Abstract
Invasive plants have wide-ranging impacts on native systems including reducing native plant richness and altering soil chemistry, microbes, and nutrient cycling. Increasingly, these effects are found to linger long after removal of the invader. We examined how soil chemistry, bacterial communities, and litter decomposition varied with cover of Euonymus fortunei, an invasive evergreen liana, in two central Kentucky deciduous forests. In one forest, E. fortunei invaded in the late 1990s but invasion remained patchy and we paired invaded and uninvaded plots to examine the associations between E. fortunei cover and our response variables. In the second forest, E. fortunei had completely invaded the forest by 2005; areas where it had been selectively removed by 2010 were paired with an adjacent invaded plot. Where E. fortunei had patchily invaded, E. fortunei patches had up to 3.5× nitrogen, 2.7× carbon, and 1.9× more labile glomalin in soils than uninvaded plots, whereas there were no differences in soil characteristics between invaded and removal plots. In the patchily invaded forest, bacterial community composition varied among invaded and non-invaded plots, whereas bacterial communities did not vary among invaded and removal plots. Finally, E. fortunei leaf litter decomposed faster (k = 4.91 year−1) than the native liana (k = 3.77 year−1), Vitis vulpina; decomposition of both E. fortunei and V. vulpina was faster in invaded (k = 7.10 year−1) than removal plots (k = 4.77 year−1). Our findings suggest that E. fortunei invasion increases the rate of leaf litter decomposition via high-quality litter, alters the decomposition environment, and shifts in the soil biotic communities associated with a dense mat of wintercreeper. Land managers with limited resources should target the densest mats for the greatest restoration potential and remove wintercreeper patches before they establish dense mats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exotic plant species have been shown to alter ecosystem structure through negative effects on the growth and performance of native plants (Miller and Gorchov 2004; Hartman and McCarthy 2004) resulting in reduced plant richness and evenness (Vilà et al. 2011). Such changes in plant richness and evenness alter the quantity and quality of leaf litter inputs available to detritivores and soil microbes (Liao et al. 2008; Heberling and Fridley 2013; Jo et al. 2015). Leaf litter chemistry is a key driver of decomposition rate and invasive species have repeatedly been shown to increase decomposition rate and nutrient cycling (Ashton et al. 2005; Arthur et al. 2012; but see Jo et al. 2016). While changes in ecosystem functions after invasion have, in part, been attributed to changes in litter quality and quantity, invasive plants may also alter ecosystem functions by directly altering the abiotic environment including soil pH, chemistry, and moisture (Ehrenfeld et al. 2001; Heneghan et al. 2006; Iannone et al. 2015) or indirectly by altering the decomposer community (Strickland et al. 2009; Arthur et al. 2012; Piper et al. 2015). The relative importance of invaders’ impacts on litter chemistry and quantity and the abiotic and biotic decomposition environment is not well understood and seems to vary by species (Liao et al. 2008).
Invasive lianas may have even greater potential for altering ecosystem processes and nutrient cycling than invasive shrubs and trees. Because lianas are structural parasites, they invest more in leaves relative to stems (Powers 2014) and lianas can make up to 40% of leaf litter inputs in tropical forests (Tang et al. 2012). As native liana diversity is relatively low in North America (Leicht-Young and Pavlovic 2014), invasion by non-native lianas has great potential to alter both the quantity and quality of leaf litter inputs. In the most comprehensive study of liana leaf characteristics to date, lianas were found to have greater leaf nitrogen (12%) and phosphorus (9%) than trees on a mass basis, and 11-26% less defensive compounds such as lignin, phenols, and tannins (Asner and Martin 2012). As these traits are also known to be positively correlated with decomposition rates (Cornwell et al. 2008), any increase in the abundance of lianas has great potential to alter ecosystems processes as well as soil physio-chemical properties. Extensive studies of native and non-native invasive lianas in Australia have indicated that leaves of invasive vines have lower construction costs and C:N and higher nitrogen, potassium, and phosphorus concentrations than native lianas, and non-native lianas occur in soils with greater moisture, nitrogen, phosphorus, and potassium (Osunkoya et al. 2010; Perrett et al. 2012). Most studies of the impacts of invasive lianas in North America have focused on distribution, competition, and response to disturbance (Leicht-Young and Pavlovic 2014) although there is increasing evidence that invasive lianas also alter soil conditions in North America (Leicht-Young et al. 2009; Smith and Reynolds 2012; Leicht-Young et al. 2015).
Euonymus fortunei (Turxz.) Hand-Maz [Celastraceae], herein wintercreeper, is a perennial evergreen liana native to China and known to be an invader in at least 11 states (Invasive Plant Atlas of the United States 2013). Wintercreeper grows in diverse environments from heavy-shade to full-sun, creating dense, near-monoculture ground cover that shades out natives (Hutchison 1992; Swearingen et al. 2010; Mattingly et al. 2016). Wintercreeper offers a unique case study of North American liana invasion as its density suggests potential for high-volume leaf litter inputs that may alter soil physio-chemical properties, while its evergreen nature and ability to grow in deep-shade suggest that it may possess leaf traits more consistent with long leaf-life and highly defended, low-quality litter (Reich et al. 1997; Cornwell et al. 2008). Few studies have examined plant–soil interactions of wintercreeper; however, wintercreeper has been found to grow better in soil conditioned by conspecifics than by native species, while natives grew better in soil conditioned by other species (Smith and Reynolds 2012, 2015). Thus, wintercreeper’s unique life history as an invasive evergreen liana along with indicators that wintercreeper alters soil conditions to its advantage make it a unique study system to understand how invasive lianas may alter soil characteristics, soil biota, and ecosystem processes.
