Abstract
In this work, the removal of the worldwide non-steroidal anti-inflammatory drugs ibuprofen (IBP) and ketoprofen (KTP) by emulsion liquid membrane (ELM) was carried out. An ELM system is made up of hexane as diluent, Span 80 as the surfactant and sodium carbonate as the inner aqueous solution. Effect of experimental conditions that affect the extraction of IBP such as surfactant concentration, emulsification time, sulfuric acid concentration in external phase, acid type in external phase, internal phase concentration, type of internal phase, stirring speed, volume ratio of internal phase to membrane phase, treatment ratio, IBP initial concentration, diluent type and salt was investigated. The obtained results showed that by appropriate selection of the operational parameters, it was possible to extract nearly all of IBP molecules from the feed solution even in the presence of high concentration of salt. Under optimum operating conditions, the efficiencies of IBP removal from distilled water (99.3 %), natural mineral water (97.3 %) and sea water (94.0 %) were comparable, which shows that the ELM treatment process represents a very interesting advanced separation process for the removal of IBP from complex matrices such as natural and sea waters. Under the optimized experimental conditions, approximately 97.4 % KTP was removed in less than 20 min of contact time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last decade, the presence of various pharmaceuticals in water has received more attention due to the unknown environmental impact, possible damages to the botany and fauna present in aquatic systems and human health effects. The major sources of pharmaceuticals are the continuous disposal of wastewaters released from the pharmaceutical industries, excretory products of medically treated humans and animals (Madhavan et al. 2010). A remarkable group of such pharmaceutical pollutants comprises non-steroidal anti-inflammatory drugs (NSAIDs) with more than 70 million annual prescriptions in the world (Méndez-Arriaga et al. 2010; Takagi et al. 2006). Moreover, several recent reports have confirmed the presence of the NSAIDs ibuprofen (IBP) and ketoprofen (KTP), in effluents of wastewater treatment plants (Alonso et al. 2006; Andreozzi et al. 2006).
The compound 2-[3-(2-methylpropyl)phenyl]propanoic acid, commercially available as IBP, is widely used as an anti-inflammatory and antipyretic drug especially prescribed for the treatment of fever, migraine, muscle aches, arthritis and tooth aches (Takagi et al. 2006). Several kilotons of this compound are produced worldwide each year, part of which is rejected to the effluents, excreted by patients in its original form or as metabolites from human biodegradation (Méndez-Arriaga et al. 2010). Concentrations of IBP in the environment are reported between 10 ng/l and 169 μg/l (Santos et al. 2007). IBP is a persistent pollutant because it is not destroyed in a municipal water treating station (Fent et al. 2006). IBP concentrations in the effluents leaving several sewage-treatment plants are reported between 0.002 and 24.6 μg/l (Méndez-Arriaga et al. 2008; Miege et al. 2007).
Ketoprofen (2-(3-benzoylphenyl)-propanoic acid), a type of NSAID that is extensively used as a non-prescription drug, has been detected in surface waters in concentrations ranging from ng/l up to μg/l (Metcalfe et al. 2003; Tixier et al. 2003). Due to its heavy medical use, KTP is regularly detected in municipal wastewaters and is not completely removed in most of sewage treatments (Marco-Urrea et al. 2010).
Although IBP and KTP, which are present in the aquatic environment in low concentrations, are still harmful humans and animals, only highly concentrated, polluted solutions have been investigated so far (Choina et al. 2013; Illés et al. 2012; Méndez-Arriaga et al. 2008; Mozia and Morawski 2012; Zheng et al. 2011).
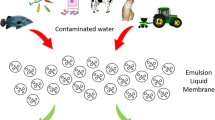
Most frequently, conventional treatment processes applied at domestic wastewater treatment plants fail to remove completely pharmaceutical substances. Therefore, the integration of conventional wastewater treatments with advanced technologies has become of great interest. Recently, emulsion liquid membrane (ELM) or surfactant liquid membrane has gained much attention as an advanced extraction process for the removal of contaminants present in wastewater. Liquid membranes are not only an important technique for concentration, separation and recovery, but also are of fundamental importance from an environmental engineering point of view in understanding the transport mechanisms.
