series i-10). The energy consumed per vibration is then 8.83 kW (G. Johnson, New Logic Research Inc., 2003, personal communication), as it is not much affected by the number of compartments. Energy consumed by the feed pump is also small as its flow rate does not need to be much larger than the permeate flow rate. They estimated the energy consumed by the recirculation pump at 9.4 kW h m−3 of permeate in a 154-m2 membrane area unit. This could be reduced to 6 kW h m−3 if plane ceramic membranes were used. Energetic calculations based on these data indicate that large VSEP units will consume significantly less energy per m3 of permeate than traditional cross-flow filtration. Nevertheless, energy consumption and economic performance remain critical points of attention in evaluating membrane technology.
4.4 Agricultural and Economic Value of Concentrates
Membrane technology allows handlers to concentrate nutrients recovered in the liquid fraction of digestate in a small volume that can be transported to agricultural fields. Concentrates produced by the first membrane filtration step could potentially be re-used as inorganic fertilizers, rich in nitrogen and potassium. The nitrogen content was 7.3 ± 1.6 kg N t−1 FW, comparable to that of conventional pig manure (5–10 kg N t−1 FW; Lemmens et al. 2007). The potassium content was 3.5 ± 0.0 kg K2O t−1 FW, which is lower than predicted literature data (6–12 kg K2O t−1 FW; Melse and Verdoes 2002), but slightly higher than that of conventional pig manure (3.3 kg K2O t−1 FW; Lemmens et al. 2007). As expected, the amount of phosphorous in the concentrates was rather low, because most of the phosphorous ends up in the separated thick fraction during the pretreatment. Regarding the phosphorous restrictions that become more and more stringent in high-nutrient regions, the use of this phosphorous-poor fertilizer could benefit important advantages. Concentrates produced by the second membrane filtration step were poor in macronutrients and have therefore little/no potential for re-use as a fertilizer. This flow is currently recycled within the process.
Although potassium is an important element for crop production, high ratios of potassium over nitrogen and phosphorous are not preferred in every agricultural sector. In particular, livestock farmers rather use potassium-poor fertilizers, because of the potential health risks for cattle (head illness) at high potassium fertilization (>50 t ha−1 year−1; Hillel 2008; Romheld and Kirkby 2010). Also, high ratios of monovalent cations, such as K and Na, to divalent bases, such as Ca and Mg, may cause degradation of the soil structure, especially when soils are rich in clay (USEPA 2004). Hence, depending on the composition of the base fertilizer and the soil characteristics, more or less concentrate can be applied as mineral fertilizer, with a maximum advised dose of 70 kg K2O ha−1 year−1 (Hillel 2008; Romheld and Kirkby 2010). Furthermore, concentrates produced by the first membrane filtration could have higher salt contents (66 mS cm−1) compared to conventional animal manure (30–50 mS cm−1; Moral et al. 2008). This results in high salt/N ratios (±6) for this product. Too high salt contents can cause soil degradation and can dramatically reduce crop production (Verlinden 2005). Therefore, when using concentrates in agriculture, it will also be important to pay attention to the salt doses per unit nitrogen that is applied to the soil. Extensive greenhouse and field testing will be required to investigate the impact of concentrates on soil and crop production.
Next to the potential ecological benefits, re-use of concentrates as a green fertilizer and/or soil conditioner in agriculture could also result in significant economic benefits. Nowadays, the anaerobic digestion plant has to pay high disposal or treatment costs for the off-set of the produced concentrates. In the meantime, prices for artificial mineral fertilizers are increasing and nutrient resources are depleting (Öborn et al. 2005; Ruddock et al. 2003; Smit et al. 2009; Vilalba et al. 2008). Re-use of valuable nutrients coming from digestate processing could therefore also convert the digestate problem into an economic opportunity.
The economic value of concentrates is calculated based on the current cost price for fossil based artificial fertilizers/soil conditioners (Table 2). The application of concentrates in agriculture could have a value of €6.3 ± 1.1 t−1 FW, if both nitrogen and potassium are appreciated by the agriculturist. If only nitrogen is appreciated, the economic value is €4.5 ± 1.0 t−1 FW, whereas it amounts to €1.8 ± 0.1 t−1 FW if only potassium is of relevance. Unlike as mineral fertilizers, these concentrates could also contain significant amounts of organic carbon (24 ± 1%). Application of concentrates could therefore also have additional values in organic carbon recycling.
