Abstract
Photodegradation of four pharmaceuticals (i.e. carbamazepine, ibuprofen, ketoprofen and 17α-ethinylestradiol) in aqueous media was studied using a solar light simulator (Xe lamp irradiation) and sunlight experiments. These experiments were carried out in river and seawater and compared to distilled water. The latter was used to evaluate the direct photodegradation pathways. Irradiation time was up to 400 min and 24 days for the solar light simulator and sunlight assays, respectively. Pharmaceutical photodegradation followed a first-order kinetics and their half-lives calculated in every aqueous matrix. Moreover, the sensitizing effect of DOC was evaluated by comparison with the kinetics obtained in distilled waters. Ketoprofen was rapidly transformed via direct photolysis in all the waters under both sunlight (t 1/2 = 2.4 min) and simulated solar light simulator test (t 1/2 = 0.54 min). Under xenon lamp radiation, ibuprofen and 17α-ethinylestradiol were photodegraded at moderate rate with half-lives from 1 to 5 h. Finally, carbamazepine had the lowest photodegradation rate (t 1/2 = 8–39 h) attributable to indirect photodegradation. Indeed, its elimination was strongly dependent on the DOC concentration present in solution. Finally, several ketoprofen photoproducts were identified and plotted against solar light simulator irradiation time. Accordingly, the photodegradation pathway of ketoprofen was postulated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pharmaceuticals have been continuously discharged into the aquatic environment for more than a century without any restrictions (Daugthon and Ternes 1999; Sedlak et al. 2000). Pharmaceutical products have been detected in effluents from a variety of wastewater treatment plants (WWTPs) in concentrations from parts per trillion to low parts per billion depending on their usage and removal efficiency (Heberer 2002; Ternes et al. 2004; Matamoros et al. 2008). In addition to the direct release from WWTPs, pharmaceuticals enter into the environment when digested sewage sludge is used as fertilizer in agricultural land or from landfill disposal sites leading to groundwater pollution (Ternes et al. 2004). Consequently, variable concentrations of pharmaceutical products are detected in surface, ground, and coastal waters receiving treated sewage effluents (Daugthon et al. 2001).
In surface waters these contaminants are exposed to solar radiation leading to photodegradation, which is one of the potential significant removal mechanisms for pharmaceuticals in the aquatic environment (Blough and Sulzberger 2003). In this respect, some compounds such as ketoprofen and diclofenac are generally removed with high efficiencies (Lin and Reinhard 2005) but others, such as carbamazepine and clofibric acid, are not efficiently photodegraded (Andreozzi et al. 2003). Consequently, carbamazepine and clofibric acid are the most frequently detected in seawater (Buser and Müller 1998).
Laboratory studies using sunlight and lamps of different characteristics have shown that several pharmaceuticals are sensitive to photodegradation in different water matrixes (Lin and Reinhard 2005). Direct and indirect photolysis are the primary pathways for abiotic transformation of pharmaceuticals in surface waters. While direct photolysis of chemical species is caused by direct absorption of solar light, the indirect photolysis occurs via light absorption by photosensitizers, such as nitrate and dissolved organic matter (DOM) (Zeep and Cline 1977). Excited photosensitizes can generate extremely reactive oxygenated radicals (e.g. 1O2 and ∙OH) and other photoreactants that leads to indirect photolysis pathways (Zika et al. 1989).
The aim of this work was to evaluate the photodegradation of carbamazepine, ibuprofen, ketoprofen and 17α-ethinylestradiol under solar simulated radiation and sunlight conditions. Photodegradtion kinetics has been evaluated in different aqueous matrixes aiming to distinguish between direct or indirect photolysis. In addition, the major photoproducts from ketoprofen were identified and plotted against irradiation time.