Our study took place in the north-central Bluegrass Physiographic Region of Kentucky. Most forests in this region exhibit a binary of invasion: sites with nearly 100% invasive cover and sites uninvaded by wintercreeper. We located a 20-ha rural forest, ‘Scotts Grove’ (Bloom et al. 2002), in which wintercreeper invasion was patchy with some areas reaching close to 100% cover while other areas remain uninvaded. This site allowed for an observational study of the association of wintercreeper with soil characteristics, decomposition and nitrogen loss from litter, and bacterial community composition. We paired this rural, patchily invaded forest with an urban forest fragment with known history where wintercreeper had formed a continuous layer and that has been experimentally removed in specific sections. Preliminary studies in the urban forest fragment suggested that wintercreeper decomposed very quickly, on par with invasive Lonicera maackii (unpublished data, Arthur et al. 2012); in that preliminary study, however, we did not have a functionally equivalent native liana. In the current study, we sought to compare the decomposition of wintercreeper to that of a native deciduous liana, Vitis vulpina (frost grape). We hypothesized that: (H1) despite its evergreen life history, wintercreeper litter would have higher litter quality and faster decomposition rates than the native frost grape; (H2) wintercreeper cover would be associated with differences in soil physical, chemical, and biotic environments relative to plots without cover; (H3) decomposition rate would be faster under wintercreeper cover compared to plots without cover due to wintercreeper’s litter quality and reduced soil drying; and (H4) due to different land-use and invasion histories, associations of wintercreeper cover with soil biotic and abiotic conditions and decomposition rate would be site specific.
Methods
The study was conducted in two forested sites located in the Bluegrass Physiographic Region of central Kentucky, where soils are moderately to well-drained silt loams of high pH underlain by Middle Ordovician Lexington Limestone (Wharton and Barbour 1991). The regional land use is a mix of agriculture and urban/suburban development, with forest fragments interspersed throughout.
The University of Kentucky Lexington-Fayette Arboretum forest is a mixed eastern deciduous forest remnant measuring 5.8 ha, in Fayette County, Kentucky. The canopy is primarily composed of Celtis occidentalis, Juglans nigra, Fraxinus americana, Acer negundo, Prunus serotina, and Quercus macrocarpa (Mattingly et al. 2016). In the decades preceding this study, the site was managed with grazing and understory mowing, which ceased in 1980. Wintercreeper was already present when mowing ceased, but the site was then quickly invaded by a dense stand of Amur honeysuckle. Honeysuckle removal in 2005 revealed a dense cover of wintercreeper which had spread throughout the understory. Since then it has been the site of ongoing wintercreeper removal with removal efforts used in this study completed by 2010. Plots at the Arboretum without wintercreeper cover are “removal” areas, with potential legacy effects of wintercreeper on soil structure, chemistry, and bacterial communities, and are referred to as “removal” plots.
The forest at Scott’s Grove, located in Jessamine County, KY, is dominated by Acer saccharum, Carya glabra, C. ovata, Fraxinus americana, F. quadrangulata, Quercus alba, Q. muhlenbergii, Q. shumardii, Q. velutina, and Ulmus rubra (J. Campbell, pers. comm.). The site has been in private ownership and primarily used for hunting for the past 50 years; aerial photos by the USDA suggest that it has not been logged during this time (Bloom et al. 2002). Wintercreeper began invading Scotts Grove in the late 1990s and was well established by 2004 (C. Baskin, pers. comm.). Wintercreeper invasion is variable and highly patchy; hence, in this site we have true “uninvaded” plots. These uninvaded plots typically occur in close proximity to invaded locations (within 5 m) and do not vary in slope or canopy composition and cover.
We examined the effect of wintercreeper cover on the rate of decomposition of non-native and native species of lianas (wintercreeper and frost grape), in paired plots with and without ground cover of wintercreeper. Removal/invaded plot pairs were spread throughout the Arboretum woods, but paired plots were no more than 3 m apart (see Supplemental Materials Fig. 1). The area of wintercreeper removed ranged from 3 m × 3 m to 8 m × 8 m with all samplings occurring in the center 1.5 m × 1.5 m. Wintercreeper was removed from plots over the period of 2005–2010 with winter application of glyphosate used to remove wintercreeper in plots 1, 4, and 5 and a plastic tarp covering the ground for plots 2 and 3. We collected senescent leaves at the Arboretum in the winter of 2013 and fall of 2014. Leaves were deemed senescent when an abscission line had formed and they fell freely from the plant. Ten gram samples of air-dried leaves were incubated in bags with mesh size of 1 mm2. Samples were placed in 10 plots (5 with wintercreeper cover and 5 with wintercreeper removed or uninvaded) at each study site. Samples were placed in an approximately 1.5 m × 1.5 m array in each of the ten plots at the Arboretum site on February 21, 2014 and Scott’s Grove samples were placed on February 24, 2014 (t = 0). Samples were picked up after 4, 8, 12, 26, and 52 weeks and mass and nutrients remaining were measured.
Five subsamples of initial leaf litter were analyzed for total C and N using an Elementar vario MAX CNS analyzer (Elementar, Germany). Carbon fractions from 5 subsamples of initial litter of each species were extracted with increasingly acidic solutions (van Soest 1963) using an Ankom 220 Fiber Analyzer (Ankom, Macadon, NY, USA). We also analyzed initial concentrations of cations (Ca, Mg, and K) and phosphorus (P) in leaf litter. Samples of 0.25 g were ashed overnight at 500 °C, then acidified with 6 N HNO3 and heated, filtered, and the eluent analyzed for Ca, Mg, and K on a GBC Avanta atomic absorption spectrophotometer (GBC Scientific Equipment, Hampshire, IL). Samples were analyzed for P using the malachite green micro-plate method (D’Angelo et al. 2001).