4.5 Conclusions and Future Perspectives
The performance of the VSEP filtration system technically and mechanically proved not yet satisfactory to allow for a reliable, continuous operation. Further technical/mechanical optimization of the process is now ongoing in order to implement the VSEP system in full-scale installations.
One VSEP filtration step resulted in an average removal of 93% N and 59% P, which was not sufficient to achieve the Flemish legal discharge criteria of 15 mg N l−1 and 2 mg P l−1. A second VSEP filtration step allowed handlers to achieve a total average removal efficiency of 95% N and 69% P, which was still not sufficient to meet the discharge criteria. A subsequent treatment in an aerated lagoon allowed operators to produce dischargeable water. However, also in the lagoon, the discharge criteria were regularly exceeded due to instability of the VSEP performance. Optimization of process parameters, such as membrane type, pH, temperature, as well as condition and prefiltration of the feed, is therefore advised. On the upside, salt content and total hardness in the permeate of the second VSEP filtration step were low, indicating that it could potentially be a water source for re-use in high quality applications.
Concentrates produced by the first VSEP filtration step were rich in macronutrients and could potentially be re-used as a sustainable substitute for fossil-based mineral fertilizers. However, pot and field experiments are required to evaluate its impact on plant growth and soil quality. Re-use of nutrient rich concentrates produced by VSEP membrane filtration in a sustainable cradle-to-cradle approach, might so benefit the economic performance of anaerobic digestion in Flanders, thereby stimulating the production of bio-energy in frame of the 2020 objectives.
The VSEP filtration system has potential for use in conversion of the liquid fraction of digestates into dischargeable/re-usable water and green fertilizers, although further optimization and testing in full-scale installations is required.
References
Akoum, O., Jaffrin, M. Y., & Ding, L. H. (2005). Concentration of total milk proteins by high shear ultrafiltration in a vibrating membrane module. Journal of Membrane Science, 247(1–2), 211–220.
Atkinson, S. (2005). Vibratory membrane filtration system treats hog manure. Membrane Technology, 2005(1), 10–11.
Bian, R., Yamamoto, K., & Watanabe, Y. (2000). The effect of shear rate on controlling the concentration polarization and membrane fouling. Proceedings of the Conference on Membranes in Drinking and Industrial Water Production, 1, 421–432.
Bilstad, T., Madland, M., Espedal, E., & Hanssen, P. H. (1992). Membrane separation of raw and anaerobically digested pig manure. Water Science and Technology, 25, 19–26.
Cheryan, M. (1998). Ultrafiltration and microfiltration handbook. Lancaster: Technomic Pub.
Culkin, B., Plotkin, A., & Monroe, M. (1998). Solve membrane fouling problems with high-shear filtration. Chemical Engineering Progress, 94(1), 29–33.
Europe, F. (2009). Forecast of food, farming and fertilizer use in the European Union 2009–2019. Brussels: European Fertilizer Manufacturers Association.
FEA. (2010). Prognose on renewable energy for 2020. Brussels: Flemish Energy Agency.
Fehrenbach, H., Giegrich, J., Reinhardt, G., Schmitz, J., Sayer, U., Gretz, M., et al. (2008). Criteria for a sustainable use of bio-energy on a global scale. Germany: Federal Environment Agency.
Frappart, M., Jaffrin, M. Y., Ding, L. H., & Espina, V. (2008). Effect of vibration frequency and membrane shear rate on nanofiltration of diluted milk, using a vibratory dynamic filtration system. Separation and Purification Technology, 62(1), 212–221.
Gagliardo, P., Samer, A., Rodes, T., & Adam, O. (1998). Water repurification via reversed osmosis. Desalination, 117(1–3), 73–83.
Hillel, D. (2008). Soil in the environment. Crucible of terrestrial life. New York: Academia Press.
Hjorth, M., Christensen, K. V., Christensen, M. L., & Sommer, S. G. (2010). Solid–liquid separation of animal slurry in theory and practice. A review. Agronomy for Sustainable Development, 30, 153–180.
Johnson, G., Culkin, B., & Stowell, L. (2004). Membrane filtration of manure wastewater. A comparison of conventional treatment methods and VSEP, a vibratory RO membrane system. Emeryville: New Logic Research.
