Abstract
Non-native invasive species are often more productive aboveground than co-occurring natives. Because aboveground productivity is closely tied to plant nitrogen (N) uptake and use, high invader leaf productivity should be associated with root growth and plant N use strategies. However, little is known about the above- and belowground carbon (C) and N use strategies of native and invasive plants. We measured shoot and root attributes and soil properties associated with 10 native and 14 non-native invasive forest shrubs and lianas of the Eastern U.S. in a common garden in Syracuse, New York (USA), including leaf growth and chemistry (C, N), root growth, specific root length (SRL), root tissue density, and associated soil C and N concentration, each determined at 2-month intervals (July–November). Non-native species had greater leaf and root production, leaf N concentration, and SRL, but lower leaf N resorption rates and root N concentration than natives. Soil N concentration associated with non-natives was significantly lower than that of native species. Our results suggest that greater aboveground productivity of invasive forest species is linked to greater production of fine roots that may increase the capacity of invaders to take up soil resources. In addition, our findings suggest that invaders beget more rapid plant-soil N feedbacks by promoting N cycling compared to the strategy of slow growing native species that emphasizes recycled plant N. Such differences in N use strategy between native and non-native species would significantly impact forest soil nutrient cycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive plant species, or naturalized species that establish widely outside their native range, are often found to grow faster aboveground than co-occurring natives across a wide variety of ecosystems (Liao et al. 2008; van Kleunen et al. 2010; Vilà et al. 2011), including temperate forests (Herron et al. 2007; Fridley 2012). Explanations for this successful invasion strategy have been sought in terms of aboveground traits associated with leaf economics (e.g., higher photosynthetic rate, specific leaf area [SLA], leaf nitrogen [N] concentration; as evaluated by Baruch and Goldstein 1999; Funk and Vitousek 2007; Leishman et al. 2007, 2010; Osunkoya et al. 2010; Ordonez and Olff 2013). Few if any studies, however, have examined the belowground traits presumably required to support a high rate of aboveground physiological activity. In particular, it remains unclear whether faster rates of aboveground productivity by invaders are associated with qualitatively different strategies of root production, allocation, and nutrient uptake compared to native species in the invaded habitat.
Because plant productivity is often limited by available N in terrestrial ecosystems, the way in which invasive plants harvest and use N is likely to be an important component of their success and an important component of their impacts on nutrient cycling (Laungani and Knops 2009). However, linkages between how carbon (C) and N are acquired and used by invaders are poorly understood because rooting behaviors of invasive plants have been rarely investigated. In a comparison of over 70 native and invasive shrubs and lianas in Eastern U.S. forests, Fridley (2012) found that non-native species had substantially (4-wk) delayed autumnal leaf senescence, which would seemingly limit the capacity of invaders to recycle N from senescing leaves given the time typically required for nutrient resorption in deciduous species (Weih 2009). Additional analyses by Heberling and Fridley (2013) of the leaf characteristics of a subset of these species corroborated that invaders had both more productive and longer-lived leaves with greater photosynthetic capacity and leaf N concentration, such that, on average, more C was produced per unit N over the lifetime of the leaf. If invaders are investing more C and nutrients in leaves, what are the implications for whole plant function, and particularly belowground resource allocation?
Root foraging behavior and nutrient uptake capacity in general have received scant attention in native-invader comparisons but could be a primary mechanism of invader advantage in N-limited ecosystems (Laungani and Knops 2009). In theory, C gains by more productive invaders could be invested belowground in the form of greater allocation to fine roots, higher specific root length (SRL), greater nutrient uptake kinetics, or morphological changes to roots that favor nutrient exchanges with soil microbes (Chapin 1980; Hodge 2004; Craine 2011). In temperate deciduous forests, for example, the C subsidy that invaders get from exhibiting a longer growing season (Fridley 2012) could be invested into greater soil nutrient foraging and uptake. However, there has as yet been no systematic comparison of the rooting behavior of native and invasive plants in temperate forests.
