Abstract
This study investigates removal of total nitrogen (TN) in moving bed biofilm reactor (MBBR) supported with high-density polyethylene for biofilm formation and ibuprofen (IBU) as a carbon source. At first, the microorganisms have been acclimated for 45 days. In the optimum condition, TN removal of more than 80 % was reached. Optimization results of simultaneous removal of IBU and TN showed that the obtained removal efficiencies for IBU and TN are close together and the correlation coefficients have high values. The obtained results show that MBBR bioreactor could remove 72.03 % IBU and 81.1 % TN at 145.15 h and TN concentration of 156.37 mg/L. Biodegradation constant (k biol) values were varying from 0.4 to 0.009 L/g biomass.d, which represents that IBU is a hard biodegradable or persistent substance. This study demonstrated that the proposed MBBR is highly effective for the simultaneous removal of IBU and TN in wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fertilizers, industrial effluents, and municipal wastewater are main sources of nitrate and organic compounds in the water and wastewater (Hoseinzadeh et al. 2015). In recent years, the presence of pharmaceuticals in aqueous solution and water bodies has been increased (Kruglova et al. 2014). By releasing pharmaceuticals to the environment because of their harmful effects, they could have remark effects on human health and the ecosystems. In the case of antibiotics, their presence in the aquatic environment can lead to antibiotic resistance in pathogenic bacteria. Pharmaceutical compounds can enter in the aquatic environment from manufacturing sites and excretory of hospitals and households (Sarasidis et al. 2014; Paíga et al. 2013). One of the most widely used classes of pharmaceuticals is non-steroidal anti-inflammatory drugs (NAIDs) (Kruglova et al. 2014; Sarasidis et al. 2014). They are drugs for pain mitigation and fever reducing. The most common NSAIDs are aspirin, ibuprofen (IBU), and naproxen. Unfortunately, in some countries, they are available in a large number without any prescription (Langenhoff et al. 2013). IBU is a NSAID and one of the “essential drugs” listed by World Health Organization (WHO), which is especially prescribed in case of fever reducing, and mitigation of migraine, muscle aches, arthritis, and tooth aches (Langenhoff et al. 2013; Kruglova et al. 2014). Different dosage formulations and forms of IBU are available and consumed in large quantities. According to the available reports, the annual world production of IBU is several kilotons. Some parts of IBU are rejected to the effluents or discarded as a waste. Also, the IBU could be excreted from the body of patients in a non-consumed form or as metabolites caused by human biodegradation (Falås et al. 2013). Entrance of IBU in the environment could have negative effects on reproduction of vertebrates and invertebrates and growth of bacterium, fungus, and human fetal cells. According to the available scientific reports, it is genotoxic for fish by accumulation in fatty tissues (Bendz et al. 2005; Jeffries et al. 2015). Also, IBU could have harmful effects on aquatic and terrestrial environments (Bendz et al. 2005). Various treatment techniques have been proposed for IBU removal in the aqueous environment such as photo-degradation, solar photodegradation, biological process, advanced oxidation processes, ultrafiltration/reverse osmosis and membrane base techniques, polymeric adsorbents, membrane bioreactors (MBRs), and gamma irradiation (Rivera-Utrilla et al. 2013; Ambuludi et al. 2013; Dâas and Hamdaoui 2014; Singh et al. 2012; Garcia-Rodríguez et al. 2014; Matamoros et al. 2009; D. Camacho-Muñoz et al. 2012; Ifelebuegu and Ezenwa 2011; Tijani et al. 2013). However, there are some drawbacks as a result of the buildup of dead end products, non-selectivity for target contaminants, and high treatment cost (Rivera-Utrilla et al. 2013). On the other hand, nitrogen compound such as nitrate and nitrite ions with manmade origin are one of the main water pollutants. There are many industries that produce wastewater with high level of nitrate and organic compounds, simultaneously. Coexistence of both IBU and total nitrogen (TN) in wastewater or water could have harmful effects on human and environmental health. Nitrate pollution has various harmful effects on human such as cancer, methemoglobinemia, or blue baby syndrome and harmful effects on environments by creating eutrophication (Hossini et al. 2015). Many different processes have been proposed for total nitrogen and organic compound removal, simultaneously. The biological process