Abstract
Comparisons between invasive and native species may not characterize the traits of invasive species, as native species might be invasive elsewhere if they were introduced. In this study, invasive Oxalis corymbosa and Peperomia pellucida were compared with their respective noninvasive alien congeners. We hypothesized that the invasive species have higher specific leaf (SLA) than their respective noninvasive alien congeners, and analyzed the physiological and ecological consequences of the higher SLA. Higher SLA was indeed the most important trait for the two invaders, which was associated with their lower leaf construction cost, higher nitrogen (N) allocation to photosynthesis and photosynthetic N use efficiency (PNUE). The higher N allocation to photosynthesis of the invaders in turn increased their PNUE, N content in photosynthesis, biochemical capacity for photosynthesis, and therefore light-saturated photosynthetic rate. The above resource capture-, use- and growth-related traits may facilitate the two invaders’ invasion, while further comparative studies on a wider range of invasive and noninvasive congeners are needed to understand the generality of this pattern and to fully assess the competitive advantages afforded by these traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many species have intentionally been introduced outside their native range for different purposes (Reichard and White 2001; Xu et al. 2006). A few of them have established and spread in their introduced range, becoming invasive species, while most of introduced species are widely cultivated without problems (Williamson and Fitter 1996). Invasive species can alter species composition, structure and function of the invaded ecosystems, and often cause significant environmental damage and huge economic loss worldwide (Pimentel et al. 2000; D’Antonio and Kark 2002; Xu et al. 2006). It is important to predict potentially invasive species before introduction to reduce the possibility of introducing new invasive species. Thus, it is necessary to identify the traits that may be associated with invasiveness of alien species. Knowing these traits is also helpful for controlling existing invasive species. However, little is known about these traits and the mechanisms underlying biological invasions (Smith and Knapp 2001; Burns 2004).
For identifying the traits associated with invasiveness, a few ecophysiological comparisons across invasive and native species have been conducted. It has been found that some invasive species have significantly higher light-saturated photosynthetic rate (P max), photosynthetic nitrogen (N) use efficiency (PNUE), water use efficiency (WUE), and higher specific leaf area (SLA) but lower leaf construction cost (CC) than native species (Durand and Goldstein 2001; Nagel and Griffin 2001; Smith and Knapp 2001; McDowell 2002; Ewe and Sternberg 2003; Niinemets et al. 2003; Feng et al. 2007a; Xu et al. 2007). However, invasive species were not always higher in P max, PNUE, WUE and SLA (see review of Daehler 2003). For example, 24 photosynthetic comparisons between invasive and native species were reviewed by Daehler (2003): 11 found similar photosynthesis for invasive and native species; seven found higher while six found lower photosynthesis for invasive species. One of the reasons for the inconsistent results in the literature is that most of the studies (20 of the 24 photosynthetic comparisons) compared phylogenetically unrelated invasive and native species. Phylogenetic relatedness may constrain morphological and physiological variations of plants; closely related plants may share more common traits and more overlapping resource requirements than unrelated species (Goldberg 1987). Therefore, comparisons between related invasive and native species are more meaningful in explaining invasiveness of alien species (Harvey and Purvis 1991; Mack 1996; McDowell 2002). Another reason for the inconsistent results is the intrinsic weakness of the approach of invasive and native comparison (Burns 2004, 2006; Grotkopp and Rejmánek 2007). Some native species might be invasive when introduced outside their native range. In this case, comparison between invasive and native species may not elucidate the traits associated with invasiveness, while it may be helpful for understanding why the invasive species outcompete the native species (Burns 2004).
Comparisons across invasive and noninvasive alien species are an effective approach to detect the potential traits associated with invasiveness, while this approach is seldom used (but see Burns 2004, 2006; Grotkopp and Rejmánek 2007; Feng et al. 2007b). Knowing the differences between invasive and noninvasive alien species may help to understand the mechanisms that allow some alien species to spread rapidly and even dominate communities (Mack 1996; Radford and Cousens 2000; Smith and Knapp 2001). With this approach, it has been found that invasive species have higher relative growth rate (RGR) and SLA than their noninvasive alien congeners (Grotkopp et al. 2002; Burns 2004, 2006; Grotkopp and Rejmánek 2007). However, the higher RGR and SLA were not found for invasive species when compared with phylogenetically unrelated noninvasive alien species (Smith and Knapp 2001; Feng et al. 2007c), indicating again the importance of the relatedness of the compared invasive and noninvasive alien species.