Here we report a comparative analysis of above- and belowground traits and resource foraging behaviors of 10 native and 14 non-native, invasive shrub and lianas of Eastern U.S. deciduous forests, focusing on a subset of those reported in Fridley’s (2012) study of leaf phenology and Heberling and Fridley’s (2013) study of leaf-level metabolism. Our objective was to test the hypothesis that the higher aboveground productivity of invaders is supported by greater investment in root structures associated with high rates of N uptake (fine root production and SRL). Secondarily, we aimed to integrate leaf-level traits (photosynthetic capacity, N concentration, SLA, and N resorption rate) and seasonal root growth and morphology to address whether native and invasive species in this ecosystem have different coupled C–N use strategies that could drive large changes in forest nutrient dynamics as a result of increasing invader dominance.
Materials and methods
Study design and species
Our study was conducted in 2011 at an experimental garden in Syracuse, New York, USA (43°03′N, 76°09′W), on plants established in 2006–2007 (Fridley 2012). Plants were covered by shade cloth (80 % light reduction) from May 20 to October 24 annually to simulate forest understory conditions. From the garden collection of over 70 species of native and non-native species present in deciduous forests of the Eastern U.S., we selected 10 native and 14 non-native invasive shrub and liana species of 10 genera and nine families, many of which are widespread and locally abundant in the northeast US forests (Table 1). This assortment of species provided an opportunity to explore the effects of nativity on above- and belowground traits among ecologically important species across a wide breadth of taxonomic groups. Each species was represented by individuals present in three replicate blocks (N = 3), except for Lonicera morrowii (N = 2).
Leaf and root sampling
Three to five healthy fully developed leaves were collected at random from each plant every 2 months, July to November, to determine leaf N and C concentration. Ten leaves were sampled from Berberis thunbergii due to their small size. Leaves were pooled for each individual and sample date for analysis. To determine leaf N resorption, abscised leaves were collected after branches of each plant were gently shaken. Leaves were sampled every other day from October to November. Because of a marked increase in the rate of leaf abscission after the first frost date (October 27), leaves that abscised before and after this date were analyzed separately.
Root production was determined using point-in-space ingrowth cores, which allow for sequential root sampling from the same locations, to predict root production during the measurement period (Milchunas et al. 2005). Ingrowth cores (4 cm diameter × 10 cm height) were constructed with plastic netting (1 × 1 cm mesh). Two ingrowth cores were installed on opposite sides and 15 cm from the main stem of each plant in May 2011. After installation, cores were filled with root-free soil collected from within the garden. To prevent root intrusion from neighboring plants, a 45 cm wide × 15 cm deep aluminum shield was installed 20 cm on the outside, relative to the target individual, of each ingrowth core to a 12 cm depth. Soil cores were sampled every 2 months, July to November, using a stainless core sampler (4 cm diameter). There was no significant soil disturbance around any of the ingrowth cores during the experiment. After sampling, ingrowth cores were refilled with root-free soil collected during the previous sample date. Soil cores were kept frozen until processed.
Leaf traits
The total leaf area of each individual was measured in July, September, and November 2011. We selected five branches randomly and counted the number of leaves attached to each branch. Leaf area was measured using a portable leaf area meter (LI-3000C, LI-COR Biosciences, Lincoln, Nebraska, USA) on three leaves evenly distributed between the tip and base of each branch. Total branch length was measured for each individual plant. Total leaf area for each of the five branches was calculated by multiplying average leaf area of the three selected leaves and the total leaf number of each branch. Leaf area per unit branch length for each branch was calculated by dividing total leaf area by branch length. Total leaf area for each individual (m2 plant−1) was calculated by multiplying total branch length and average leaf area per unit branch length. For small plants, leaf area was measured for six leaves randomly selected from the plant and total leaf number was determined for the entire plant.