is an interesting technique, because it has high effectiveness and is an environmentally friendly method. Generally, in biological removal of nitrogen, an external carbon source is needed to add in wastewater (Luo et al. 2014; Sun et al. 2014; Wu et al. 2012). If wastewaters be contain organic compounds any external carbon source in need to do the process. Additionally, nitrogen and organic carbon removal will be performed using single step and plant. This approach is accepted economically and technically. There are few scientific reports in biological nitrogen removal using low biodegradable compound such as IBU. In this study, we have proposed a moving bed biological reactor (MBBR) for IBU and TN removal in wastewater. MBBR is an integration of activated sludge and trickling filters, in which microbial biomass exists in the form of suspended and attached form (Luo et al. 2014). MBBR has low sensitivity to hydraulic and organic extra-loading. This process could be applied for the removal of organic substances as well as nitrification and denitrification at a single stage or multi-stage (Chu and Wang 2011). According to the scientific reports, MBBR has a potential for removal of aromatic and pharmaceutical compounds from wastewater in laboratory and full scales. Luo et al. (2014) reported a batch sponge-based moving bed bioreactor for removal of micropollutants. Their study showed that acclimatized sponge could have high potential for IBU removal, because of its hydrophobic properties. The micropollutant removal potential of activated sludge and biofilm carriers was studied by Falås et al. (2012) in a batch scale. Both activated sludge and MBBR demonstrated similar removal rate constants for ibuprofen removal (around 2–5 L/g biomass.d). The novelty of the present study is to use a hard biodegradable and toxic organic compound as an organic carbon source for biological removal of total nitrogen. Therefore, this study was aimed to investigate the effectiveness of a MBBR with 2H-BCN carries for removing total nitrogen with IBU as an organic carbon source.
1.1 Experimental
1.1.1 Nitrifying and Denitrifying Bacteria Enrichment
Inoculum for nitrifying and denitrifying bacterial growth was collected from a wastewater treatment plant, Tehran, Iran. For enrichment of the bacteria, a synthetic medium was used as follows: 0.3 g/L KH2PO4, 1 g/L Na2HPO4·12H2O, 0.5 g/L NaCl, 2 g/L NaHCO3, and 0.1 g/L MgSO4·7H2O in tap water. The carbon source for denitrifying bacteria was IBU that considered according to the required carbon/nitrogen (C/N) in influent. NH4 + was added to the bioreactor (100– 200 mg/L). All used chemicals in the present study were in analytical reagent grade without further purification.
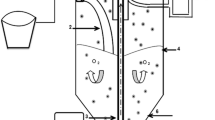
1.1.2 Experimental setup
A Plexiglas column was used (32 cm height × 10 cm internal diameter). Airflow rate was maintained at approximately 1.8 L/min. Peristaltic pumps (Hidolph, Model 5201) with the flow of 0.1 L/min were used for feeding and its discharge. In start-up time, 2H medium (Germany) was added to the reactor and nitrifying and denitrifying bacteria were inoculated. Characteristics of the 2H media are shown in Table 1. The MBBR bioreactor contained a mixed microbial culture both as nitrifying and denitrifying bacteria; those exist as suspended biomass and biofilm attached to the carrier media. Over 10 days, effluent of the bioreactor had very low suspended film, indicating that the biofilm had formed successfully. Feeding containing ammonium was maintained at 60 mg/L. When the ammonium and nitrate elimination reached more than 80 %, the bioreactor start-up was completed. In the total nitrogen removal study till reaching the pseudo steady state condition, the experimental conditions (hydraulic retention time (HRT), C/N) for each experiment were maintained. Each sample was taken for at least three successive HRTs. The experiments were operated at room temperature (25 ± 2 °C).
1.1.3 Experimental Design and Modeling
A Design-Expert regression program (Design-Expert 7.0) was applied for determining of the response function coefficients for different dependent variables. Central composite design (CCD) was used to present the model as a specific design. The CCD of the main parameters (x 1, IBU concentrations; and x 2, hydraulic retention time (HRT)) has been presented in Table 2. According to the proposed design by the software, 13 experiments were conducted (a 4 (22) factorial design, 4 (2×2) axial points with 5 central points). After the main experiments were conducted, the experiments were repeated followed by the order of experiments.