SLA is associated with N allocation to photosynthesis. Poorter and Evans (1998) found that high-SLA species allocate a higher fraction of leaf N to photosynthesis and have a higher PNUE than low-SLA species. Thus, invasive species may allocate a higher fraction of leaf N to photosynthesis than related noninvasive alien species, provided they have a higher SLA. However, few studies determined leaf N allocation to photosynthesis in invasive species (but see Feng et al. 2007a, b), which is an important factor explaining interspecific differences in P max, PNUE and WUE (Onoda et al. 2004). In a recent study, we found that invasive Buddleja davidii allocates a higher fraction of leaf N to photosynthesis than five unrelated native species (Feng et al. 2007a), while in another study, the higher N allocation to photosynthesis was not detected for invasive Ageratina adenophora and Chromolaena odorata compared to an unrelated noninvasive alien species (Feng et al. 2007b).
In this study, we compared ecophysiological traits of two invasive species with their respective noninvasive alien congeners at a partially shady site. We hypothesized that compared to their respective noninvasive alien congeners, invasive alien species (1) have higher SLA, (2) allocate a higher fraction of leaf N to photosynthesis and (3) have higher P max, PNUE, and WUE but lower CC. We focused on SLA in this study because of its pivotal roles in determining other variables such as RGR, CC, P max and N allocation (Reich et al. 1997; Feng et al. 2007a, c). This study is the first that compares ecophysiological traits of two pairs of invasive and noninvasive alien congeners and hence would contribute to the critical evaluation of the role of ecophysiological traits in facilitating alien plant invasion.
Materials and methods
Study site and species
This study was carried out in Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (21°56′ N, 101°15′ E, 600 m above see level), which is located in the southern part of Yunnan Province, southwest China. Here, the mean annual temperature is 21.7°C, with a mean of 25.3°C in the hottest month (July) and 15.6°C in the coolest month (January); the mean annual precipitation is 1,557 mm; and the prevalent soil type is latosol.
There are many invasive plant species in the studied area. However, only two of them were found to have noninvasive alien congeners in this area. Thus, in this study, the two invasive species, Oxalis corymbosa DC. (Oxalidaceae) and Peperomia pellucida (Linn.) Kunth (Piperaceae), were compared with their noninvasive alien congeners, O. triangularis cv. Purpurea A. St. Hil. and P. caperata Yunck., respectively. Oxalis corymbosa, a perennial forb, was introduced into China as an ornamental plant in the middle of 19th century; P. pellucida, an annual forb, was introduced unintentionally at the beginning of 20th century. Both the invasive species have naturalized in the subtropics and tropics of many countries. They invade crop field, garden, orchard, plantation, wasteland and roadside. Oxalis triangularis and P. caperata, perennial forbs, were introduced into China more than 10 years ago. They are widely grown as ornamental plants in China and other counties, but are not recognized as invasive species in any country. All the four species are native to tropical America and can propagate sexually and asexually. Peperomia pellucid can asexually propagate by forming adventitious roots at stems; the other species can propagate with rhizomes.
Irradiance influences plant growth, morphology, biomass allocation and physiology, including N allocation (Feng et al. 2007b, c). Thus, we compared each invasive and noninvasive alien congeneric species pair at similar irradiance to exclude its influence. Soil conditions, plant age and size (20-cm-tall current year seedlings) were also similar for the invasive and noninvasive alien species of each genus. In October 2006, 11 individuals for each of O. corymbosa, O. triangularis and P. pellucida were chosen at 25% irradiance in a Murraya exotica-dominated community beside a road. However, only four individuals were found for P. caperata. The invasive species were naturally grown in the community, while the noninvasive species were transplanted to the community 1 month before the study. Light intensity above each sample individual and in an open site was simultaneously recorded in cloudy mornings with quantum sensors and a Li-1400 datalogger (Li-Cor, Lincoln, NE, USA). Relative irradiance above each sample individual was calculated as the ratio of the light intensity above it to that in the open site.