Leaves sampled for C and N concentration were dried at 60 °C for >2 days and ground with a hand mill to a fine powder. Total C and N concentration were determined using an elemental CN analyzer (NC 2100, Thermo Quest CE Instruments, Milan, Italy). Leaf N resorption rate was determined by the following equation (Vergutz et al. 2012):
where Nmax = maximum leaf N concentration of leaves collected in July and September, Nabscised = leaf N concentration of abscised leaves, and MLCF = mass loss correction factor for each species calculated from changes in leaf mass per unit area between fresh leaves sampled in August and abscised leaves collected at the end of the growing season in 2013. Leaf N resorption rates before and after the first frost were determined separately. To obtain an estimate of the maximum leaf N resorption potential of each species, we used the maximum resorption value of calculations using abscised leaves before and after the first frost date.
Root traits
We pooled roots present in paired ingrowth cores for each individual and sample date. Roots were picked with forceps from the soil collected from the cores and washed gently with distilled water. Plants that had no roots in their ingrowth cores for all three sampling periods were excluded from the analyses. This only changed the number of replicates per species and all the species were included in the analyses. After removing roots and organic debris, soils were sieved (2 mm), dried, and stored at room temperature until used to refill cores in the field. A subset of each soil sample was used to determine C and N concentration. Live roots were separated based on root morphology and color, scanned with a transparency scanner (Umax Power Look II, Umax Technologies, Inc., Taiwan) and analyzed for length and volume using DELTA-T SCAN software (Kirchhof and Pendar 1993). We measured traits on roots ≤1 mm in diameter (representing 98.7 % of roots collected from ingrowth cores) that were younger than 2 months and assumed to be involved in resource foraging rather than storage. Separated roots were dried at 60 °C for >2 days to measure biomass and total C and N concentration was determined using same method for leaf tissue analysis. Root growth (length and biomass) for each ingrowth period, SRL (m g−1), and root tissue density (RTD; g cm−3) were calculated based on root biomass and image analyses.
Statistical analyses
Plant and soil traits were compared across native and non-native species using linear mixed effects (LME) models. Nativity was treated as a fixed effect and block, genus, and individual plants were treated as random effects. Genus was included as a random effect to account for correlated trait variation contributed by shared phylogeny. Frangula and Rhamnus are sister genera in the Rhamnaceae (Richardson et al. 2000) and were treated as one group in LMEs. We tested for fixed effects by comparing full models to a null model with only the random effects based on maximum likelihood with the ‘lme4’ package for R (Bates 2010). Total leaf area, root production (total root length), and SRL data were normalized with log transformation. Post-hoc tests were conducted to evaluate pair-wise differences in measured traits between sampling times (Table 2) using the glht function in the R ‘multicomp’ package (Hothorn et al. 2012). We performed a principal component analysis (PCA) to determine multivariate trait patterns of native and non-native species using all measured variables plus SLA, leaf dry matter content (LDMC), and maximum C assimilation rate (Amax) measured on the same individuals in a previous study (Fridley 2012). Total N and C concentrations of plant tissue and soil in July were used for the PCA analysis because majority of plants showed a peak above- and belowground growth during that period and excluding September and November data did not change ordination patterns. A bivariate relationship of SRL and leaf N resorption rate was analyzed via standardized major axis (SMA) regression. We tested for differences in elevation and slope between fitting lines for each group and a shift between groups along their common axis using the ‘smatr’ package for R (Warton and Warton 2007; Warton et al. 2012). All statistical tests were performed in R version 2.14.1 (R Development Core Team 2011).
Results
Leaf traits
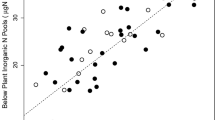
Non-native species produced greater total leaf area (m2) than natives and had higher leaf N concentration and a lower leaf C:N ratio (Table 2). Leaf N decreased and the C:N ratio increased from July to November for both native and non-native species (Table 2). Natives had significantly greater leaf N resorption rates (P = 0.018, Fig. 1). Rates of resorption ranged more widely among invasive species compared to native species; invasive honeysuckles including L. fragrantissima, L. japonica, and L. morrowii had particularly low leaf N resorption rates (<50 %), while Celastrus spp., Viburnum spp., Frangula caroliniana, L. canadensis, and the common native shrubs Hamamelis virginiana and Lindera benzoin had high resorption rates (>65 %) (Fig. 1).