Kertesz, S., Beszedes, S., Laszlo, Z., Szabo, G., & Hodur, C. (2010). Nanofiltration and reverse osmosis of pig manure: comparison of results from vibratory and classical modules. Desalination and Water Treatment, 14(1–3), 233–238.
Lemmens, E., Ceulemans, J., Elslander, H., Vanassche, S., Brauns, E., & Vrancken, K. (2007). Best available techniques (BAT) for animal manure processing. Ghent: Academia Press.
Masse, L., Masse, D. I., & Pellerin, Y. (2007). The use of membranes for the treatment of manure: a critical review. Biosystems Engineering, 98(4), 371–380.
Melse, R., & Verdoes, N. (2002). In dierlijke mest is ook Kalium van belang. Varkens, 16, 18–19.
Mira-T. (2010). Flanders Environment Report: Indicator Report. Erembodegem: Flemish Environment Agency.
Moral, R., Perez-Espiosa, A., Moreno-Casselles, J., Paredes, C., & Rufete, B. (2008). Salinity, organic content, micronutrients and heavy metals in pig slurries from southeastern Spain. Waste Management, 28(2), 367–371.
New Logic Research Inc. (2008). Membrane filtration of hog manure: A cost-effective and environmentally sound solution. Emeryville: New Logic Research Inc.
Öborn, I., Andrist-Rangel, Y., Askekaard, M., Grant, C., Watson, C., & Edwards, A. (2005). Critical aspects of potassium management in agricultural systems. Soil Use and Management, 21, 102–112.
Petala, M. D., & Zouboulis, A. I. (2006). Vibratory shear enhanced processing membrane filtration applies for the removal of natural organic matter from surface waters. Journal of Membrane Science, 269(1–2), 1–14.
Roeper, H., Bade, O., Streese-Kleeberg, J., & Stegman, R. (2007). Integrated concept for decentralized wastewater and biowaste treatment—Experiences at the pilot plant stage. In Eleventh International Waste Management and Landfill Symposium. Italy: CISA Publisher.
Romheld, V., & Kirkby, E. A. (2010). Research on potassium in agriculture: needs and prospects. Plant and Soil, 335(1–2), 155–180.
Ruddock, J., Short, T. D., & Brudenell, K. (2003). Energy integration in ammonia production. Energy and the Environment, Sustainable World, 7, 267–276.
Smit, A. L., Bindraban, P. S., Schröder, J. J., Conijn, J. G., & Van Der Meer, H. G. (2009). Phosphorous in agriculture: Global resources, trends and developments. Plant Research International: Wageningen.
Tam, L. S., Tang, T. W., Lau, G. N., Sharma, K. N., & Chen, G. H. (2007). A pilot study for wastewater reclamation and reuse with MBR/RO and MF/RO systems. Desalination, 202(1–3), 106–113.
USEPA. (2004). Guidelines for water reuse. Cincinnati: United States Environmental Protection Agency.
Van Ranst, E., Verloo, M., Demeyer, A., & Pauwels, J. M. (1999). Manual for the soil chemistry and fertility laboratory: Analytical methods for soils and plants, equipment and management of consumables. Ghent: Faculty of Agricultural and Applied Biological Sciences.
Verlinden, G. (2005). Valorisation of effluents from manure processing. Heverlee: Belgian Service for Soil Science.
Vilalba, G., Liu, Y., Schroder, H., & Ayres, R. U. (2008). Global phosphorous flows in the industrial economy from a production perspective. Journal of Industrial Ecology, 12(4), 557–569.
Wei, S., & Mark, M. B. (2008). Fouling of RO membranes in a vibratory shear enhanced filtration process (VSEP) system. Journal of Membrane Science, 331(1–2), 11–20.
Acknowledgements
The authors thank Mr. J. Neri (University of Ghent) for laboratory assistance and Mr. F. Vanden Abeele (Eneco Energy) for technical assistance in the pilot plant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaneeckhaute, C., Meers, E., Michels, E. et al. Fate of Macronutrients in Water Treatment of Digestate Using Vibrating Reversed Osmosis. Water Air Soil Pollut 223, 1593–1603 (2012). https://doi.org/10.1007/s11270-011-0967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0967-6