Measurements
Photosynthetic response to intercellular CO2 concentration (C i) was determined on the youngest fully expanded leaves of the field-grown plants with a Li-6400 Portable Photosynthesis System (Li-Cor). Under saturated photosynthetic photon flux density (PPFD) determined by the preliminary experiments, net photosynthetic rate (P n) was measured at 380, 300, 260, 220, 180, 140, 110, 80, 50 and 0 μmol mol−1 CO2 in the reference chamber. Relative humidity of the air in leaf chamber was controlled at 55%, leaf temperature at 25°C. Stomatal conductance (G s), C i and P n were recorded when the sample leaf was balanced for 200 s under each PPFD and CO2 step. In this study, P n measured at 380 μmol mol−1 CO2 and 2,000 μmol m−2 s−1 PPFD was considered as P max and the ratio of P max to G s as WUE. Afterwards, light- and CO2-saturated photosynthetic rate ( \( P^{'}_{{\max }} \)) was detected after 500 s under 2,000 μmol m−2 s−1 PPFD and 1,500 μmol mol−1 CO2. Before measurement, samples were illuminated with saturated PPFD provided by the LED light source of the equipment for 5–30 min to achieve full photosynthetic induction. No photoinhibition occurred during the measurements.
Leaf discs with a definite area were taken from each sample leaf, oven-dried at 60°C for 48°h. SLA was calculated as the ratio of leaf area to mass. Leaf N and carbon contents were determined with Kjeldahl (Büchi Auto Kjeldahl Unit K-370, Büchi Labortechnik AG, Zürich, Schweiz) and H2SO4/K2Cr2O7 oxidization–FeSO4 titration methods, respectively. The measurements were performed by the Biogeochemistry Laboratory of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences. Leaf CC was calculated according to McDowell (2002). The same leaf of each sample plant was used if possible for measurements of photosynthesis, SLA and carbon content and area (N A)- and mass (N M)-based N content.
Calculations of P n–C i curve-related variables
The P n–C i curve was fitted with a linear equation (P n = kC i + i) within 50–200 μmol mol−1 C i. Maximum carboxylation rate (V cmax) and dark respiration rate (R d) were calculated according to Farquhar and Sharkey (1982) as follows:
where K c and K o were the Michaelis–Menten constants of Rubisco for carboxylation and oxidation, respectively; Г * was CO2 compensation point; O was the intercellular oxygen concentration, close to 210 mmol mol−1. The values of K c, K o, and Г * were temperature-dependent (Bernacchi et al. 2001).
Maximum electron transport rate (J max) was calculated according to Loustau et al. (1999) as:
The fractions of total leaf N allocated to carboxylation (P C, g g−1) and bioenergetics (P B, g g−1) of the photosynthetic apparatus were calculated as:
where V cr and J mc are the specific activities of Rubisco (μmol CO2 g−1 Rubisco s−1) and cyt f (mol electrons mol−1 cyt f s−1), respectively (Niinemets and Tenhunen 1997). Nitrogen contents in carboxylation (N C) and bioenergetics (N B) were calculated as the products of N A and P C, P B, respectively.
Statistical analyses
The differences between each invasive species and its noninvasive congener in the variables presented in Table 1 were analyzed with independent-sample t tests. A one-way ANCOVA was used to determine the difference between each invader and its noninvasive congener in the correlation between each pair of the variables presented in Figs. 1, 2, 3 and 4. If the above difference was not significant, the invasive and noninvasive species of the same genus were pooled together and the difference between the two genera was determined further. If the difference between congeners was significant, the difference between the invasive or noninvasive species of different genera was determined further. If the difference between the invasive or noninvasive species of different genera was not significant, the invasive or noninvasive species of different genera were pooled together and the difference between the pooled invasive and the pooled noninvasive species was determined further. All the analyses were carried out using SPSS 13.0 (SPSS, Chicago, IL, USA).