2 Materials and Methods
2.1 Chemicals
HPLC grade methanol, acetonitrile, and water were obtained from Merck (Darmstadt, Germany). Analytical grade formic acid and trifluoroacetic acid were obtained from Panreac (Barcelona, Spain). Suprasolv grade ethyl acetate was obtained from Merck. Triphenylamine, carbamazepine, ibuprofen, ketoprofen and 17α-ethinylestradiol were purchased from Sigma-Aldrich (Steinheim, Germany). Mecoprop was obtained from Reidel-de-Haen (Seelze, Germany).
2.2 Experimental Design
Irradiation experiments were conduced in a solar simulator apparatus (SUNTEST® CPS, ATLAS, Chicago, IL, USA) equipped with a Xenon lamp 1500B NrB4 (507.5 W m-2) with a wavelength range of 300–800 nm. IR filter was employed for elimination of infrared radiation. In this device, the temperature is stabilized at 45°C. Solutions of selected pharmaceuticals with initial concentrations of 10 to 40 mg l−1 were prepared in purified (Milli Q) water, filtered seawater, Ebre and Besòs river waters (Table 1).
The 15 ml samples subjected to irradiation were kept in a capped cylindrical quartz tubes (20 ml) of 100 × 20 mm (length × diameter). Tubes were positioned vertically at 15 cm from the lamp by using a metallic holder. Dark control experiments were conduced in parallel for each pharmaceutical. Irradiation time was 400 min for each compound. Briefly, 1 ml of water was sampled for kinetic studies in each experiment and time.
Additional experiments included a sunlight irradiation of target compounds during 1 month (from 2nd to 29th may 2007) at Barcelona city (41°23′ N, 2°6′ E) by using the same cylindrical quartz tubes described above. Sunny weather occurred all over this period. Sunlight radiation was daily time dependant from 0 to 1,000 W m−2 (Fig. 1) and with day average of 270 W m−2.
Twenty-four hours daily sunlight intensity from 2nd to 29th of May 2007 (obtained from http://www.meteocat.com). Discontinuous line represents daily average radiation intensity
2.3 Analytical Methodologies
Prior to chemical analysis, three replicate aliquots of the filtered sample were acidified (pH = 2 with HCl) and then were determined for dissolved organic carbon (DOC). DOC was measured in the filtrate using a TOC-5000 Shimadzu instrument, following the EPA Method 9060A.
The used analytical procedure for pharmaceutical products analysis has been previously described (Matamoros et al. 2005). Briefly, 1 ml of each irradiated sample was spiked with 25 μg of mecoprop as internal standard. The resulting samples were analyzed using a dual pump HPLC equipped with a UV diode array detector from Shimadzu (Kyoto, Japan) at 230 nm. Injections were carried out using a Rheodyne valve (Rohnert Park, CA) with a sample loop volume of 25 μl fitted to a Shimadzu autosampler. 0.5 ml of chromatographic separation was performed on a Lichro-CART column 125 × 4 mm packed with Lichrospher 100 RP-18 (5 μm) from Merck. The mobile phase used in the chromatographic separation consisted of a binary mixture of solvents A (acetonitrile) and B (water adjusted to pH 3 with formic acid) at a flow rate of 0.8 ml min−1. Initial conditions were 40% A for 20 min, then linearly programmed up to 80% A for 10 min and then followed by a linear program to 100% A for 5 min, and then held for 5 min. The LOD and LOQ (mg l−1) were compound dependent, ranging from 0.15 to 0.24. Relative standard deviations (RSDs) of spiked Besòs river sample (n = 3; 25 mg l−1) were from 1.4% to 4.2%. LOD and LOQ of the analytical procedure were carried out by using a water effluent from a microcosm pilot plant effluent without pharmaceuticals. LOD was calculated three times for the area of the procedural blank and ten times for the LOQ. Linear regression coefficients from a ten-point linear calibration curve (concentration range 0.6–50 mg l−1) were always higher than 0.998 for all the target analytes.