Relationships between specific root length (SRL) and leaf N resorption rate. The dark gray arrow indicates the shifted distribution of non-natives and the light gray arrow indicates the shifted distribution of native species along a common slope (solid line). Point symbols indicate species identity as listed in Table 1. Error bars are ± SE. In box plots, white boxes represent natives and gray boxes represent non-natives. Asterisks on the box plots represent significance level of mean differences between native and non-native species (*P < 0.05, **P < 0.01, and ***P < 0.001)
Root traits and associated soil properties
We found significant differences between native and non-native species in all root traits measured (Table 2). Non-native species had greater fine root production, SRL, RTD, and root C:N ratio, and lower root N concentration. Several traits varied seasonally, such as root production; however, SRL, RTD, root N, and root C:N ratio did not (Table 2). Soil N concentration was significantly higher under native shrubs and lianas and lower in July compared to September and November. Soil N concentrations among roots of non-native species were on average 11 % lower than those associated with natives during the growing season (July and September), but recovered to the similar level as those of native species in November (Table 2). Soil C:N ratio was highest in July and decreased in September and November (Table 2).
Multivariate trait analysis
A principal components analysis that included all the plant and soil characteristics showed significant separation between native and non-native species along PC1 (P = 0.022) and 2 (P < 0.001) axes, but not axis 3 (P = 0.54) (Fig. 2). The PC1 axis, which accounted for 25.2 % of trait variation, separated species according to traits associated with tissue chemistry and leaf morphology (leaf N and CN ratio, root N and CN ratio, SLA, and LDMC; Fig. 2 and Table S1 [Online Resource]). The PC2 axis, which accounted for 13.9 % of trait variation, discriminated species based on their belowground N foraging ability (fine root production and SRL), tissue chemistry (root N and CN ratio, leaf C), and RTD (Fig. 2 and Table S1 [Online Resource]). The PC3 axis accounted for 11.0 % of trait variation and was most closely associated with soil chemistry (soil C, N, and CN ratio) (Table S1 [Online Resource]). On the PC1 and PC2 plane, invaders were clustered toward a suite of traits linked to higher above- and belowground growth rates (leaf N, SLA, total leaf area, photosynthetic rate, fine root production, and SRL) as opposed to natives, which exhibited traits related to a more conservative growth strategy (higher LDMC, leaf N resorption rate, leaf C, and CN ratio) (Fig. 2).
Principal Components Analysis of leaf and root traits of native and non-native shrubs and lianas from a common garden experiment. a Species scores along two major principal components (PC1 and PC2) and b vectors representing the coefficients of the traits on the principal components. See Table 2 and Table S1 (Online Resource) for descriptions of the trait abbreviations (“LNrsp” denotes leaf N resorption rate). The symbol beside each point indicates species identity (see Table 1). Error bars are ± SE. Box plots indicate a separation of species scores for each principal component by nativity. White boxes represent natives and gray boxes represent non-natives. Asterisks on the box plots represent significance level of mean differences between native and non-native species (*P < 0.05, **P < 0.01, and ***P < 0.001)
Leaf N resorption and root foraging ability
SRL declined with increased leaf N resorption rate, and SMA analysis revealed a significant shift (P < 0.001) along a common slope for native and non-native species (r 2 = 0.21, P < 0.001, Fig. 1), although this shift was dominated by native and non-native species of Lonicera. Invasive honeysuckles (L. fragrantissima, L. japonica, and L. morrowii) had low leaf N resorption rates, but high SRL, in contrast to native shrubs (e.g., H. virginiana, L. benzoin, F. caroliniana, and native Viburnum spp.) that had relatively high resorption rates and low SRL (Fig. 1). We did not find any other significant bivariate correlations between above- and belowground traits.