The ELM process is carried out by combining extraction and stripping steps in one stage, which leads to simultaneous purification and concentration of the solute. ELMs consist of an aqueous internal phase stabilized by a surfactant and dispersed as very fine droplets inside a membrane (organic) phase. The resulting liquid membrane, or water-in-oil (W/O) emulsion, is further dispersed as emulsion globules in external feed phase (a second aqueous phase). Target solute in the external feed phase is transferred across the membrane phase into the internal phase during an extraction process. In this water-in-oil-in-water (W/O/W) ELM, the organic phase functions as a barrier, or membrane. The solute mass transfer is driven by the concentration difference between the external feed phase and the internal phase. The solute is converted to ion state which cannot transfer through the membrane phase back to the external phase by the internal phase agent. After the subsequent separation of the external phase from the W/O emulsion by gravity (settling), splitting of the emulsion (demulsification) is carried out. Demulsification is performed in order to separate the phases that makeup the emulsion, the internal aqueous phase and the organic membrane phase. At the end of the process, the membrane phase can be reused and the receiving phase (enriched in the recovered solute) can be recycled or recovered for solute.
Immobilized solvent and membrane extraction of IBP and its metabolite has been reported in the literature (Williams et al. 2012, 2013). To the best of our knowledge, data on the removal of IBP and KTP from contaminated water by ELM have not been reported previously. Additionally, it is of considerable practical interest to examine the removal of IBP in complex matrices such as natural waters. Therefore, in this study, ELM system was developed for the extraction of IBP and KTP from aqueous solutions. The influence of operational parameters such as surfactant concentration, emulsification time, sulfuric acid concentration in external phase, acid type in external phase, internal phase concentration, type of internal phase, stirring speed, volume ratio of internal phase to membrane phase, treatment ratio, IBP initial concentration, diluent type and salt on the extraction of IBP by ELM was examined. Additionally, permeation of IBP by ELM in pure and natural waters was compared. The removal of KTP using the optimized ELM system was also studied.
Experimental
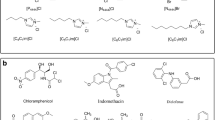
IBP sodium salt (CAS number 31121-93-4) and KTP (CAS number 22071-15-4) were purchased from Sigma-Aldrich and used as received. Though IBP and KTP occur in concentrations ranging from ng/l up to μg/l in water, we chose higher concentrations to check the feasibility of the ELM process. The solubility limits are 100 mg/ml for IBP sodium salt (Sigma-Aldrich) and 230 mg/l for KTP (Bundgaard and Nielsen 1988; Yalkowski et al. 2010). The molecular structures of IBP sodium salt and KTP are shown in Fig. 1.
All other chemicals used were of the highest available purity and were purchased from Sigma-Aldrich.
The extraction of IBP and KTP using liquid emulsion membrane involves three steps: preparation of liquid membrane emulsion, extraction of the solute from feed by contacting the emulsion, and separation of liquid emulsion from the external phase by settling (Fig. 2).
Internal aqueous standard solutions were prepared by taking the required amount of alkaline solution (Na2CO3, NaOH or NH4OH) in distilled water. The organic membrane phase was prepared by dissolving the appropriate amount of Span 80 as a surfactant in hexane under a gentle mixing by a magnetic stirrer. The emulsion was prepared by mixing the internal aqueous solution with the organic membrane phase using a high-speed disperser for a fixed mixing time. The volume ratio of internal aqueous phase to organic phase was changed from 1:2 to 2:1.
The three-phase dispersion (W/O/W) was stirred with a mechanic stirrer at 250 rpm (except when the effect of stirring speed was studied). A volume of the prepared W/O emulsion was added to 600 ml of external aqueous solution (IBP or KTP) in a cylindrical thermostated vessel that was attached to an overhead mechanical stirrer. The agitator used was a 45° pitch four blades down pumping impeller (diameter, 5 cm). The content of the vessel was stirred in order to disperse the W/O emulsion in the external phase at variable speeds for different contact times to make the W/O/W double emulsions. The external phase solution was periodically sampled at various time intervals. The concentration of pollutant in the solutions was determined by a UV–visible spectrophotometer set at the wavelength corresponding to maximum absorbance of the studied pollutant.