The coefficients of the polynomial model were calculated using the following equation and experiments data:
where Y is the considered response, β i , β 1, β ii , and β ij are the coefficients of regression, ε is the error value of the system, and x j is the coded variables.
Three-dimensional (3D) plots and their respective contour plots were obtained based on the effect of the levels of two factors. Simultaneous interaction of the two factors on the responses was evaluated by 3D diagram.
1.1.4 Analytical Methods
The samples were taken from the reactor using a 10-mL syringe. Ammonia and nitrite were determined in accordance with the standard method (Urtiaga et al. 2013). The colorimetric method was applied according to phenate method at λmax = 640 nm. Nitrate was determined using a spectrophotometer (UV/Vis Spectrophotometer, Perkin-Elmer 550 SE, USA) at λmax 220 and 275 nm. IBU in analytical grade (99.6 %) was prepared from a local pharmaceutical industry (Sobhan Darou, Iran). A stock solution of IBU was prepared by dissolving the accurate weight of 100 mg ibuprofen in 15 mL methanol, and volume was made with distilled water. The contents of the flasks were mixed manually to ensure complete mixing. The final solution was stored at 4 °C in dark. Typical UV/Vis absorbance spectra of IBU were obtained by a UV/Vis spectrophotometer with 1-cm quartz cells and a scan rate of 1000 nm/min, and the slit width of 2 nm was used along with a blank solution applied as the reference. The UV spectra of the mixtures were recorded over the wavelength 200–400 nm with one data point per nanometer. IBU showed λmax values at 222 nm. The amount of residual IBU in samples was determined by the calibration curve at 222 nm. Also, pH (Hach, model HQ40d), oxidation/reduction potential (ORP meter Eutech, Singapore), and medium temperature were evaluated for each sample.
2 Results and Discussion
2.1 Bioreactor Start-up
The start-up period lasted for 45 days, and TN removal of more than 80 % was obtained. The IBU removal rate in two different concentrations is represented in Fig. 1 after 31 to 45 days. After this incubation period, high surface area available for the media resulted in the appearance of sufficient homologous biofilm growth on the media surface (Fig. 1a, b). However, homologous biofilm presence on the media could speed up the biochemical reactions. Accordingly, there was a slow removal rate of IBU in the first step, I. Acclimation of microorganisms to a micropollutant such as IBU to be used as the carbon source is a very important part of biodegradation (Langenhoff et al. 2013). After an initial lag time, the biodegradation rate of most micropollutants can increase. Start-up stage of the present study showed the lag time required for IBU in MBBR might be 168 h that was equal to or greater than 7 days of incubation period. However, these time periods were greater than the retention periods of conventional activated sludge wastewater treatment process for treating municipal wastewater.
During the next step (step II), a higher IBU removal rate or diapering was observed after 8 h. After that, IPU appeared at half initial concentration and then complete removal was obtained. However, representing of IBU at some time points occurred; but, the residual IBU was removed finally. On the other hand, IBU might be sorbed firstly to biosolids (in suspension and on the formed biofilm on the carrier) and returned to the solution due to suspended solids degradation or stayed attached to the solids in case of resistant biodegradable compounds (Falås et al. 2013; Langenhoff et al. 2013). Sorption is a more rapid process than biodegradation. To perform the acclimatization stage properly, sorption sites were fully occupied; therefore, it may result in limited sorption rate. Pharmaceutical drugs with low hydrophobicity (log D < 2.5) have negligible sorption on suspended biosolids; so, biodegradation is the dominant and major mechanism for its removal (Langenhoff et al. 2013). In MBBR, because of the presence of more varied microbial community and different sludge characteristics on and inside of the carrier, better biodegradation capacity is achievable compared to other systems. Table 3 presents IBU (as an organic compound) removal efficiencies, and treatment process in batch, laboratory, and full scale.
2.2 Effect of TN Concentrations and HRTs
TN removal efficiencies with the different influent concentrations of NH4 +-N/L in the presence of IBU are presented in Fig. 2a, b. Obtained results showed 85 % of TN removal and 100 % ammonia removal. TN had no unique removal trend against the HRT. By increasing HRT, decreasing of TN removal appeared. TN removal was achieved in nitrification and denitrification at two sequence stages. The low rate of removal as a result of increasing HRT is attributed to the effect of low residual dissolved oxygen concentration that is consumed for nitrification. Low residual dissolved oxygen leads to failure to do denitrification, or reversely, it may be due to less denitrification. Different ammonia residuals along with produced nitrite and nitrate at various intervals confirmed the issue (data not shown). The occurrence of nitrification and denitrification simultaneously and in a reactor can produce a variety of enzymes that help IBU removal or catalyze its biodegradation (Kruglova et al., 2014; Langenhoff et al., 2013) and may be a major biodegradation pathway for removal of IBU at the present study.