Characterization of decomposition environment
We examined soil texture, moisture, percent carbon and nitrogen, and carbon fractions in all plots. Soil texture was determined using the micro-pipette method (Miller and Miller 1987). Soil carbon fractions were determined on soils collected at depths of 0–5 and 5–10 cm in late February and early March, 2015. Soil carbon was extracted using cold water extraction followed by hot-water extraction which represents labile carbon using the methods of Ghani et al. (2003). Total organic carbon in both cold- and hot-water extractions was determined on a Shimadzu TOC-V total organic carbon analyzer (model TOC-VCSN). Total carbohydrate concentration for both extractions was determined via phenol method without acid hydrolysis (Ghani et al. 2003) followed by colorimetric quantification. Acid hydrolysis of soil for 1 h under 15 psi was followed by colormetric quantification (Lowe 1993). Easily extractable (labile) and total glomalin were extracted using the Broadford assay procedure (Wright and Upadhyaya 1998) and quantified colorimetrically. All colorimetric quantification was performed on a Genesys 20 spectrophotometer (Thermo Scientific). Total soil carbon and nitrogen of soils were determined using an Elementar vario MAX CNS analyzer (Elementar, Germany). Soil moisture samples for both 0–5 cm depth and 5–10 cm depth were collected approximately bi-weekly from all plots for the duration of the study. On the last pickup date (February 14 at Scott’s Grove and March 11, 2015 at the Arboretum), the height of wintercreeper cover from the forest floor was measured at five points in each invaded plot and averaged. The final pick up dates were different at the two sites due to a large snowstorm that arrived on February 15, 2015.
Soil bacterial community sequencing
We collected three 10-cm-depth soil cores at the margins of each plot at the four-week collection date. Cores were combined by plot and passed through a 2-mm sieve. Total DNA was extracted from 0.25 g of soil from each sample using the PowerSoil DNA Isolation Kit (MoBio, Carlsbad, CA). We followed the manufacturer’s protocol with the exception of adding 60 µL of 50 mg/mL lysozyme and incubating at 37 °C for 1 h prior to the first step of the manufacturer’s protocol. We amplified the 16S rRNA gene using barcoded primers designed for Illumina MiSeq (Caporaso et al. 2012). Amplicons were sequenced using 250 × 250 paired-end sequencing with an Illumina MiSeq at the Indiana University Center for Genomics and Bioinformatics. Contig assembly, quality trimming, alignment, and chimeric sequence removal were completed with mothur v. 1.31.2 (Schloss et al. 2009) using the methods of Muscarella et al. (2014). We removed mitochondrial, archaeal, and eukaryotic sequences by identifying them with the Ribosomal Data Base Project’s 16S rRNA reference sequences and taxonomy version 7 (Cole et al. 2009). We clustered remaining sequences into operational taxonomic units (OTUs) using nearest-neighbor distance and 97% sequence similarity in mothur. Because not all samples had equal sequencing coverage, we subsampled 60,000 sequence observations from each sample before estimating OTU richness and evenness. After 1000 subsampling iterations, we calculated average richness, Pielou’s evenness, and Shannon–Wiener index for each sample. Richness and evenness estimates were performed in R v. 3.1.0 (R Core Team 2015).
Statistical analysis
To compare litter decay rates between species and wintercreeper cover treatments, decomposition rate constants (k) for each litter type were determined using a negative exponential model:
where X t equals the amount of mass left at time = t, X 0 is the initial mass of litter, and t = time in years (Olson 1963). Because the native cover plots at the Arboretum and Scotts Grove represent removal and uninvaded treatments, respectively, all analyses were performed separately for each site. Differences in initial litter chemistry of frost grape and wintercreeper and wintercreeper cover depth between sites were examined using a Student’s t test. Bacterial richness and evenness between cover (invaded and removal/uninvaded) were examined with Student’s paired t-test at each site. Differences in soil texture between cover types within a site were compared with a paired Wilcoxon sign test as the proportion of each soil texture category which must, by definition, add up to 1.0. Differences in soil conditions among cover (invaded, uninvaded/removal) and soil depth (0–5, 5–10 cm) were determined using two-way ANOVA. When significant interaction effects were identified, we followed up with a Tukey HSD to determine differences among all groups. Soil moisture was averaged by season and differences in soil moisture were examined with a three-way blocked ANOVA (cover, depth, and season). The effects of litter type (wintercreeper/frost grape) and cover (invaded, removal/uninvaded) on mass loss and nitrogen dynamics of litter were examined with two-way blocked ANOVA. Nitrogen dynamics in litter were examined using a blocked three-way ANOVA (species, cover, and time). To examine the response of soil bacterial community composition to wintercreeper invasion or removal, we used Principle Coordinates Analysis (PCoA) on Bray–Curtis distance matrices of log-transformed relativized OTU abundances. To determine if soil bacterial community composition varied as a function of plant cover, we ran a PERMANOVA with the adonis function in the ‘vegan’ package v. 2.3-0 (Oksanen et al. 2015) of R. When differences between bacterial communities of invaded and removal/uninvaded plots were discovered, indicator species analysis was used to determine which OTUs were indicative of cover type with indval function in the ‘labdsv’ package (Roberts 2015). All statistical analyses were performed in R v. 3.1.0 (R Core Team 2015) except for analyses of differences in litter cation and phosphorus concentrations which were analyzed with JMP v. 10.0 (SAS Institute 2012).
Results
Litter chemistry and decomposition
Wintercreeper had generally higher litter quality than frost grape. Wintercreeper had higher initial %carbon, (t = 3.79, p < 0.001) and %nitrogen (t = 23.3, p < 0.001) than frost grape, and lower C:N (t = 18, p < 0.001, Table 1). Frost grape had greater initial calcium (t = 9.04, p < 0.001) and magnesium (t = 3.07, p < 0.01), while wintercreeper had higher potassium (t = 12.5, p < 0.001, Table 1). Species did not differ in initial phosphorus (t = 0.06, p > 0.05). There were no differences between species in the most labile carbon fractions, but frost grape had a greater proportion of hemicellulose (t = 3.27, p < 0.01) and lignin (t = 10.2, p < 0.001), while wintercreeper had a greater proportion of cellulose (t = 9.9, p < 0.001, Table 1).