A well-known procedure for determining IBP and KTP concentrations, based on Beer's law calibration plots, was applied using a UV–visible spectrophotometer (Jenway 6405). The wavelength resolution and the bandwidth were, respectively, 1 and 0.5 nm. The length of the optical path in glass cell was 1 cm. The maximum absorption wavelength was determined as equal to 220 and 260 nm for IBP and KTP, respectively. Then, the calibration plot was constructed. The calibration was repeated five times during the period of measurements. The linearization of this plot usually provided a determination coefficient close to 99.99 %. These data were used to determine the remaining IBP or KTP concentration in aqueous solution.
All the experiments were carried out in duplicate at least and the mean values are presented. The maximum standard deviation was 3 %.
Results and discussion
The influence of experimental conditions such as surfactant concentration, emulsification time, sulfuric acid concentration in external phase, acid type in external phase, internal phase concentration, type of internal phase, stirring speed, volume ratio of internal phase to membrane phase, treatment ratio, IBP initial concentration, diluent type and salt on the extraction of IBP by ELM was examined and optimum conditions for maximum removal of IBP were established. The effects of these parameters have been discussed in the following sections.
Transport mechanism
The pK a values of IBP and KTP are 4.9 and 4.4, respectively. Therefore, the overall extraction was greatly affected by the external phase pH. When the pH was kept low, IBP and KTP are present in neutral protonated form. Strong basic agent used inside the emulsion droplets as internal stripping phase (Na2CO3) serves to convert unionized IBP and KTP, which had diffused through the liquid membrane, into ionized forms. The latter compounds, that are charged specie in basic aqueous phase, could not diffuse back to the external phase and thus were retained in the internal aqueous phase (Fig. 3). However, the difference of electrolyte concentrations between the internal and external phases is increased by increasing Na2CO3 concentration, which causes an osmotic pressure between these two phases. Water in the external phase is then transferred to the internal phase that results in swelling and breakage of the emulsion, and thus decreasing in removal efficiency. Therefore, sulfuric acid is used in the external phase in order to avoid swelling phenomenon and enhance the extraction efficiency.
Effect of surfactant concentration
In the ELM process, surfactant concentration affects not only the stability of the liquid membrane but also mass transfer. Thus, it is very important to ascertain the effect of surfactant concentration on the behavior of IBP extraction by ELM.
The experimental conditions were that conducting to meta-stable W/O emulsion and are summarized as follows: emulsion volume, 60 ml; external feed phase (IBP solution) volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; diluent, hexane.
The variation in the extent of extraction of IBP from aqueous external phase for various Span 80 concentrations ranging from 1 % to 9 % (w/w) is represented in Fig. 4. It was observed that increasing surfactant concentration from 1 % to 3 % (w/w) increased the removal efficiency. Emulsion with 1 % Span 80 was not stable for the total duration of run time leading to poor extraction. This is due to insufficient surfactant molecules at the aqueous internal phase–organic phase interface (Peng et al. 2012; Dâas and Hamdaoui 2010a, b). Increasing the surfactant concentration to 3 % (w/w) resulted in a stable membrane throughout the duration of the experiment with negligible leakage. Excessive amount of surfactant (>3 % [(w/w)) decreases the extraction extent. Higher amount of surfactant tends to increase the resistance at the interface and this can be attributed to a number of possible factors caused by high interfacial occupancy of the surfactant that includes decrease in IBP extraction at the membrane phase–feed phase interface, increase in interfacial viscosity and decrease in movement of inner droplets within the emulsion globule (Yang et al. 2005). Therefore, the amount of surfactant in the membrane phase must be minimal, yet enough to stabilize the emulsion. Hence, the optimal surfactant concentration was 3 % (w/w).
Effect of surfactant concentration on the extraction of IBP (50 mg/l) by ELM (experimental conditions: emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of emulsification time
Experiments were conducted under the same operating conditions as mentioned previously, using a surfactant concentration of 3 % (w/w) and varying the emulsification time from 1 to 9 min. The effect of emulsification time on the removal of IBP from aqueous solution by ELM is shown in Fig. 5. These results show that the higher extraction efficiency was obtained for an emulsification time of 3 min. This is due to the formation of a stable emulsion and the diminution of the size of Na2CO3 containing internal phase droplets and enhances the homogeneity of the dispersed phase. Further increase in emulsifying time decreased the removal of IBP. For insufficient emulsification time (1 min), the breakage was great because the droplets have a large size, which leads to their coalescence. In contrast, for a long emulsification time, the breakage is important because of high internal shearing conducive to a very high number of small droplets by volume unit. This increases the collision frequency between small droplets conducting to emulsion breakage (Dâas and Hamdaoui 2010a, b; Ng et al. 2010). Therefore, an emulsification time of 3 min was chosen throughout this study.