Pharmaceutical metabolites were processed as follows, 0.5 ml of each irradiated sample was spiked with 25 μg of mecoprop as surrogate, and then pharmaceutical metabolites were extracted by sonication in an ultrasonic bath with 2 × 0.5 ml of ethyl acetate. The obtained extracts were evaporated to ca. 20 μl under a gentle stream of nitrogen, and 186 ng of triphenylamine as internal standard were added. Then, the vial was reconstituted to 200 μl with ethyl acetate. Briefly 2 μl of extracts were injected onto a TRACE GC-MS (Thermo-Finnigan, Dreieich, Germany) in the electron impact mode (70 eV ionization energy) fitted with a 30 m × 0.25 mm i.d. × 0.25 μm DB-5 (JW Scientific, Folsom, CA). Detailed description of temperature program and other GC-MS parameters are reported previously (Matamoros and Bayona 2006). Recoveries were higher than 90%.
3 Results and Discussion
3.1 Matrix Effects on Photodegradation Kinetics
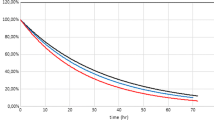
Figure 2 shows the photodegradation rates of pharmaceuticals studied in distilled, sea and river waters that fitted to first-order kinetics as it is observed at the plots shown.
The fast ketoprofen photodegradation rates are consistent with the previously reported studies in similar conditions (Lin and Reinhard 2005). Comparing the obtained photolysis rates in Milli-Q water with the rates obtained in river and sea waters, similar photodegradation rates were observed for all compounds with the exception of carbamazepine. Therefore, pseudo-first order kinetic was assigned to this compound. Its photodegradation rate increases with DOC concentration from Milli Q water to Besòs river water (Table 1). Consequently, indirect photolysis was suggested for this product because the low (Doll and Frimmel 2003)
Table 2 shows the kinetic data according to a first-order kinetic model and half-lives of the target pharmaceutical products. As expected, kinetic rates and half-lives were slightly higher than the ones observed in other studies using similar Xe lamp (Lam and Mabury 2005; Lin and Reinhard 2005) but lower than the ones with Hg lamp (Packer et al. 2003). In fact xenon lamps show a spectrum closer to sunlight than Hg lamps with less radiation intensity in the UV range.
Carbamazepine showed a half-life in the Ebre and Besòs river waters from two to four times lower than in pure water, respectively, which is consistent with the high DOC content in river water promoting the indirect photodegradation by generation of photoreactants (e.g. 1O2 and ∙OH) that can react with carbamazepine. In this regard, Doll and Frimmel (2003) observed that the presence of humic acid increases the carbamazepine photodegradation.
3.2 Comparison between Solar Simulated vs. Sunlight Kinetics
Besòs river water was used as a model of high DOC containing waters in the sunlight assays because it yielded high photodegradation rates in the solar simulated experiments. Figure 3 shows higher 17α-ethinylestradiol photodegradation rates than carbamazepine at the first irradiation days and lower at the end. This is consistent with the higher interaction of 17α-ethinylestradiol with the organic matter (log Kow = 4), suggesting low incidence of irradiation on this substance at the end of the irradiation experiment and lower interaction at the first irradiation days. That process could be attributable to the generation of photoproducts from DOC with high interaction with 17α-ethinylestradiol. Consequently, low correlation coefficients were observed for these two compounds attending to the interaction with the organic matter (Table 3).
Table 3 contains a summary of the sunlight photolysis results obtained including the first order rate constants, half-lives and comparison with those obtained in the solar simulator device. As expected, ketoprofen showed the highest photodegradation rate. Nevertheless, the comparison of sunlight with the solar simulated studies resulted to be compound dependent, ranging from five to 111 times lower. These differences are in part explained by daily light variation, considering night–day, radiation intensity (Fig. 1) and spectral emission Other studies in similar latitudes reported a half-live for carbamazepine nearly to 100 days using glass vessels attributable to glass UV filtering (Andreozzi et al. 2003). In this work, quartz vessels were used in order to be closer to real conditions (e.g. transparent to UV) obtaining a half-live about 3 days. These results, point out the relevance of the experimental set up in the photooxidation kinetics.