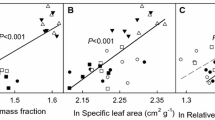
Leaf construction cost (CC, a), the fractions of leaf nitrogen allocated to carboxylation (P C, b) and to bioenergetics (P B, c), and photosynthetic nitrogen use efficiency (PNUE, d) as a function of specific leaf area (SLA) in invasive Oxalis corymbosa (open circles) and Peperomia pellucida (open triangles), and noninvasive alien O. triangularis (filled circles) and P. caperata (filled triangles)
Photosynthetic nitrogen use efficiency (PNUE) as a function of the fractions of leaf nitrogen allocated to carboxylation (P C, a) and to bioenergetics (P B, b), leaf nitrogen content (N A, c), and light-saturated photosynthetic rate (P max, d) in invasive Oxalis corymbosa (open circles) and Peperomia pellucida (open triangles), and noninvasive alien O. triangularis (filled circles) and P. caperata (filled triangles). The lines fitted for the invasive and noninvasive alien species are significantly different in plots c and d (P < 0.001) according to the result of a one-way ANCOVA with PNUE as dependent variable, species category (invasive vs. noninvasive) as fixed factor, and N A and P max as covariate, respectively
Light-saturated photosynthetic rate (P max) as a function of the fractions of leaf nitrogen allocated to carboxylation (P C, a) and to bioenergetics (P B, b), maximum carboxylation rate (V cmax, c) and maximum electron transport rate (J max, d) in invasive Oxalis corymbosa (open circles) and Peperomia pellucida (open triangles), and noninvasive alien O. triangularis (filled circles) and P. caperata (filled triangles). The lines fitted for Oxalis and Peperomia are significantly different in plots a and b (P < 0.001) according to the result of a one-way ANCOVA with P max as dependent variable, genus (Oxalis vs. Peperomia) as fixed factor, and P C and P B as covariate, respectively
Light-saturated photosynthetic rate (P max) as a function of leaf nitrogen content (N A, a), and stomatal conductance (G s, b) in invasive Oxalis corymbosa (open circles) and Peperomia pellucida (open triangles), and noninvasive alien O. triangularis (filled circles) and P. caperata (filled triangles). The outlier was not included in the correlation between P max and G s for O. triangularis. The lines fitted for the invasive and noninvasive alien species are significantly different (P < 0.001) according to the result of a one-way ANCOVA
Results
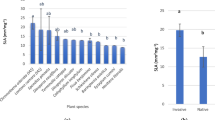
The two studied invasive species had significantly higher P max, V cmax, J max, CE, PNUE, P C, P B, N C, N B and SLA, but lower N A and CC than their noninvasive alien congeners, respectively (Table 1). Stomatal conductance was significantly higher for invasive O. corymbosa than for noninvasive O. triangularis, while C i was similar between the congeners. Invasive P. pellucida showed lower G s and C i than noninvasive P. caperata.
Except P. caperata (P = 0.183), other studied species showed significant correlation between CC and SLA (P < 0.001). Leaf CC decreased exponentially with the increase of SLA (Fig. 1a). Oxalis triangularis showed significant correlations between SLA and P C (P = 0.003), P B (P < 0.001) and PNUE (P < 0.001); P. pellucida exhibited significant correlation between P B and SLA (P = 0.007). However, P C, P B and PNUE increased significantly with the increase of SLA for the pooled invasive and noninvasive alien species (Fig. 1b–d).
Photosynthetic N use efficiency was correlated positively with P C and P B for all the studied species (P < 0.05) except P. caperata, in which the correlation between PNUE and P C was not significant (P = 0.170). With the increase of P C or P B, PNUE showed similar increase pattern for all the studied species (Fig. 2a, b). Oxalis corymbosa (P = 0.036) and P. pellucida (P < 0.001) showed significant correlation between PNUE and P max, while only O. triangularis showed significant correlation between PNUE and N A (P = 0.003). However, PNUE decreased with increasing N A, but increased with increasing P max for the pooled invasive and the pooled noninvasive species (Fig. 2c, d). At the same value of N A or P max, PNUE was significantly higher in the invasive species than in the noninvasive species.
With the increase of P C or P B, P max also increased significantly for the pooled Oxalis and the pooled Peperomia species (Fig. 3a, b), although only P. pellucida showed significantly positive correlation between P max and P C (P < 0.001), P B (P = 0.007). At the same value of P C or P B, P max was significantly higher in Oxalis than in Peperomia. Light-saturated photosynthetic rate was correlated positively with V cmax for all the studied species (P < 0.01) except P. caperata (P = 0.598). Among the four studied species, only O. corymbosa (P = 0.001) and P. pellucida (P < 0.001) showed significant correlation between P max and J max. With the increase of V cmax or J max, P max showed similar increase pattern for all the studied species (Fig. 3c, d).
No studied species showed significant correlation between P max and N A (P > 0.05). However, P max increased significantly with increasing N A for the pooled invasive species, while the correlation between P max and N A was still not significant for the pooled noninvasive species (Fig. 4a). Oxalis triangularis (P = 0.006) and P. pellucida (P < 0.001) exhibited significant correlation between P max and G s. With the increase of G s, P max showed similar increase pattern for the pooled invasive and the pooled noninvasive (the outlier was removed for P. caperata) species (Fig. 4b). At the same value of G s, the invasive species showed significantly higher P max than the noninvasive species, indicating their higher WUE. However, the difference in WUE between O. corymbosa and O. triangularis was not significant according to independent samples t test (Table 1).