Effect of emulsification time on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; Span 80 concentration, 3 % (w/w); stirring speed, 250 rpm; volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of sulfuric acid concentration in external phase
In order to mitigate the problem of membrane swelling and explore the significant role being played by acid concentration in the external phase during the transport of the solute in ELM system, the extraction of IBP were carried out at different sulfuric acid concentration in the feed phase. The permeation studies were performed with various sulfuric acid concentrations in the range 0.01–2 N, and the obtained results are shown in Fig. 6. As can be seen from this figure, IBP extraction increased with the increase of sulfuric acid concentration from 0.01 to 0.1 N and then decreased due to deterioration in the emulsion stability. Maximum extraction as well as maximum transport was achieved at 0.1 N H2SO4. However, at higher acid concentrations, IBP transport efficiency diminished appreciably as shown in Fig. 6. This might be due to the fact that at very high acid concentration the properties of the surfactant are reduced (Sabry et al. 2007) that leads to a destabilization of the emulsion and a diminution in the extraction efficiency. It has been observed that in the absence of sulfuric acid, the phenomenon of swelling take place conducting to the breakage of the emulsion and thus decreasing in extraction efficiency. This transport of water from external phase to internal phase is mainly driven by the difference in osmotic pressure between the external and the internal phases. Thus, the 0.1 N H2SO4 solution was selected as the best concentration for feed phase.
Effect of sulfuric acid concentration in the external phase on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; diluent, hexane)
Effect of acid type in external phase
Although in the preliminary experiments sulfuric acid solution was used as the external solution, other acids were also tested. The effect of the presence of 0.1 N of different acids namely sulfuric acid, hydrochloric acid and perchloric acid in the external feed phase on the extraction of IBP was studied. Figure 7 compares the extraction data of IBP for different acidic external solutions. The obtained results indicate that sulfuric acid showed slightly higher extraction extent, whereas hydrochloric acid and perchloric acid yielded near quantitative extraction. Consequently, sulfuric acid was accepted as the best acid and was used as the external solution in the following experiments.
Effect of acid type in the external phase on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; diluent, hexane)
Effect of internal phase concentration
Stripping phase concentration plays an important role in the transport of solute from feed solution to stripping phase through ELM. Consequently, the effect of Na2CO3 concentration of the stripping solution on extraction of IBP was studied at different concentration levels from 0.001 to 2 N. The results are shown in Fig. 8. From Fig. 8, it appeared that the extraction increased when Na2CO3 concentration was varied from 0.001 to 0.1 N. However, when the concentration of Na2CO3 in the stripping solution was varied from 0.1 to 2 N, the level of IBP removal was reduced. It was expected that increasing the amount of Na2CO3 in the internal phase decreased the difference of densities and increased the emulsion viscosity. In addition, difference in ionic strength between external to internal phase led to internal phase volume increasing, which promotes excess emulsion leakage (Dâas and Hamdaoui 2010a, b; Balasubramanian and Venkatesan 2012). The increasing of emulsion viscosity reflected in an increasing in the size of drops. Therefore, 0.1 N Na2CO3 solution was chosen as the best internal phase concentration.
Effect of internal phase concentration (Na2CO3) on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of type of internal phase
The stripping reaction at the membrane-stripping solution interface plays a vital role in the extraction of solute from the feed solution to the stripping solution in ELM process. Although in the preliminary experiments sodium carbonate solution was used as the internal aqueous phase, other alkaline substances were also tested. The effect of the presence of 0.1 N of different stripping solutions, namely, Na2CO3, NaOH and NH4OH, in the stripping phase on the extraction of IBP was studied. The obtained results were shown in Fig. 9. As shown in Fig. 9, when three different solutions Na2CO3, NaOH and NH4OH with the same concentration (0.1 N) was used in the stripping solution, the extraction efficiencies showed the following tendency: Na2CO3 > NaOH > NH4OH. Thus, the 0.1 N Na2CO3 solution was selected as the suitable stripping agent.