Discussion
Across a wide variety of ecosystems, non-native invasive species typically exhibit higher rates of productivity than co-occurring natives (Liao et al. 2008; van Kleunen et al. 2010; Vilà et al. 2011). This is generally true for invaders in Eastern U.S. forests. Results of our work on this group of deciduous forest species (Fridley 2012; Heberling and Fridley 2013; this study) show that, compared to both widespread and closely related native species, invaders on average have higher maximum photosynthetic capacity, higher leaf N concentration, faster rates of leaf production and shoot elongation, and a greater total amount of root production. Greater whole-plant productivity of invaders begs the question as to how such rates of production are maintained under the same resource conditions as natives. One possibility is that where plant growth is limited by soil N supply, invaders exhibit greater photosynthetic N use efficiency at the leaf level (Funk and Vitousek 2007; Leishman et al. 2010; Ordonez et al. 2010; Ordonez and Olff 2013). This is true in our study system only as a consequence of the greater leaf longevity of invaders (Heberling and Fridley 2013), and comes with the apparent cost of lower leaf N resorption. If invaders are investing more photosynthate in leaves to promote longevity but are losing more leaf N as a result of delayed senescence, how are they able to maintain such high leaf N over the growing season?
In this study we focus on the hypothesis that greater invader productivity is part of an integrated strategy of shoot and root foraging behavior, where greater light harvesting ability is driven by differences in N uptake and use throughout the growing season. Very few studies have addressed differences in root traits and foraging behavior between native and invasive species or have attempted to integrate above- and belowground resource foraging strategies for invaders of high productivity (Craine and Lee 2003). Our measurements on 10 native and 14 non-native invasive woody species common to Eastern U.S. forests revealed greater rates of fine root proliferation, higher SRL, and lower root N in invaders. The higher root N concentrations of native species may be indicative of more effective mycorrhizal symbioses. However, as most of our study species, including non-natives, have mycorrhizal roots (Brundrett et al. 1990; Wang et al. 2006; Akhmetzhanova et al. 2012), whether non-native species associate with more effective N foraging mycorrhizal symbionts remains to be tested. Allocation to fine roots with high SRL is associated with nutrient foraging ability (Eissenstat 1991; Reich et al. 1998; Comas and Eissenstat 2004; Hodge 2004), suggesting invaders are more effective foragers for soil nutrients including N (Liao et al. 2008; van Kleunen et al. 2010; Vilà et al. 2011). To our knowledge, these are the first results suggesting a distinct belowground growth strategy for invaders across a taxonomcally diverse sample of native and non-native species.
The negative relationship between SRL and leaf N resorption may indicate an overall tradeoff between the production of fine, physiologically active roots for efficient root N foraging (Reich et al. 1998) and plant N retention. Dispersion around the linear function in Fig. 1 may in part be due to a relatively large phylogenetic effect on SRL (high between-genus effect in Table S2 [Online Resource]), and much of the noted shift in the SRL-N resorption tradeoff was due to our largest single clade of Lonicera, suggesting this tradeoff is most apparent among closely related species and weakens in larger phylogenetic contrasts. However, overall, invaders in our study exhibited significantly lower leaf N resorption rates during leaf senescence than natives. These results are consistent with recent meta-analyses of leaf nutrient resorption rates showing that species of lower leaf N have higher N resorption rates (Kobe et al. 2005; Vergutz et al. 2012).
Why should invaders exhibit higher rates of N uptake, along with corresponding lower N resorption rates, than native species in Eastern U.S. forests? We suggest that the explanation may hinge on the time required for nutrient resorption (Weih 2009), which necessitates relatively early initiation of autumnal leaf senescence and results in reduced C gain at the end of the growing season. Fridley (2012) showed that, with only a few exceptions, invaders in our study exhibited later leaf senescence and greater autumnal C gain than native species. With reduced time for senescence before damaging frosts, invaders lose a greater amount of leaf N than natives but in return get a C subsidy that can be up to a fourth of annual C gain (Fridley 2012). In turn, this added energetic resource could fuel greater N foraging ability of invaders, allowing more effective recapture of lost N before the next growing season. We expect this strategy to be more associated with species adapted to habitats of high N supply rates, where re-uptake of lost N would be less costly (Chapin 1980; Craine 2011). If true, it remains a mystery why invaders would adopt this strategy in contrast to the N conservation strategy adopted by natives, although enhanced supply rates of N across Eastern North America in the twentieth Century from industrial and agricultural pollution (Aber et al. 1989) or nitrification-stimulating earthworm invasions (Nuzzo et al. 2009) may be contributing factors. Future studies of native-invader performance across a N gradient would help resolve this issue.