Wintercreeper decomposed faster than frost grape at the Arboretum (F1,12: 7.25, p = 0.02) with 50% of its mass lost in 37 days, while it took 49 days for frost grape to lose 50% of its mass (Table 2) and decomposition was 1.5x faster in invaded than removal cover plots (F1,12: 14.7, p = 0.002, Fig. 1a). At Scotts Grove, although wintercreeper lost 50% of its mass in 81 days and frost grape took 103 days (Fig. 1b) and initial mass remaining was lower for wintercreeper than frost grape for times 1–4 (F range 5.5–92.4, p < 0.05), by the end of the study there were no differences in decomposition rate constants as a function of litter species (F1,12: 2.44, p > 0.1, Table 2). There was no difference in decomposition rate as a function of vegetation cover at Scotts Grove (F1,12: 0.81, p > 0.1).
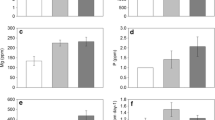
Nitrogen concentrations in litter increased through time (F4,56: 20.5, p < 0.001) at the Arboretum with frost grape (F1,56: 386, p < 0.001) and invaded plots (F1,56: 10.5, p = 0.002) having the highest nitrogen concentrations (Fig. 2a). There was limited immobilization of nitrogen in frost grape (species x time F4,56: 16.5, p < 0.001) decomposing in invaded cover plots (species × cover F1,56: 4.49, p = 0.04, Fig. 2b), with initial nitrogen remaining generally declining through time (F4,56: 40.6, p < 0.001). Similarly at Scotts Grove, nitrogen concentration increased through time (Fig. 2c, F4,70: 47.0, p < 0.001), with higher nitrogen concentrations under invaded cover (F1,70: 20.7, p < 0.001). Differences between invaded and uninvaded cover increased with time (cover × time interaction F4,70: 4.00, p = 0.005). There was some immobilization of nitrogen in frost grape samples (species × time F4, 70: 18.5, p < 0.001) with greater initial nitrogen remaining in invaded cover plots (F1,70: 12.1, p < 0.001). Cation and phosphorus dynamics showed similar patterns to nitrogen dynamics (Supplemental Materials, Figs. 2, 3, 4, and 5).
Nitrogen dynamics of litter incubated in invaded (closed symbols) and removal/uninvaded plots (open symbols). Nitrogen dynamics of wintercreeper (diamonds), frost grape (squares), at the Arboretum (a and b) and Scotts Grove (c and d). Panels a and c represent N concentration of litter. Panels b and d represent N mass remaining (percent of initial litter N remaining). Error bars show ±SE
Decomposition environment
Soil moisture varied with depth, cover, and season at both sites (Fig. 3, Table 3). At the Arboretum, soil moisture was higher in the top 5 cm and these differences were greatest in spring and winter (significant depth x season interaction, Table 3). Invaded plots at the Arboretum had slightly higher soil moisture than removal plots (Fig. 3a). Soil moisture at Scotts Grove was also highest in the top 5 cm (Fig. 3b), with differences between depths greatest in spring and winter (Table 3). Soil moisture content was 16% greater in invaded plots than uninvaded plots at Scotts Grove in the spring. Soils in the Arboretum were classified as having ‘silt’ texture, whereas those at Scott’s Grove, where there was higher percent sand and lower percent clay, were classified as silt-loam, clay-loam, and silty-clay-loam (Table 4). Soil texture did not vary between treatments within site. Wintercreeper groundcover depth was significantly greater at the Arboretum (18.6 ± 0.3 cm) than at Scott’s Grove (13.0 ± 1.2, t = 4.48, p < 0.01).
Soil carbon and nitrogen concentrations also varied according to vegetation cover and depth, in ways that differed between the two sites (Table 5). At Scotts Grove, soil nitrogen levels were 1.8x (0–5 cm) to 3.5x (5–10 cm) higher in invaded plots than non-invaded plots (F1,12: 57.3, p < 0.001). Total soil %C (F1,12:, 45.9, p < 0.001) and labile glomalin (F1,11:, 22.6, p < 0.001) were 1.6x and 2.3x greater in invaded plots at 0-5 cm and 2.7x and 4.6x greater at 5–10 cm, respectively. Labile polysaccharides (F1,11: 7.53, p = 0.02) and total glomalin (F1,11: 7.82, p < 0.001) levels at the 5–10 cm depth were 2x those of non-invaded plots although there were no differences at the most shallow depth. Unlike Scotts Grove, soil variables examined did not vary between invaded and removal plots at the Arboretum (p > 0.05, Table 5). Both sites saw a decrease in several soil abiotic characteristics with depth. At Scotts Grove, %C (F1,12: 28.5, p < 0.001), %N (F1,12: 20.2, p < 0.001), and hot-extracted carbohydrates (F1,12: 33.6, p < 0.001), hot-extracted DOC (F1,12: 8.25, p = 0.02), labile polysaccharides (F1,11: 32.5, p < 0.001), labile glomalin (F1,11: 10.1, p = 0.008), and total glomalin (F1,11: 33.6, p < 0.001) decreased with depth (Table 5). At the Arboretum, %C (F1,12: 42.1, p < 0.001), %N (F1,12: 35.3, p < 0.001), hot-extracted carbohydrates (F1,12: 11.7, p < 0.001), hot-extracted DOC (F1,12: 5.06, p = 0.04), labile polysaccharides (F1,12: 21.4, p < 0.001), labile glomalin (F1,12: 15.5, p = 0.002), and total glomalin (F1,12: 29.3, p < 0.001) all decreased with depth.