Effect of type of internal phase on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of stirring speed
The stirring speed is a key factor in the rate of mass transfer through ELM. In order to obtain optimal stirring speed that allows effective extraction of IBP in the ELM process, the feed and emulsion were mixed at stirring speeds ranging from 150 to 350 rpm. The obtained results are shown in Fig. 10. For the lower stirring speed (150 rpm), the extraction rate was low because of the formation of larger emulsion globules involving a decrease of the area for mass transfer. By increasing the rotational speed from 150 to 250 rpm, IBP in the external phase is removed more rapidly during the treatment. This might be due to the fact that the size of droplets and the membrane thickness are both reduced by increasing the stirring intensity. Emulsion liquid droplets with smaller size lead to better dispersion. At the same time, more interfacial surface area is available between the external phase and the membrane phase, so the removal efficiency and transfer rate are higher. Further increase in speed of mixing from 250 to 350 rpm resulted in an improvement of both the rate of transport and the extent of extraction during the first few minutes. However, the removal efficiency starts to decrease after certain treatment time (10 min for 300 rpm, 4 min for 350 rpm). Increasing the stirring speed above a critical value not only decreases the extraction efficiency, but also affects the stability of the emulsion and makes the emulsion unstable. In addition, the shear induced breakage of fragile emulsion droplets near the tip of the impeller or impact on the wall of a contactor imposes upper limit on the speed of agitation (Kulkarni and Mahajani 2012). For the higher stirring speed (350 rpm), the swelling phenomenon becomes notable, which resulted in greater amounts of water to permeate through the membrane causing the internal droplets to swell and coalesce. Thus, 250 rpm was recommended as the most appropriate stirring speed.
Effect of stirring speed on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1/1; emulsification time, 3 min; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of volume ratio of internal phase to membrane phase
Volume ratio of internal phase to membrane phase plays an important role in determining the effectiveness of ELM system. The effect of volume ratios of the internal solution to membrane phase varied between 1:2 and 2:1, by maintaining membrane volume constant, on the removal of IBP is presented in Fig. 11. At a low volume ratio, membrane thickness and viscosity of the emulsion phase is high due to the relatively high organic content. Also, a low volume ratio means less stripping agents are available for solute stripping. From Fig. 11, it is observed that with increase of volume ratio from 1:2 to 1:1, the transport rate and removal efficiency of IBP increases. This can be attributed to the fact that an increase in the internal phase volume fraction shifts the internal drop size distribution toward larger size and decreases the thickness of the membrane phase resulting in enhanced mass transfer. This results in increase in the capacity of the membrane for enhanced permeation of the solute. However, there is a limit to which the size of the droplet can increase with an increase in internal aqueous phase to membrane phase ratio. Beyond the ratio of 1:1, a further increase in the volume of internal aqueous solution decreases both the rate and efficiency of extraction. This may be due to an increase of the emulsion viscosity and also an increase of the diameter of internal droplets (Chiha et al. 2006; Djenouhat et al. 2008a). The increase of droplets diameter decreases the interfacial contact area between the emulsion and feed solution and thereby decreases the extraction efficiency. Additionally, for higher volume ratios, the volume of membrane solution is not enough for enclosing all the stripping solution (Juang and Lin 2004). Thus, in order to obtain a uniform and homogeneous distribution of the stripping solution droplets in the membrane solution with high extraction efficiency, the volume ratio of internal aqueous phase to membrane phase of 1:1 was selected as the best volume ratio.
Effect of volume ratio of internal phase to organic phase on the extraction of IBP (50 mg/l) by ELM (experimental conditions—emulsion volume, 60 ml; external phase [IBP solution] volume, 600 ml; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of treatment ratio
The volume ratio of the emulsion to the aqueous external solution, defined as treatment ratio, controls interfacial mass transfer and plays an important role in determining the efficiency of ELMs. The effect of the volume ratio of emulsion to external feed phase on the extraction efficiency was studied in the range 10:600–300:600 and the obtained results are given in Fig. 12. The figure shows that the removal efficiency increases by increasing the volume ratio of emulsion to external phase from 10:600 to 60:600. This is because with the increasing amount of emulsion in the external phase, the amount of available globules and interfacial surface area per unit volume of external phase increase and hence the transfer rate of IBP and removal efficiency are accelerated. Beyond 60:600, further increase in the ratio caused less extraction efficiency due to improper mixing of phases because of higher proportion of the more viscous emulsion phase. With the increasing of the volume ratio, the size of globules increases and in turn results in the membrane thickening, which resists the transportation of IBP. Furthermore, the influence of the volume ratio of emulsion to the external feed solution on the stability was not significant, because the breakage increases very slightly with an increase of this volume ratio (Djenouhat et al. 2008a, b). With the consideration of the economy of the ELM process, a lower volume ratio is favorable because it can increase the capacity per unit volume of emulsion membrane and save the cost. Consequently, volume ratio of emulsion to external feed solution of 60:600 was selected as the best treatment ratio.