Replacement of more nutrient-conserving native species with non-native species that have both more nutrient-rich leaf litter and greater capacity for nutrient uptake is likely to shift rates of nutrient cycling in invaded deciduous forests (Liao et al. 2008). In this study, invaders reduced the soil N concentration 11 % more than natives during the growing season. We note that our study soils were not subject to the same rate and type of leaf litter input found under canopy trees and likely did not support the same microbial communities as natural forest stands. Nevertheless, we predict that rates of forest nutrient cycling have increased and the competition for mineralized N has strengthened significantly as a result of increasing dominance of non-native shrubs and lianas, potentially changing ecosystem C and nutrient fluxes and shifting the composition of microbial communities (Kourtev et al. 2002; Ashton et al. 2005; Liao et al. 2008; Lee et al. 2012). Experiments designed to isolate long-term plant-soil feedbacks in stands dominated by native and invasive understory species would go a long way toward improving our understanding of changes in ecosystem functioning in temperate forests as a result of species invasions.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM (1989) Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–386. doi:10.2307/1311067
Akhmetzhanova AA, Soudzilovskaia NA, Onipchenko VG, Cornwell WK, Agafonov VA, Selivanov IA, Cornelissen JHC (2012) A rediscovered treasure: mycorrhizal intensity database for 3000 vascular plant species across the former Soviet Union: ecological Archives E093-059. Ecology 93:689–690. doi:10.1890/11-1749.1
Ashton I, Hyatt L, Howe K, Gurevitch J, Lerdau M (2005) Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol Appl 15:1263–1272. doi:10.1890/04-0741
Baruch Z, Goldstein G (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121:183–192. doi:10.1007/s004420050920
Bates DM (2010) lme4: Mixed-effects modeling with R. Springer, Berlin
Brundrett M, Murase G, Kendrick B (1990) Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can J Bot 68:551–578. doi:10.1139/b90-076
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260. doi:10.1146/annurev.es.11.110180.001313
Comas LH, Eissenstat DM (2004) Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct Ecol 18:388–397. doi:10.1111/j.0269-8463.2004.00835.x
Craine JM (2011) Resource strategies of wild plants. Princeton University Press, Princeton
Craine JM, Lee WG (2003) Covariation in leaf and root traits for native and non-native grasses along an altitudinal gradient in New Zealand. Oecologia 134:471–478. doi:10.1007/s00442-002-1155-6
Eissenstat DM (1991) On the relationship between specific root length and the rate of root proliferation: a field study using citrus rootstocks. New Phytol 118:63–68. doi:10.1111/j.1469-8137.1991.tb00565.x
Fridley JD (2008) Of Asian forests and European fields: eastern U.S. plant invasions in a global floristic context. PLoS ONE 3:e3630. doi:10.1371/journal.pone.0003630
Fridley JD (2012) Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485:359–362. doi:10.1038/nature11056
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081. doi:10.1038/nature05719
Heberling JM, Fridley JD (2013) Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol 200:523–533. doi:10.1111/nph.12388
Herron PM, Martine CT, Latimer AM, Leicht-Young SA (2007) Invasive plants and their ecological strategies: prediction and explanation of woody plant invasion in New England. Divers Distrib 13:633–644. doi:10.1111/j.1472-4642.2007.00381.x
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. doi:10.1111/j.1469-8137.2004.01015.x
Hothorn T, Bretz F, Westfal P (2012) Package ‘multcomp’: simultaneous inference in general parametric models. R package version 1.2–13. Available at: http://cran.r-project.org/web/packages/multcomp/ Last Accessed 15 March 2013