Bacterial community composition
A total of 31,931 OTUs were classified at the 97% similarity level. There were no differences in taxa richness or evenness (Table 6) between cover types within a site. After removal of zero sum OTUs, bacterial community composition was examined on the relative abundance of remaining OTUs (Arboretum: 21,730, Scotts Grove: 22,413). At the Arboretum, bacterial communities tended to be most similar among spatially paired plots (Fig. 4a), and pH was negatively and positively correlated with PCoA Axes 1 and 2, respectively. There was no difference in the composition of the bacterial communities between invaded and removal plots (PERMANOVA, R 2 = 0.066, p = 0.8). At Scotts Grove, bacterial communities tended to be similar in invaded plots (Fig. 4b) which also tended to have higher soil water content and pH. One uninvaded plot (N4) clustered with invaded plots and did have low, but some, wintercreeper cover. There was a significant difference in bacterial community composition among invaded and uninvaded plots (PERMANOVA, R2 = 0.23, p = 0.03). Of the 22,413 OTUs found at Scotts Grove, 3.6% of them were indicator species for either uninvaded or invaded plots. Indicator taxa represented 12 phyla with the most coming from Proteobacteria, followed by Acidobacteria and Actinobacteria (Table 7). Only wintercreeper plots had indicator taxa from the genus Nitrospira, known nitrite-oxidizers.
Principle Coordinates Analysis of relative abundance of OTUs at a the Arboretum and b Scotts Grove. Filled symbols (invaded plots); open symbols (removal/uninvaded plots). Spatially paired plots have the same number, while N indicates removal/uninvaded plots and WC indicated wintercreeper-invaded plots. Vectors represent the strength and direction of correlation (p < 0.05) between soil abiotic characteristics (A = 0–5 cm depth, B = 5–10 cm depth) and PCoA axes. Percentages on each axis represent percent of variation in the distance matrix described by that axis
Discussion
We hypothesized (H1) that wintercreeper would possess higher litter quality and decompose faster than the native liana, frost grape, due to preliminary data suggesting high decomposition rates for wintercreeper. Consistent with studies comparing invasive and native vines, wintercreeper did have higher litter quality than the native frost grape (Osunkoya et al. 2010; Perrett et al. 2012). Although we predicted that as an invader wintercreeper would have higher litter quality than frost grape, evergreen leaves generally contain more defensive compounds and lower quality litter compared to deciduous leaves (Reich et al. 1997; Cornwell et al. 2008). The low quality of evergreen litter is due to the negative correlations of leaf traits such as maximum photosynthetic rate and leaf nitrogen with leaf age (Reich et al. 1992). While we are aware that some wintercreeper leaf turn over occurs in the fall, it is possible that leaves turn over throughout the year. This may mean that average leaf lifespan of wintercreeper is not longer than frost grape although more investigation is needed. Alternatively, wintercreeper’s lower investment defensive compounds such as hemicellulose and lignin and lower C:N ratio may also suggest a plastic response of wintercreeper to reduced herbivory in its introduced range (Pysek and Richardson 2007; Feng et al. 2008). Anecdotally, we have not observed much herbivory damage on wintercreeper, but no data currently exist in the literature on herbivory pressure on wintercreeper in its introduced range. Consistent with our hypothesis and with the two species’ litter quality, wintercreeper did decompose more rapidly than the native frost grape in early decomposition at Scotts Grove and throughout decomposition at the Arboretum. These differences were likely due to the lower C:N, hemicellulose, and lignin found in wintercreeper compared to frost grape.
We also hypothesized (H2) that wintercreeper-covered plots would be associated with higher nutrient- and carbon levels in the soil as well as a unique bacterial community. We only found differences in decomposition environment between invaded and uninvaded plots at Scotts Grove where wintercreeper presence was associated with higher soil moisture content, soil carbon and nitrogen, and labile polysaccharides and glomalin. The higher sand content of Scotts Grove soils may be responsible for the greater variability in soil moisture and greater differences in soil moisture in relation to vegetation cover. Many invasive plants have been shown to increase nutrients and nutrient cycling due to high-quality litter (Ehrenfeld et al. 2001; Ashton et al. 2005; Arthur et al. 2012). Invasive lianas, in particular, have great potential for altering soil nutrients as they exhibit fast-growth life history traits such as high photosynthetic rate (Leicht-Young and Pavlovic 2014) that are correlated with high litter quality traits (Cornelissen and Thompson 1997). Celastrus orbiculatus (oriental bittersweet), also a liana in the Celastraceae family, increased soil nutrient level and nitrogen mineralization (Leicht-Young et al. 2009). Both Celastrus and wintercreeper may be changing soil conditions due to their relatively high leaf N and low C:N content (Leicht-Young et al. 2009, Table 6). The litter may be influencing soil conditions either via litter inputs and their decomposition or via leaching of minerals and soluble carbon compounds from the living wintercreeper mat (Levia and Frost 2003). Higher soil carbon levels found in invaded plots, particularly labile glomalin, are likely derived from root production and leachates. Higher soil C and labile carbon compounds may serve as a resource for the soil microbial community.