Thirteen runs were conducted to do optimization of simultaneous removal of IBU and TN. The predicted values were calculated by the final coded model equation (Eq. 2). With regard to the results, the response surface model for predicting IBU and nitrogen removal efficiency was considered reasonable. The final second-order polynomial regressions in terms of coded factor were represented by the following equations (Eqs. 2 and 3).
The obtained removal efficiencies for IBU and TN were close and the correlation coefficients had high values. R-square and adjusted R-square for IBU and TN removal were obtained as 0.95 and 0.91, and 0.98 and 0.97, respectively. The R 2 values indicated the reasonability of the experiments. ANOVA analysis results for regression parameters of predicted response surface quadratic and cubic models used to model of IBU and TN removal efficiency are given in Table 4. According to obtained results, the models’ F-value (IBU ~26.7 and TN ~53.19) and low probability values (P value = 0.0002) suggest that the models were significant. The lower probability value (P value <0.05) implies that the model terms were significant. Generally, the acceptability of the model can be evaluated by diagnostic plots, such as a normal probability plot of residuals and a plot of predicted versus actual values.
2.3 Utilization of IBU as an Organic Carbon Source at Different C/N Ratios
As Fig. 3 represents, influent IBU was down from 75 to 25.5 % of its initial concentrations with a C/N from 2 to 0.5. The result means that the IBU can be suitable for denitrification as a carbon source when the C/N ratio is 2. It may be possible when the C/N is lower than 2, the nitrifying bacteria or autotrophic denitrifying bacteria are dominant, and the organic carbon consumption decreased. When IBU utilization rate was 75 % (at C/N = 2), the IBU was not completely used by the bacteria. Furthermore, incomplete use of carbon source (IBU) has been occurred and it may be considerable in secondary treatment need.
2.4 Kinetic Study
Biodegradation rate of pollutants is classified in three classes based on the obtained k biol (Kruglova et al. 2014); if k biol is higher than 5 L/g ss.d, the compound is very highly biodegradable; if 1 < k biol < 5 L/g ss.d, the compound is highly biodegradable, while for 0.5 < k biol < 1 L/g ss.d and k biol < 0.5 L/g ss.d, the compound is fair and hard to be biodegraded, respectively. In a batch reactor, the relative quantity used for biodegradation rate constant k biol (L g MLSS−1 day−1) is calculated using the following equation:
where C 0 and C t are the initial and residual concentrations of IBU at time point t, respectively. SS is mixed liquor suspended solids (MLSS) of batch reactor at time t. By plotting ln(C 0/C t) against time, the reaction rate constant k’ (day−1) is obtained as the slope of the line. The calculated biodegradable constant as a result of the obtained removal rate of IBU is presented in Table 5 and Fig. 4.
The mean k biol obtained for IBU at the concentration below 100 mg/L was lower than 100 < concentration < 200 mg/L. In the study of Kruglova et al., the calculated k biol value for IBU at the concentration of 20 μg L−1 was 5.0 L/g ss.d, which was about 13 times higher than that in the present study. k biol was found from the experimental results to be varying from 0.4 to 0.009 L/g ss.d, which represents IBU as a low biodegradable or persistent substance in the studied treatment system. Lower values of k biol for the present study compared to others can be explained by a lower SRT period of our system. Furthermore, MLSS represents the food-to-microorganism ratio (F/M); hence, it can affect k’ and k biol. Lowe k biol in the present study may be due to the MLSS concentrations.