Discussion
As expected, O. corymbosa and P. pellucida had significantly higher SLA than their respective noninvasive alien congeners (Table 1). Higher SLA was also found for other invasive species compared with their noninvasive congeners by Grotkopp et al. (2002), Burns (2006) and Grotkopp and Remánek (2007). In addition, most studies found that invasive species have higher SLA than their co-occurring native species (Daehler 2003). The higher SLA of the studied invaders may translate to a higher RGR compared to their respective noninvasive congeners, as SLA is the most important determinant of RGR, especially at low irradiance (Shipley 2006; Feng et al. 2007c). The higher P max and PNUE of the invaders supported the above prediction. Feng et al. (2007c) found that P max is positively correlated with net assimilation rate, which is another factor influencing RGR (Goodger et al. 2006; Shipley 2006). Pattison et al. (1998) found that P max is positively correlated with RGR. Species with high PNUE and SLA usually have high RGR (Lambers and Poorter 1992; Reich et al. 1997; Schieving and Poorter 1999). Both RGR and SLA are important in capturing available resources (Grotkopp and Remánek 2007), and the ability to opportunistically capture resources is important for alien plant invasion (Davis et al. 2000). With 29 invasive and noninvasive pine species, Grotkopp et al. (2002) found that both RGR and SLA are positively correlated with invasiveness.
On the other hand, the higher SLA of the two studied invaders contributed to their lower CC (Table 1). Leaf CC decreased exponentially with increasing SLA (Fig. 1a), while in another study, the negative correlation between CC and SLA is linear (Feng et al. 2007a). With lower CC but higher P max, the leaves of the two invaders may have shorter payback time than those of their respective noninvasive alien congeners, i.e., they can fix the amount of energy required to construct themselves through photosynthesis in a shorter time, leaving more energy for defense, growth and reproduction. It has been documented that low CC is often associated with high RGR and small difference in CC can lead to big difference in RGR (Griffin 1994; Poorter and Villar 1997). Nagel and Griffin (2001) and Tsialtas et al. (2002) further reported that species with low CC have high field abundances.
Furthermore, the higher SLA of the two studied invaders also contributed to their higher N allocation to photosynthesis (Fig. 1). SLA is the reciprocal of the product of leaf density and thickness. Leaf density is positively associated with the fraction of leaf mass in structural tissue (mainly cell walls). Leaves with high SLA may have lower fraction of leaf mass in structural tissue than leaves with low SLA. Onoda et al. (2004) indeed found that SLA is negatively associated with cell wall mass. Cell walls contain many kinds of proteins, and about 5–10% of the primary cell wall mass is protein (Loomis 1997). Nitrogen allocation to carboxylation and bioenergetics increased significantly with increasing SLA (Fig. 1b, c). Thus, the higher SLA of the two invaders may contribute to their higher N allocation to photosynthesis by reducing N allocation to cell walls. There is a trade-off between N allocation to cell walls and to photosynthesis. With native species, Takashima et al. (2004) found that SLA is positively correlated with the fraction of leaf N in Rubisco.
The positive correlation between SLA and PNUE (Fig. 1d) may be due to the influence of SLA on N allocation to photosynthesis, indicating that SLA may influence PNUE by influencing N allocation. It is understandable that increasing N allocation to photosynthesis translates to increasing PNUE. Nitrogen allocation to photosynthesis may influence PNUE by contributing to P max (Fig. 3a, b), which was positively correlated with PNUE (Fig. 2d). The similar increasing pattern of PNUE with P C and P B indicated that the higher PNUE of the two invaders in comparison with their respective noninvasive alien congeners was related to their higher P C and P B (Fig. 2a, b). The higher PNUE of the two invaders might also be associated with their lower N A. At high level of N A, PNUE is negatively correlated with N A (Hikosaka and Terashima 1995). In our study, PNUE indeed showed a decreasing trend with increasing N A, but was significant only for the noninvasive species (Fig. 2c).