Effect of volume ratio of emulsion to external phase on the extraction of IBP (50 mg/l) by ELM (experimental conditions—external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Effect of IBP initial concentration
The effect of initial concentration of IBP ranging from 10 to 150 mg/l on the degree of extraction was studied. The concentration of IBP in the feed solution was reduced from 10 to 0.4 mg/l, from 20 to 0.8 mg/l, from 50 to 0.4 mg/l, from 100 to 0.8 mg/l and from 150 to 2 mg/l. After 20 min of treatment, the extraction efficiencies were approximately 96 % for initial IBP concentrations of 10 and 20 mg/l and 99 % for initial IBP concentrations of 50, 100 and 150 mg/l. The removal efficiency increases with the increase of solute concentration. This may be attributed to an increase in the driving force, the concentration gradient, with the increase in the initial IBP concentration.
Effect of diluent type
The transfer of solute through the membrane phase might be considered as an important parameter. It is recognized that the organic diluent influences the performance of an ELM system. The viscosity of the diluent is the main determinant of the thickness and permeability of the membrane for the solute. Therefore, the type of diluent may have a controlling effect on the removal efficiency. The extraction kinetics of IBP using hexane, heptane or kerosene as diluents is presented in Fig. 13. This figure shows that hexane provided better extraction performances than heptane and kerosene. This behavior is due to the viscosity of hexane (0.200 mPa s) that is lower than that of heptane (0.387 mPa s) and kerosene (1.383 mPa s), which conducted to a higher rate of transfer. On the other hand, emulsion prepared using hexane is more stable than those produced using heptane and kerosene. Thus, hexane was the best choice as the diluent in the present ELM system.
Effect of diluent type on the extraction of IBP (50 mg/l) by ELM (experimental conditions—external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N)
Effect of salt
Salt could be present in a various range of concentration in wastewater and influence the ELM process by changing the ionic strength in the external phase, which affects the membrane stability (Yan and Pal 2004). The effect of salt concentration (ionic strength) in the external feed phase on the degree of IBP removal was analyzed over NaCl concentration range from 0 to 35 g/l. The influence of sodium chloride concentration on the extraction of IBP is illustrated in Fig. 14. It is observed that the removal efficiency is slightly affected by sodium chloride, which indicates that the membrane has an acceptable stability against strong electrolyte and may be used for the treatment of water contaminated with IBP.
Effect of salt (NaCl) on the extraction of IBP (50 mg/l) by ELM (experimental conditions—external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1/1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Extraction of IBP from natural and sea waters
The removal efficiency of IBP was examined by dissolving the pollutant in a natural mineral water and in sea water. Experiments were carried out using an initial IBP concentration of 50 mg/l. The obtained results are shown in Fig. 15. The main characteristics of the natural water were: pH = 7.2, Ca2+ = 81 mg/l, Mg2+ = 24 mg/l, Na+ = 15.8 mg/l, Cl− =72 mg/l, SO4 2− = 53 mg/l, HCO3 − = 265 mg/l. In all cases, the removal kinetics of IBP in distilled water was slightly higher than those obtained in natural and sea waters. The concentration of IBP in the feed solution was reduced from 50 to 0.4 mg/l in distilled water, to 1.4 mg/l in natural mineral water and to 3 mg/l in sea water. After 20 min, the removal efficiencies were 99.3 % in distilled water, 97.3 % in natural mineral water and 94.0 % in sea water. The total loss on removal efficiency is less than 5.3 %, which shows that the ELM treatment process represents a very interesting advanced separation process for the removal of IBP in complex matrices such as natural and sea waters.