2.5 Effects of pH, EC, and ORP
As shown in Fig. 5a, pH values have no wide variation (±0.6 unit) throughout the experiments (pH = 8.18 ± 0.6). Nitrification and denitrification are able to change the pH value of the system; H+ producing in nitrification can decrease pH. Also, increasing pH may be as a result of denitrification and anammox deammunification (anammox bacteria can place inside the biofilm that not studied here). ORP was variable from −38 to +215 mV during the experiments. The ORP values indicate that the system had a large oxidation and reduction capacity, simultaneously. Decreasing oxygen supply or increasing TN loading leads to low oxidation potential. It results nitrification/denitrification taking place in the system, simultaneously. Linear correlation between ammonium and conductivity values was reported by Yang et al. (2011). No similar result was obtained at the present study. The EC showed almost stable value through the operation period (4.5 ± 1.2 mS/cm). Low rate oxygen supply may be the cause of EC and ORP decreasing.
2.6 Suggested Pathways of IBU Removal in the MBBR
Generally, three pathways of biodegradation, sorption, and air stripping might occur for removing IBU throughout MBBR treatment (Falås et al. 2013). Based on IBU k H, 1.39 × 10−4, it has no tendency for volatilization; therefore, air stripping is not its major removal pathway. Consequently, it is believed that IBU elimination is caused by sorption and biological degradation processes. The removal amount of IBU by adsorption on anaerobic sludge was reported from 30 to 60 % (Carballa et al. 2008). Suggested biodegradation pathways of ibuprofen in literatures have been presented in Table 6.
Ammonia oxidizer bacteria are one of oxygenase-expressing microorganism groups with monooxygenase and dioxygenase enzymes. These enzymes can oxidize many different compounds through co-metabolic reactions (Murdoch and Hay 2013). Additionally, hydroxylamine oxidoreductase (HAO) and ammonia monooxygenase (AMO) are two major enzymes with a complementary function to convert ammonia into nitrite. AMO, an enzyme, can degrade aromatic hydrocarbons, alkanes, alkenes, and chlorinated hydrocarbons and also oxidize ammonia to hydroxylamine. The AMO enzyme either initiates a dehydrogenase or oxidase reaction or a reductive dehalogenation reaction. The mentioned pathway that can form meanwhile nitrification–denitrification is assumed as the possible pathway for IBU biodegradation in the studied system. As a well-nitrifying activated sludge was used for the stating up, a diversity of bacteria exists, surely. Due to aerating in the MBBR reactor, a DO gradient takes place in the center of the biofilm carriers and anoxic/anaerobic situation forms within it and, by this means, facilitated partial denitrification. As a result, the MBBR system was able to achieve the TN removal of 75.5 ± 24.6 %. The nitrification–denitrification with diverse bacteria and IBU as an uneasily accessible carbon source could be the motivating force for most of the bacteria to use co-metabolic processes to be able to use their carbons. In spite of the removal rate for ibuprofen dependency on the nitrification–denitrification ability, there was an obviously positive trend between the nitrification–denitrification ability. The production of nitrate started a day or two after ammonia oxidation, suggesting the presence of other organisms which could have utilized ammonia (Kruglova et al. 2014). The alteration in the absorption spectrum (both at UV and visible area) at various time intervals during the MBBR operation has been presented in Fig. 5b. The peak observed in the ultraviolet region was as a result of IBU and nitrate (222 vs. 220 nm). Nitrate formed as a result of nitrification reaction. As Fig. 5 represents, the peak of IBU in effluent is in accordance to the parent (influent), so the use of IBU in MBBR as a carbon source can remove it without producing intermediates.
3 Conclusions
The nitrogen removal was investigated in the MBBR operated with ibuprofen as an organic carbon source. The removal was strongly dependent on HRT. Based on obtained k biol for IBU, it can be concluded that IBU is a hard biodegradable or persistent organic substance that leads to higher HRT demand to obtain effectiveness in TN removal. The MBBR is an efficient, reliable, and compact system for simultaneous IBU and TN removal. We think that further research should be done to find the exact removal mechanisms of IBU degradation and its metabolite fate.
References
Ambuludi, S., Panizza, M., Oturan, N., Özcan, A., & Oturan, M. (2013). Kinetic behavior of anti-inflammatory drug ibuprofen in aqueous medium during its degradation by electrochemical advanced oxidation. Environmental Science and Pollution Research, 20, 2381–2389. doi:10.1007/s11356-012-1123-6.
Atkinson AJ (2012) Principles of clinical pharmacology: Academic Press
Behera, S. K., Kim, H. W., Oh, J. E., & Park, H. S. (2011). Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Science of the Total Environment, 409, 4351–4360.