The composition of the soil bacterial community in invaded areas was significantly different from bacterial communities in uninvaded areas of Scotts Grove although richness did not vary. Invasive plants have frequently been found to alter microbial communities (Arthur et al. 2012; Lin et al. 2014; Piper et al. 2015). We found that bacterial communities were more similar to each other among invaded plots than communities in uninvaded plots were to one another. Piper et al. (2015) similarly found that as the percent cover of invasive smooth brome (Bromus inermis) increased, bacterial communities became more similar. In the smooth brome bacterial communities, this similarity in bacterial communities was driven by an increase in rare bacterial species and an overall increase in species richness (Piper et al. 2015). We did not observe differences in species richness or evenness. Instead, it seems most likely that wintercreeper altered the abundance of common bacteria rather than increasing rare species. While the plurality of indicator species for both invaded and uninvaded plots was Proteobacteria, Actinobacteria were the 2nd most represented phyla in invaded plots, while Acidobacteria were the 2nd most represented phyla in uninvaded plots. As Actinobacteria are considered to be copiotrophic and Acidobacteria oligotrophic (Fierer et al. 2012), it appears that the higher level of labile carbon compounds and nitrogen associated with wintercreeper invasion is favoring a more copiotrophic bacterial community. Copiotrophic bacteria in the soil have been hypothesized to have high growth rates, brief lags to substrate additions, high maintenance requirements, and low C:N and C:P ratios (Fierer et al. 2007). Such a bacterial community may translate to faster decomposition and nutrient cycling rates but may also result in greater competition between plants and bacteria for soil nutrients. Although we did not examine fungi in this study, the higher levels of glomalin in wintercreeper-invaded areas suggest higher biomass of mycorrhizal fungi and levels of exudates. The combination of a shift in the bacterial community and a possible increase in mycorrhizal fungi with wintercreeper may, in part, explain the positive soil-feedback on wintercreeper growth that Smith and Reynolds (2015) found. Although Smith and Reynolds (2015) did not explicitly examine mycorrhizae, mycorrhizae are known to be agents of both positive feedback on invasive plants and negative feedback on native plants (Bever 2002; Bray et al. 2003; Reinhart and Callaway 2006).
We hypothesized (H3) that differences in decomposition environment where wintercreeper was present would result in faster decomposition of all species under wintercreeper mat. The presence of wintercreeper in a plot increased decomposition of litter only at the Arboretum. Interestingly, while there were changes in the biotic and abiotic soil environment with invasion at Scotts Grove, there was no wintercreeper cover effect on decomposition, whereas at the Arboretum there were no soil effects, but decomposition was faster under the wintercreeper mat. The wintercreeper mat at the Arboretum was deeper than at Scotts Grove. The thicker mat at the Arboretum may have maintained a moister environment with more constant temperature between the soil surface and the mat, particularly during winter months, that would increase decomposition rate (Swift et al. 1979; Couteaux et al. 1995). The association of higher decomposition rate with thicker wintercreeper mats also has management implications as it suggests that targeting the thickest, deepest wintercreeper mats would reduce changes in decomposition rates in invaded forests.
Finally, we hypothesized (H4) that differences in the sites’ land-use histories would result in site-specific differences in the association of wintercreeper with the response variables examined. Because our removal and uninvaded plots were nested within sites, we are unable to separate site effects from legacy effects. In general, we found differences between wintercreeper-invaded and uninvaded plots at Scotts Grove in soil abiotic and biotic conditions but not at the Arboretum, whereas we only found differences in decomposition rate as a function of wintercreeper cover at the Arboretum. These site-specific differences in soils may suggest a possible legacy effect of wintercreeper invasion on soil biotic and abiotic conditions at the Arboretum. At the Arboretum, C, N, and labile polysaccharides and glomalin levels did not vary between invaded and removal plots and levels were intermediate between invaded and uninvaded areas of Scotts Grove. It is possible that C, N, polysaccharides, and glomalin levels were increased with invasion as was seen at Scotts Grove, but have not returned to pre-invasion levels since wintercreeper removal at the Arboretum. As with most approaches to invasion control, wintercreeper removal was achieved by removing and killing the aboveground biomass. Previous work manipulating above- and belowground biomass has found that belowground biomass has a larger impact on long-term soil legacies (Holub et al. 2005; Keith et al. 2009; Elgersma et al. 2011). We also found that bacterial communities did not differ between invaded and removal plots within the Arboretum. This joins a growing body of literature that suggests removal of invaders or short-term modification of litter inputs does not restore microbial communities (Elgersma et al. 2011; Kulmatiski and Beard 2011; Lankau et al. 2014; but see Carey et al. 2015). In the case of wintercreeper, a microbial legacy after removal is particularly concerning as wintercreeper is known to benefit from positive feedback via wintercreeper-conditioned soil (Smith and Reynolds 2012, 2015).
Our data were generated from a natural invasion with experimental removal, and thus, it is difficult to eliminate the alternative hypothesis that wintercreeper does not change decomposition environment and rate; rather, it preferentially grows in such soil conditions. Wintercreeper has previously been found to alter the soil environment in Indiana forests (Swedo et al. 2008); thus, our findings confirm those results. Additionally, once established, wintercreeper expands to cover most of the forest floor except for seasonally flooded areas (pers. obs.) which do not exist in either of our sites suggesting wintercreeper has not yet reached the uninvaded areas of Scotts Grove. It is also possible that the legacy effects that we observed at the Arboretum are not legacy of wintercreeper presence, but previous land-use history. While we cannot eliminate this alternative hypothesis, our data do suggest wintercreeper has the potential to create legacy effects. Carbon, nitrogen, and labile glomalin levels in both invaded and removed plots at the Arboretum have values between those in invaded and uninvaded plots at Scotts Grove. This may indicate that carbon, nitrogen, and labile glomalin increased with wintercreeper and have not returned to pre-invasion levels.
Conclusions
Our study shows wintercreeper invasion is associated with altered soil and ecosystem processes. Resources for soil microbes are higher where wintercreeper is present and bacterial communities are composed of members with more copiotrophic life histories. Our finding of increased glomalin in wintercreeper-invaded plots requires additional direct study of the response of mycorrhizal fungi to wintercreeper invasion. Although our study suggests there is a potential for a legacy effect of wintercreeper after removal, more manipulative approaches are needed to determine the extent of legacy effects. Together with work by Smith and Reynolds (2012, 2015), our research suggests that wintercreeper modifies free-living bacterial communities and potentially mycorrhizal fungi to the benefit of wintercreeper. This benefit may last beyond the removal of wintercreeper, impeding restoration of native plant communities.