Removal of IBP (50 mg/l) from distilled water, natural mineral water and sea water by ELM (experimental conditions—external phase [IBP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1/1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Extraction of KTP
Optimum experimental conditions obtained from the earlier experiments are summarized as follows: emulsion volume, 60 ml; external phase volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; diluent, hexane; concentration of H2SO4 in the external phase, 0.1 N.
The extraction kinetics of KTP in aqueous solution at the optimum operating conditions is illustrated in Fig. 16. From Fig. 16, it is clear that the degree of KTP removal increases gradually with increasing contact time and reached a plateau afterward. After only 6 min of contact time, extraction efficiency was 97.4 %. A removal efficiency of 98.8 % was attained after 20 min.
Removal of KTP (50 mg/l) by ELM (experimental conditions—external phase [KTP solution] volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; sulfuric acid concentration in the feed phase, 0.1 N; diluent, hexane)
Conclusions
An ELM was developed to remove NSAIDs IBP and KTP from water. The optimum experimental conditions for the extraction of IBP were summarized as follows: emulsion volume, 60 ml; external phase volume, 600 ml; volume ratio of internal phase to organic phase, 1:1; emulsification time, 3 min; stirring speed, 250 rpm; concentration of Span 80, 3 % (w/w); volume ratio of W/O emulsion to external phase, 60:600; internal phase concentration (Na2CO3), 0.1 N; diluent, hexane; concentration of H2SO4 in the external phase, 0.1 N. Under the best operating parameters, it was possible to extract nearly all of IBP molecules from the feed solution even in the presence of high concentration of salt. At the optimum experimental conditions, about 97.4 % KTP was removed in less than 20 min of contact time.
This study demonstrates that ELM treatment in comparison with other techniques that are hindered by the presence of salts is a promising process for the elimination of NSAIDs IBP and KTP from complex matrices such as natural and sea waters.
References
Alonso E, Villar P, Santos A, Aparicio I (2006) Fractionation of heavy metals in sludge from anaerobic wastewater stabilization ponds in southern Spain. Waste Manage 26:1270–1276
Andreozzi R, Canterino M, Marotta R (2006) Fe(III) homogeneous photocatalysis for the removal of 1,2-dichlorobenzene in aqueous solution by means UV lamp and solar light. Water Res 40:3785–3792
Balasubramanian A, Venkatesan S (2012) Removal of phenolic compounds from aqueous solutions by emulsion liquid membrane containing ionic liquid [BMIM]+[PF6]− in tributyl phosphate. Desalination 289:27–34
Bundgaard H, Nielsen NM (1988) Glycolamide esters as a novel biolabile prodrug type for non-steroidal anti-inflammatory carboxylic acid drugs. Int J Pharm 43:101–110
Chiha M, Samar MH, Hamdaoui O (2006) Extraction of chromium(VI) from sulphuric acid aqueous solutions by a liquid surfactant membrane (LSM). Desalination 194:69–80
Choina J, Kosslick H, Fischer C, Flechsig G-U, Frunza L, Schulz A (2013) Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl Catal B-Environ 129:589–598
Dâas A, Hamdaoui O (2010a) Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. J Hazard Mater 178:973–981
Dâas A, Hamdaoui O (2010b) Extraction of bisphenol A from aqueous solutions by emulsion liquid membrane. J Membr Sci 348:360–368
Djenouhat M, Hamdaoui O, Chiha M, Samar MH (2008a) Ultrasonication-assisted preparation of water-in-oil emulsions and application to the removal of cationic dyes from water by emulsion liquid membrane: Part 1. Membrane stability. Sep Purif Technol 62:636–641
Djenouhat M, Hamdaoui O, Chiha M, Samar MH (2008b) Ultrasonication-assisted preparation of water-in-oil emulsions and application to the removal of cationic dyes from water by emulsion liquid membrane: Part 2. Permeation and stripping. Sep Purif Technol 63:231–238
Fent K, Weston A, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76:122–159
Illés E, Takacs E, Dombi A, Gajda-Schrantz K, Gonter K, Wojnarovits L (2012) Radiation induced degradation of ketoprofen in dilute aqueous solution. Radiat Phys Chem 81:1479–1483