3.3 Environmental Relevance
The data obtained from these photodegradation assays show clearly the positive effect of photodegradation on the removal of some recalcitrant pharmaceuticals in WWTPs (Daugthon and Ternes 1999). Ketoprofen is slightly removed in WWTPs but in surface treatment systems such as stabilization ponds or surface flow constructed wetlands, it could be efficiently removed according with their long hydraulic retention time of these wastewater treatment systems. On the other hand, carbamazepine is also a recalcitrant compound but its low photodegradation rate observed in sunlight studies produce a low removal in surface treatment systems (Matamoros et al. 2008). Therefore, effluent storage in sunlight exposed systems could increase the removal of some pharmaceutical compounds by photodegradation. In continental surface waters, the relevance of photodegradation is limited by water turbidity and light attenuation in the upper meters from surface. Nevertheless, in seawater, photooxidation processes can be of relevance depending on the water column primary productivity but it is expected to be of certain relevance through the photic zone.
3.4 Photodegradation Intermediates
Figure 4 shows the Ketoprofen metabolites identified according to the NIST library match. The photodegradation pathway described in this study involves a decarboxylation (–CO2) (2) (Martinez and Scaiano 1997; Nakajima et al. 2005). 2 can react with O2 and after that with radical OH• to produce compound 3 (Pietta et al. 1987). Then demethylation and subsequent cleavage of the two rings may occur, to get more labile structures that are easily removed by biotic or abiotic pathways. However, some photoproducts were more persistent than the parent ketoprofen. The time-concentration plots show the intermediate metabolites with low stability (2) and intermediate metabolites with high stability (3, 4 and 5). Hence in ketoprofen contaminated surface waters, photoproducts 3 and 4 might occur.
4 Conclusions
Photodegradation experiments described in this work demonstrated that ketoprofen is the most photolabile among the four pharmaceuticals studied with a half-life from 2.4 to 0.54 min exposed to sunlight or solar simulated light (i.e. Xe lamp) respectively. The other compounds presented half-lives ranging from 1 to 39 h in solar simulated experiments. The comparison of sunlight with the solar simulated studies resulted to be compound dependent ranging from five to 111 times lower. Carbamazepine increased its photodegradation rate by indirect photolysis mediated by DOC. Photodegradation intermediates of ketoprofen show a decarboxylation, followed by a demethylation and subsequent ring cleavage. In conclusion, pharmaceutical photodegradation in surface waters is a significant removal pathway that needs to be taken into account, mainly for those compounds exhibiting a high recalcitrance to biodegradation.
References
Andreozzi, R., Raffaele, M., & Nicklas, P. (2003). Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere, 50, 1319–1330. doi:10.1016/S0045-6535(02)00769-5.
Blough, N. V., & Sulzberger, B. (2003). Impact of photochemical processes in the hydrosphere. Aquatic Sciences, 65, 317–319. doi:10.1007/s00027-003-0003-z.
Buser, H.-R., & Müller, M. D. (1998). Occurrence of pharmaceutical drug clofibric acid and the herbicide mecoprop in various Swiss lakes and in the North Sea. Environmental Science & Technology, 32, 188–192. doi:10.1021/es9705811.
Daugthon, C. G., & Jones-Lep, T. (2001). Pharmaceuticals and personal care products in environment: Scientific and regulatory issues. Washington, DC: American Chemical Society.
Daugthon, C. G., & Ternes, T. A. (1999). Pharmaceuticals and personal care products in the environment: Agent of subtable change? Environmental Health Perspectives, 107, 907–938. doi:10.2307/3434573.
Doll, T. E., & Frimmel, F. H. (2003). Fate of pharmaceuticals—Photodegradation by simulated solar UV-light. Chemosphere, 52, 1757–1769. doi:10.1016/S0045-6535(03)00446-6.
Heberer, T. (2002). Tracking persistent pharmaceutical residues from municipal sewage to drinking water. Journal of Hydrology (Amsterdam), 266, 175–189. doi:10.1016/S0022-1694(02)00165-8.