References
Arthur M, Bray S, Kuchle C, McEwan R (2012) The influence of the invasive shrub, Lonicera maackii, on leaf decomposition and microbial community dynamics. Plant Ecol 213:1571–1582
Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT (2005) Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous. Ecol Appl 15:1263–1272. doi:10.1890/04-0741
Asner GP, Martin RE (2012) Contrasting leaf chemical traits in tropical lianas and trees: implications for future forest composition. Ecol Lett 15:1001–1007. doi:10.1111/j.1461-0248.2012.01821.x
Bergmann GT, Bates ST, Eilers KG, Lauber CL, Caporaso JG, Walters WA, Knight R, Fierer N (2011) The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol Biochem 43:1450–1455. doi:10.1016/j.soilbio.2011.03.012
Bever JD (2002) Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc R Soc B Biol Sci 269:2595–2601. doi:10.1098/rspb.2002.2162
Bloom TC, Baskin JM, Baskin CC (2002) Ecological life history of the facultative woodland biennial Arabis laevigata variety laevigata (Brassicaceae): seed dispersal. J Torrey Bot Soc 129:21–28. doi:10.2307/3088679
Bray SR, Kitajima K, Sylvia DM (2003) Mycorrhizae differentially alter growth, physiology, and competitive ability of an invasive shrub. Ecol Appl 13:565–574. doi:10.1890/1051-0761(2003)013[0565:MDAGPA]2.0.CO;2
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi:10.1038/ismej.2012.8
Carey CJ, Beman JM, Eviner VT, Malmstrom CM, Hart SC (2015) Soil microbial community structure is unaltered by plant invasion, vegetation clipping, and nitrogen fertilization in experimental semi-arid grasslands. Front Microbiol. doi:10.3389/fmicb.2015.00466
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:141–145. doi:10.1093/nar/gkn879
Cornelissen JHC, Thompson K (1997) Functional leaf attributes predict litter decomposition rate in herbaceous plants. New Phytol 135:109–114
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. doi:10.1111/j.1461-0248.2008.01219.x
Couteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and liter quality. Trends Ecol Evol 10:63–66. doi:10.1016/S0169-5347(00)88978-8
D’Angelo E, Crutchfield J, Vandiviere M (2001) Rapid, sensitive, microscale determination of phosphate in water and soil. J Environ Qual 30:2206–2209
Ehrenfeld JG, Kourtev P, Huang WZ (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11:1287–1300
Elgersma KJ, Ehrenfeld JG, Yu S, Vor T (2011) Legacy effects overwhelm the short-term effect of exotic platn invasion and restoration on soil microbial community structure, enzyme activities, and nitrogen cycling. Oecologia 167:733–745
Feng YL, Fu GL, Zheng YL (2008) Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta 228:383–390. doi:10.1007/s00425-008-0732-2
Fierer N, Bradford M, Jackson R (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017. doi:10.1038/ismej.2011.159
Ghani A, Dexter M, Perrott KW (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol Biochem 35:1231–1243. doi:10.1016/S0038-0717(03)00186-X
Hartman KM, McCarthy BC (2004) Restoration of a forest understory after the removal of an invasive shrub, Amur Honeysuckle (Lonicera maackii). Restor Ecol 12:154–165. doi:10.1111/j.1061-2971.2004.00368.x
Heberling JM, Fridley JD (2013) Functional traits and resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol 200:523–533
Heneghan L, Fatemi F, Umek L, Grady K, Fagen K, Workman M (2006) The invasive shrub European buckthorn (Rhamnus cathartica, L.) alters soil properties in Midwestern U.S. woodlands. Appl Soil Ecol 32:142–148. doi:10.1016/j.apsoil.2005.03.009
Holub SM, Lajtha K, Spears JDH, Tóth JA, Crow SE, Caldwell BA, Papp M, Nagy PT (2005) Organic matter manipulations have little effect on gross and net nitrogen transformations in two temperate forest mineral soils in the USA and central Europe. For Ecol Manag 214:320–330. doi:10.1016/j.foreco.2005.04.016
Hutchison M (1992) Vegetation management guideline—Wintercreeper or climbing Euonymus (Euonymus-fortunei). Nat Areas J 12:220–221
Iannone BV, Heneghan L, Rijal D, Wise DH (2015) Below-ground causes and consequences of woodland shrub invasions: a novel paired-point framework reveals new insights. J Appl Ecol 52:78–88. doi:10.1111/1365-2664.12354
Jo I, Fridley JD, Frank DA (2015) Linking above- and belowground resource use strategies for native and invasive species of temperate deciduous forests. Biol Invasions 17:1545–1554. doi:10.1007/s10530-014-0814-y
Jo I, Fridley JD, Frank DA (2016) More of the same? In situ leaf and root decomposition rates do not vary between 80 native and nonnative deciduous forest species. New Phytol 209:115–122. doi:10.1111/nph.13619
Keith AM, Brooker RW, Osler GHR, Chapman SJ, Burslem DFRP, van der Wal R (2009) Strong impacts of belowground tree inputs on soil nematode trophic composition. Soil Biol Biochem 41:1060–1065. doi:10.1016/j.soilbio.2009.02.009
Kulmatiski A, Beard KH (2011) Long-term plant growth legacies overwhelm short-term plant growth effects on soil microbial community structure. Soil Biol Biochem 43:823–830. doi:10.1016/j.soilbio.2010.12.018
Lankau RA, Bauer JT, Anderson MR, Anderson RC (2014) Long-term legacies and partial recovery of mycorrhizal communities after invasive plant removal. Biol Invasions 16:1979–1990. doi:10.1007/s10530-014-0642-0
Leicht-Young SA, Pavlovic NB (2014) Lianas as invasive species in North America. In: Ecology of Lianas. pp 429–442
Leicht-Young SA, O’Donnell H, Latimer AM, Silander JA (2009) Effects of an invasive plant species, Celastrus orbiculatus, on soil composition and processes effects of an invasive plant species, Celastrus orbiculatus, on soil composition and processes. Am Midl Nat 161:219–231