Bendz, D., Paxeus, N. A., Ginn, T. R., & Loge, F. J. (2005). Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. Journal of Hazardous Materials, 122, 195–204.
Bernhard, M., Müller, J., & Knepper, T. P. (2006). Biodegradation of persistent polar pollutants in wastewater: Comparison of an optimised lab-scale membrane bioreactor and activated sludge treatment. Water Research, 40, 3419–3428. http://dx.doi.org/10.1016/j.watres.2006.07.011.
Camacho-Muñoz, D., Martín, J., Santos, J., Aparicio, I., & Alonso, E. (2012). Effectiveness of conventional and low-cost wastewater treatments in the removal of pharmaceutically active compounds. Water, Air, & Soil Pollution, 223, 2611–2621. doi:10.1007/s11270-011-1053-9.
Carballa, M., Fink, G., Omil, F., Lema, J. M., & Ternes, T. (2008). Determination of the solid–water distribution coefficient (K d) for pharmaceuticals, estrogens and musk fragrances in digested sludge. Water Research, 42, 287–295.
Chu, L., & Wang, J. (2011). Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in moving bed biofilm reactor. Chemical Engineering Journal, 170, 220–225.
Dâas, A., & Hamdaoui, O. (2014). Removal of non-steroidal anti-inflammatory drugs ibuprofen and ketoprofen from water by emulsion liquid membrane. Environmental Science and Pollution Research, 21, 2154–2164. doi:10.1007/s11356-013-2140-9.
Falås, P., Baillon-Dhumez, A., Andersen, H. R., Ledin, A., & la Cour, J. J. (2012). Suspended biofilm carrier and activated sludge removal of acidic pharmaceuticals. Water Research, 46, 1167–1175.
Falås, P., Longrée, P., la Cour, J. J., Siegrist, H., Hollender, J., & Joss, A. (2013). Micropollutant removal by attached and suspended growth in a hybrid biofilm-activated sludge process. Water Research, 47, 4498–4506.
Garcia-Rodríguez, A., Matamoros, V., Fontàs, C., & Salvadó, V. (2014). The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—a review. Environmental Science and Pollution Research, 21, 11708–11728. doi:10.1007/s11356-013-2448-5.
Hoseinzadeh, E., Khorsandi, H., Wei, C., & Alipour, M. (2015). Evaluation of Aydughmush River water quality using the National Sanitation Foundation Water Quality Index (NSFWQI), River Pollution Index (RPI), and Forestry Water Quality Index (FWQI). Desalination and Water Treatment, 54, 2994–3002.
Hossini, H., Rezaee, A., Ayati, B., & Mahvi, A. H. (2015). Simultaneous nitrification and denitrification using a polypyrrole/microbial cellulose electrode in a membraneless bio-electrochemical system. RSC Advances, 5, 72699–72708.
Ifelebuegu, A., & Ezenwa, C. (2011). Removal of endocrine disrupting chemicals in wastewater treatment by Fenton-like oxidation. Water, Air, & Soil Pollution, 217, 213–220. doi:10.1007/s11270-010-0580-0.
Jeffries K, Brander S, Britton M, Fangue N, Connon R (2015) Chronic exposures to low and high concentrations of ibuprofen elicit different gene response patterns in a euryhaline fish. Environmental Science and Pollution Research: 1–17, doi:10.1007/s11356-015-4227-y
Keener, W. K., & Arp, D. J. (1994). Transformations of aromatic compounds by Nitrosomonas europaea. Applied and Environmental Microbiology, 60, 1914–1920.
Kruglova, A., Ahlgren, P., Korhonen, N., Rantanen, P., Mikola, A., & Vahala, R. (2014). Biodegradation of ibuprofen, diclofenac and carbamazepine in nitrifying activated sludge under 12°C temperature conditions. Science of the Total Environment, 499, 394–401. http://dx.doi.org/10.1016/j.scitotenv.2014.08.069.
Langenhoff, A., Inderfurth, N., Veuskens, T., Schraa, G., Blokland, M., Kujawa-Roeleveld, K., et al. (2013). Microbial removal of the pharmaceutical compounds ibuprofen and diclofenac in wastewater. BioMed Research International, 2013, 9. doi:10.1155/2013/325806.