Kirchhof G, Pendar K (1993) Delta-T SCAN user manual. Delta-T Devices Ltd, Cambridge
Kobe RK, Lepczyk CA, Iyer M (2005) Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology 86:2780–2792. doi:10.1890/04-1830
Kourtev P, Ehrenfeld J, Häggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152–3166. doi:10.1890/0012-9658(2002)083[3152:EPSATM]2.0.CO;2
Laungani R, Knops JMH (2009) Species-driven changes in nitrogen cycling can provide a mechanism for plant invasions. Proc Nat Acad Sci USA 106:12400–12405. doi:10.1073/pnas.0900921106
Lee M, Flory S, Phillips R (2012) Positive feedbacks to growth of an invasive grass through alteration of nitrogen cycling. Oecologia 170:457–465. doi:10.1007/s00442-012-2309-9
Leishman MR, Haslehurst T, Ares A, Baruch Z (2007) Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol 176:635–643. doi:10.1111/j.1469-8137.2007.02189.x
Leishman MR, Thomson VP, Cooke J (2010) Native and exotic invasive plants have fundamentally similar carbon capture strategies. J Ecol 98:28–42. doi:10.1111/j.1365-2745.2009.01608.x
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177:706–714. doi:10.1111/j.1469-8137.2007.02290.x
Milchunas DG, Mosier AR, Morgan JA, LeCain DR, King JY, Nelson JA (2005) Root production and tissue quality in a shortgrass steppe exposed to elevated CO2: using a new ingrowth method. Plant Soil 268:111–122. doi:10.1007/s11104-004-0230-7
Nuzzo VA, Maerz JC, Blossey B (2009) Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conserv Biol 23:966–974. doi:10.1111/j.1523-1739.2009.01168.x
Ordonez A, Olff H (2013) Do alien plant species profit more from high resource supply than natives? A trait-based analysis. Glob Ecol Biogeogr 22:648–658. doi:10.1111/geb.12019
Ordonez A, Wright IJ, Olff H (2010) Functional differences between native and alien species: a global-scale comparison. Funct Ecol 24:1353–1361. doi:10.1111/j.1365-2435.2010.01739.x
Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G (2010) Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann Bot 106:371–380. doi:10.1093/aob/mcq119
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0
Reich PB, Walters MB, Tjoelker MG, Vanderklein D, Buschena C (1998) Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct Ecol 12:395–405. doi:10.1046/j.1365-2435.1998.00209.x
Richardson JE, Fay MF, Cronk QCB, Bowman D, Chase MW (2000) A phylogenetic analysis of Rhamnaceae using rbcL and trnL-F plastid DNA sequences. Am J Bot 87:1309–1324
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. doi:10.1111/j.1461-0248.2009.01418.x
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82:205–220. doi:10.1890/11-0416.1
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. doi:10.1111/j.1461-0248.2011.01628.x
Wang Z, Guo D, Wang X, Gu J, Mei L (2006) Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 288:155–171. doi:10.1007/s11104-006-9101-8
Warton D, Warton MD (2007) The smatr package: (standardised) major axis estimation and testing routines. R package version 2.1. Available at: http://cran.r-project.org/web/packages/smatr/ Last Accessed 15 March 2013
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) smatr 3-an R package for estimation and inference about allometric lines. Method Ecol Evol 3:257–259. doi:10.1111/j.2041-210X.2011.00153.x
Weih M (2009) Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): effects on shoot growth and nitrogen economy. Tree Physiol 29:1479–1490. doi:10.1093/treephys/tpp081
Acknowledgments
We thank A. Craddock and Syracuse University for support of the experimental garden.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jo, I., Fridley, J.D. & Frank, D.A. Linking above- and belowground resource use strategies for native and invasive species of temperate deciduous forests. Biol Invasions 17, 1545–1554 (2015). https://doi.org/10.1007/s10530-014-0814-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0814-y