Leicht-Young SA, Bois ST, Silander JA (2015) Impacts of Celastrus-primed soil on common native and invasive woodland species. Plant Ecol 216:503–516. doi:10.1007/s11258-015-0451-2
Levia D, Frost EE (2003) A review and evaluation of stemflow literature in the hydrologic and biogeochemical cycles of forested and agricultural ecosystems. J Hydrol 274:1–29
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714. doi:10.1111/j.1469-8137.2007.02290.x
Lin YT, Tang SL, Pai CW, Whitman WB, Coleman DC, Chiu CY (2014) Changes in the soil bacterial communities in a cedar plantation invaded by Moso Bamboo. Microb Ecol 67:421–429. doi:10.1007/s00248-013-0291-3
Lowe LE (1993) Total and labile acid extractable polysaccharide analysis of soils. In: Carter M (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, pp 373–376
Mattingly KZ, McEwan RW, Paratley RD, Bray SR, Lempke JR, Arthur MA (2016) Recovery of forest floor diversity after removal of the nonnative, invasive plant Euonymus fortunei. J Torrey Bot Soc 143:103–116. doi:10.3159/TORREY-D-14-00051
Miller KE, Gorchov DL (2004) The invasive shrub, Lonicera maackii, reduces growth and fecundity of perennial forest herbs. Oecologia 139:359–375. doi:10.1007/s00442-004-1518-2
Miller WP, Miller DM (1987) A micro-pipette method for soil mechanical analysis. Commun Soil Sci Plant Anal 18:1–15
Muscarella M, Bird K, Larsen M, Placella S, Lennon J (2014) Phosphorus resource heterogeneity in microbial food webs. Aquat Microb Ecol 73:259–272. doi:10.3354/ame01722
Oksanen AJ, Blanchet FG, Kindt R, Legendre P, Minchin PR, Hara RBO, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Vegan: Community Ecology Package. http://cran.r-project.org/web/packages/vegan/index.html
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G (2010) Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann Bot 106:371–380. doi:10.1093/aob/mcq119
Perrett C, Osunkoya OO, Clark C (2012) Cat’s claw creeper vine, Macfadyena unguis-cati (Bignoniaceae), invasion impacts: comparative leaf nutrient content and effects on soil physicochemical properties. Aust J Bot 60:539–548. doi:10.1071/BT12055
Piper CL, Siciliano SD, Winsley T, Lamb EG (2015) Smooth brome invasion increases rare soil bacterial species prevalence, bacterial species richness and evenness. J Ecol 103:386–396. doi:10.1111/1365-2745.12356
Powers JS (2014) Reciprocal interactions between lianas and forest soi. In: Schnitzer SA, Bongers F, Burnham RJ, Putz FE (eds) Ecology of Lianas, 187th edn. Wiley-Blackwell, Chichester, p 175
Pysek P, Richardson DM (2007) Traits associated with invasiveness in alien plants: where do we stand. Biological Invasions. Springer, Berlin, pp 97–126
R Core Team (2015) R: a language and environment for statistical computing. RA Lang Environ Stat Comput
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Ecology 94:13730–13734. doi:10.1073/pnas.94.25.13730
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457. doi:10.1111/j.1469-8137.2006.01715.x
Roberts DW (2015) Labdsv: ordination and multivariate analysis for ecology. http://cran.r-project.org/web/packages/labdsv/index.html
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Smith LM, Reynolds HL (2012) Positive plant-soil feedback may drive dominance of a woodland invader, Euonymus fortunei. Plant Ecol 213:853–860. doi:10.1007/s11258-012-0047-z
Smith LM, Reynolds HL (2015) Euonymus fortunei dominance over native species may be facilitated by plant–soil feedback. Plant Ecol 216:1401–1406. doi:10.1007/s11258-015-0518-0
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Swearingen J, Slattery B, Reshetiloff K, Zwicker S (2010) Invaders of mid-Atlantic natural areas, 4th edn. U. S Fish and Wlidlife Service, Washington
Swedo BL, Glinka C, Rollo DR, Reynolds HL (2008) Soil bacterial community structure under exotic versus native understory forbs in a woodland remnant in Indiana. 117, pp 7–15
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Blackwell Science Publications, Oxford
Tang Y, Kitching RL, Cao M (2012) Lianas as structural parasites: a re-evaluation. Chin Sci Bull 57:307–312. doi:10.1007/s11434-011-4690-x
van Soest PJ (1963) Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J Assoc Off Anal Chem 46:829–835
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. doi:10.1111/j.1461-0248.2011.01628.x
Wharton ME, Barbour RW (1991) Bluegrass land and life. The University of Kentukcy Press, Lexington
Wright SF, Upadhyaya A (1998) A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 198:97–107. doi:10.1023/A:1004347701584
Acknowledgements
Milinda Hamilton provided assistance in sample processing; Todd Rounsaville provided access to field sites; Nicolas Body and Kali Mattingly provided field assistance; J.T. Lennon hosted the sabbatical of SRB; and Mario Muscarella provided mothur and R assistance. SRB was supported by the Kenan Sabbatical Support Fund from Transylvania University. This is publication number 16-09-076 of the Kentucky Agricultural Experiment Station and is published with the approval of the Director. This work is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, McIntire-Stennis project under accession number 0220128.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Luke Flory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bray, S.R., Hoyt, A.M., Yang, Z. et al. Non-native liana, Euonymus fortunei, associated with increased soil nutrients, unique bacterial communities, and faster decomposition rate. Plant Ecol 218, 329–343 (2017). https://doi.org/10.1007/s11258-016-0689-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-016-0689-3