Luo, Y., Guo, W., Ngo, H. H., Nghiem, L. D., Hai, F. I., Kang, J., et al. (2014). Removal and fate of micropollutants in a sponge-based moving bed bioreactor. Bioresource Technology, 159, 311–319.
Marco-Urrea, E., Pérez-Trujillo, M., Vicent, T., & Caminal, G. (2009). Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere, 74, 765–772. http://dx.doi.org/10.1016/j.chemosphere.2008.10.040.
Matamoros, V., Duhec, A., Albaigés, J., & Bayona, J. (2009). Photodegradation of carbamazepine, ibuprofen, ketoprofen and 17α-ethinylestradiol in fresh and seawater. Water, Air, and Soil Pollution, 196, 161–168. doi:10.1007/s11270-008-9765-1.
Murdoch, R. W., & Hay, A. G. (2005). Formation of catechols via removal of acid side chains from ibuprofen and related aromatic acids. Applied and Environmental Microbiology, 71, 6121–6125.
Murdoch, R. W., & Hay, A. G. (2013). Genetic and chemical characterization of ibuprofen degradation by Sphingomonas Ibu-2. Microbiology, 159, 621–632.
Paíga, P., Santos, L. M. L. M., Amorim, C., Araújo, A., Montenegro, M. C. S. M., Pena, A., et al. (2013). Pilot monitoring study of ibuprofen in surface waters of north of Portugal. Environmental Science and Pollution Research, 20, 2410–2420. doi:10.1007/s11356-012-1128-1.
Rivera-Utrilla, J., Sánchez-Polo, M., Ferro-García, M. Á., Prados-Joya, G., & Ocampo-Pérez, R. (2013). Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere, 93, 1268–1287.
Sarasidis, V. C., Plakas, K. V., Patsios, S. I., & Karabelas, A. J. (2014). Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chemical Engineering Journal, 239, 299–311. http://dx.doi.org/10.1016/j.cej.2013.11.026.
Singh, K., Singh, A., Singh, U., & Verma, P. (2012). Optimizing removal of ibuprofen from water by magnetic nanocomposite using Box–Behnken design. Environmental Science and Pollution Research, 19, 724–738. doi:10.1007/s11356-011-0611-4.
Suarez, S., Lema, J. M., & Omil, F. (2010). Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Research, 44, 3214–3224.
Sun, Q., Lv, M., Hu, A., Yang, X., & Yu, C. P. (2014). Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. Journal of Hazardous Materials, 277, 69–75. http://dx.doi.org/10.1016/j.jhazmat.2013.11.056.
Tambosi, J. L., de Sena, R. F., Favier, M., Gebhardt, W., José, H. J., Schröder, H. F., et al. (2010). Removal of pharmaceutical compounds in membrane bioreactors (MBR) applying submerged membranes. Desalination, 261, 148–156. http://dx.doi.org/10.1016/j.desal.2010.05.014.
Tijani, J., Fatoba, O., & Petrik, L. F. (2013). A review of pharmaceuticals and endocrine-disrupting compounds: sources, effects, removal, and detections. Water, Air, & Soil Pollution, 224, 1–29. doi:10.1007/s11270-013-1770-3.
Urtiaga, A. M., Pérez, G., Ibáñez, R., & Ortiz, I. (2013). Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination, 331, 26–34. http://dx.doi.org/10.1016/j.desal.2013.10.010.
Wu, W., Yang, F., & Yang, L. (2012). Biological denitrification with a novel biodegradable polymer as carbon source and biofilm carrier. Bioresource Technology, 118, 136–140.
Yang J, Trela J, Płaza E. Influence of aeration strategy on behavior of different microorganisms in deammonification process. In Proc. of Polish-Swedish-Ukrainian Seminar “Future urban sanitation to meet new requirements for water quality in the Baltic Sea Region, 2011: 17–19.
Acknowledgments
This work is linked to PhD thesis of Mr. Edris Hoseinzadeh that was supported by Tarbiat Modares University (TMU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoseinzadeh, E., Rezaee, A. & Hossini, H. Biological Nitrogen Removal in Moving Bed Biofilm Reactor Using Ibuprofen as Carbon Source. Water Air Soil Pollut 227, 46 (2016). https://doi.org/10.1007/s11270-015-2690-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2690-1