Huertas, E., Folch, M., Salgot, M., Gonzalvo, I., & Passarell, C. (2006). Constructed wetlands effluent for streamflow augmentation in the Besos River (Spain). Desalination, 188, 141–147. doi:10.1016/j.desal.2005.04.111.
Lam, M. W., & Mabury, S. A. (2005). Photodegradation of the pharmaceuticals atorvastatin, carbamazepine, levofloxacin, and sulfamethoxazole in natural waters. Aquatic Sciences, 67, 177–188. doi:10.1007/s00027-004-0768-8.
Lin, A., & Reinhard, M. (2005). Photodegradation of common environmental pharmaceuticals and estrogens in river water. Environmental Toxicology and Chemistry, 24, 1303–1309. doi:10.1897/04-236R.1.
Martinez, L. J., & Scaiano, J. C. (1997). Transient intermediates in the laser flash photolysis of ketoprofen in aqueous solutions: Unusual photochemistry for the benzophenone chromophore. Journal of the American Chemical Society, 119, 11066–11070. doi:10.1021/ja970818t.
Matamoros, V., & Bayona, J. M. (2006). Elimination of pharmaceuticals and personal care products in subsurface flow constructed wetlands. Environmental Science & Technology, 40, 5811–5816. doi:10.1021/es0607741.
Matamoros, V., García, J., & Bayona, J. M. (2005). Behavior of selected pharmaceuticals in subsurface flow constructed wetlands: A pilot-scale study. Environmental Science & Technology, 39, 5449–5454. doi:10.1021/es050022r.
Matamoros, V., García, J., & Bayona, J. M. (2008). Organic micropollutant removal in full-scale surface flow constructed wetland fed with secondary effluent. Water Research, 42, 653–660. doi:10.1016/j.watres.2007.08.016.
Nakajima, A., Tahara, M., Yoshimura, Y., & Nakazawa, H. (2005). Determination of free radicals generated from light exposed ketoprofen. Journal of Photochemistry and Photobiology A, 174, 89–97. doi:10.1016/j.jphotochem.2005.03.015.
Packer, J. L., Werner, J. J., Latch, D. E., McNeill, K., & Arnold, W. A. (2003). Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen. Aquatic Sciences, 65, 342–351. doi:10.1007/s00027-003-0671-8.
Pietta, P., Manera, E., & Ceva, P. (1987). High-performance liquid chromatographic determination of ketoprofen degradation products. Journal of Chromatography A, 390, 454–457. doi:10.1016/S0021-9673(01)94399-7.
Prat, N., & Ibáñez, C. (2003). Avaluació crítica del Pla Hidrològic Nacional i proposta per a una gestió sostenible de l’aigua del baix Ebre. Barcelona, Institut d’Estudis Catalans. Secció de Ciències Biològiques.
Sedlak, D. L., Gray, J. L., & Pinkston, K. E. (2000). Understanding micro contaminants in recycled water. Environmental Science & Technology, 34, 509A–512A. doi:10.1021/es990024+.
Ternes, A. T., Joss, A., & Siegrist, H. (2004). Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environmental Science & Technology, 38, 393A–398A.
Zeep, R. G., & Cline, D. M. (1977). Rates of the direct photolysis in aquatic environment. Environmental Science & Technology, 11, 359–366. doi:10.1021/es60127a013.
Zika, R., Robert, G., & Fisher, A. (1989). Sunlight-induced photochemistry of humic substances in natural waters: Major reactive species. Aquatic humic substances pp. 333–361. Washington, DC: American Chemical Society.
Acknowledgments
This research was funded by the Spanish Ministry of Science and Education through the CONTWET (CTM2005-06457-CO5-04/TECNO) and CGL2005-02846/BOS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matamoros, V., Duhec, A., Albaigés, J. et al. Photodegradation of Carbamazepine, Ibuprofen, Ketoprofen and 17α-Ethinylestradiol in Fresh and Seawater. Water Air Soil Pollut 196, 161–168 (2009). https://doi.org/10.1007/s11270-008-9765-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9765-1