Juang R-S, Lin K-H (2004) Ultrasound-assisted production of W/O emulsions in liquid surfactant membrane processes. Colloids Surf A Physicochem Eng Asp 238:43–49
Kulkarni PS, Mahajani VV (2012) Application of liquid emulsion membrane (LEM) process for enrichment of molybdenum from aqueous solutions. J Membr Sci 201:123–135
Madhavan J, Grieser F, Ashokkumar M (2010) Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J Hazard Mater 178:202–208
Marco-Urrea E, Pérez-Trujillo M, Cruz-Morató C, Caminal G, Vicent T (2010) White-rot fungus-mediated degradation of the analgesic ketoprofen and identification of intermediates by HPLC–DAD–MS and NMR. Chemosphere 78:474–481
Méndez-Arriaga F, Torres-Palma RA, Pétrier C, Esplugas S, Gimenezd J, Pulgarin C (2008) Ultrasonic treatment of water contaminated with ibuprofen. Water Res 42:4243–4248
Méndez-Arriaga F, Esplugas S, Giménez J (2010) Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res 44:589–595
Metcalfe CD, Miao XS, Koenig BG, Struger J (2003) Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ Toxicol Chem 22:2881–2889
Miege C, Choubert JM, Ribeiro L, Eusebe M, Coquery M (2007) Removal efficiency of pharmaceuticals and personal care products with varying wastewater treatment processes and operating conditions – conception of a database and first results. In: Köhl A (ed) Proceedings of fifth IWA specialized conference on assessment and control of micropollutants/hazardous substances in water. Micropol & Ecohazard. DECHEMA e.V, Frankfurt/Main, pp 220–225
Mozia S, Morawski AW (2012) The performance of a hybrid photocatalysis–MD system for the treatment of tap water contaminated with ibuprofen. Catal Today 193:213–220
Ng YS, Jayakumar NS, Hashim MA (2010) Performance evaluation of organic emulsion liquid membrane on phenol removal. J Hazard Mater 184:255–260
Peng W, Jiao H, Shi H, Xu C (2012) The application of emulsion liquid membrane process and heat-induced demulsification for removal of pyridine from aqueous solutions. Desalination 286:372–378
Sabry R, Hafez A, Khedr M, El-Hassanin A (2007) Removal of lead by an emulsion liquid membrane: Part I. Desalination 212:165–175
Santos JL, Aparicio I, Alonso E (2007) Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ Int 33:596–601
Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL (2006) A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm 3:631–643
Tixier C, Singer HP, Oellers S, Müller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37:1061–1068
Williams NS, Ray MB, Gomaa HG (2012) Removal of ibuprofen and 4-isobutylacetophenone by non-dispersive solvent extraction using a hollow fibre membrane contactor. Sep Purif Technol 88:61–69
Williams NS, Gomaa HG, Ray MB (2013) Effect of solvent immobilization on membrane separation of ibuprofen metabolite: a green and organic solvent analysis. Sep Purif Technol 115:57–65
Yalkowski SH, He Y, Jain P (2010) Handbook of aqueous solubility data, 2nd edn. CRC Press, Boca Raton
Yan J, Pal R (2004) Effects of aqueous-phase acidity and salinity on isotonic swelling of W/O/W emulsion liquid membranes under agitation conditions. J Membr Sci 244:193–203
Yang L, Zhang Z, Guo Y, Gao X, Takeuchi H (2005) Uranium(VI) extraction by liquid surfactant membrane with N-alkylcaprolactams as a carrier. Sep Purif Technol 47:88–94
Zheng BG, Zheng Z, Zhang JB, Luo XZ, Wang JQ, Liu Q, Wang LH (2011) Degradation of the emerging contaminant ibuprofen in aqueous solution by gamma irradiation. Desalination 276:379–385
Acknowledgments
The financial support by the General Directorate for Scientific Research and Technological Development (PNR project no. 4/D/25) and the Ministry of Higher Education and Scientific Research of Algeria (projects No. J0101120090018 and J0101120120098) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Dâas, A., Hamdaoui, O. Removal of non-steroidal anti-inflammatory drugs ibuprofen and ketoprofen from water by emulsion liquid membrane. Environ Sci Pollut Res 21, 2154–2164 (2014). https://doi.org/10.1007/s11356-013-2140-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2140-9