Photosynthesis was positively correlated with V cmax and J max (Fig. 3c, d), which determined N C [=V cmax/(6.25V cr)] and N B [=J max/(8.06J mc)] theoretically. The results showed that P max was directly influenced by N C and N B, which are the products of N A and P C, P B, respectively. With lower N A, the two studied invaders in comparison with their respective noninvasive alien congeners had higher N C (V cmax), N B (J max) and therefore higher P max, indicating that the higher P C and P B of the invaders was the main factor explaining their higher P max (Fig. 3a, b). The higher P max of the two invaders at the same value of N A (Fig. 4a) confirmed again the importance of N allocation to photosynthesis in determing P max. Stomatal conductance was not important in explaining the higher P max of the two invaders although it was positively correlated with P max (Fig. 4b). With lower G s and C i, P. pellucida showed higher P max compared to P. caperata (Table 1). Although O. corymbosa had higher G s than O. triangularis, it did not show higher C i. In addition, P max exhibited decreasing trend with increasing C i (data not shown). The results further indicated that the ability to use intercellular CO2 (associated with N in photosynthesis) was more important than the ability to supply CO2 (associated with G s) in explaining the higher P max of the invaders, thus again confirming the importance of N allocation.
Peperomia pellucida in comparison with P. caperata had both higher PNUE and WUE (Table 1), breaking the trade-off between them. The invader increased P max and PNUE mainly by increasing N allocation to photosynthesis rather than by increasing G s, and thereby did not reduce WUE. This is different from the situation of invasive Buddleja davidii, in which the increased N allocation to photosynthesis and the increased P max do not completely compensate the negative effect of the increased G s on WUE (Feng et al. 2007a). However, the increased N allocation to photosynthesis overcompensated the negative effect of the increased G s on WUE in O. corymbosa (Table 1). Durand and Goldstein (2001), McDowell (2002) and Ewe and Sternberg (2003) also found that invasive species have both higher WUE and PNUE than native species, but they did not give mechanistic explanations to the broken trade-off.
In conclusion, the higher SLA was the most important trait for invasive O. corymbosa and P. pellucida in comparison with their respective noninvasive alien congeners, which was related directly to their lower leaf CC and indirectly to their higher N content in photosynthesis, biochemical capacity for photosynthesis, light-saturated photosynthetic rate and nitrogen use efficiency by increasing nitrogen allocation to photosynthesis. These traits may contribute to their invasiveness. Higher SLA has also been found for many other invasive species. This easily measured trait might be a predictor of some invasive species and analyzing it before introduction may be useful for reducing the possibility of introduction of new invasive species. Our study is the first that compares ecophysiological traits of two pairs of invasive and noninvasive alien congeners, while few studies have compared life-history traits of invasive and noninvasive congeners. Thus, further comparative studies on more invasive and noninvasive alien congeners at a wide range of environments are necessary for understanding the generality of the patterns found in this study and determining the extent of the competitive advantage provided by these traits. In addition, life-history traits of the invasive and noninvasive congeners compared should be considered in future study, as they may influence ecophysiological traits.
Abbreviations
- C i :
-
Intercellular CO2 concentration
- CC:
-
Leaf construction cost
- CE:
-
Carboxylation efficiency
- G s :
-
Stomatal conductance
- J max :
-
Maximum electron transport rate
- N A :
-
Total leaf nitrogen content
- N B :
-
Nitrogen content in bioenergetics
- N C :
-
Nitrogen content in carboxylation
- P B :
-
The fraction of leaf nitrogen allocated to bioenergetics
- P C :
-
The fraction of leaf nitrogen allocated to carboxylation
- P max :
-
Light-saturated photosynthetic rate
- PNUE:
-
Photosynthetic nitrogen use efficiency
- RGR:
-
Relative growth rate
- SLA:
-
Specific leaf area
- V cmax :
-
Maximum carboxylation rate
- WUE:
-
Water use efficiency
References
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259
Burns JH (2004) A comparison of invasive and non-invasive dayflowers (Commelinaceae) across experimental nutrient and water gradients. Divers Distrib 10:387–397
Burns JH (2006) Relatedness and environment affect traits associated with invasive and noninvasive introduced Commelinaceae. Ecol Appl 16:1367–1376
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
D’Antonio CM, Kark S (2002) Impacts and extent of biotic invasions in terrestrial ecosystems. Trends Ecol Evol 17:202–204
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Durand LA, Goldstein G (2001) Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 126:345–354
Ewe SML, Sternberg LSL (2003) Seasonal exchange characteristics of Schinus terebinthifolius in a native and disturbed upland community in Everglade National Park, Florida. For Ecol Manage 179:27–36
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 11:191–210
Feng Y-L, Auge H, Ebeling SK (2007a) Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia 153:501–510
Feng Y-L, Wang J-F, Sang W-G (2007b) Irradiance acclimation, capture ability, and efficiency in invasive and non-invasive alien plant species. Photosynthetica 45:245–253
Feng Y-L, Wang J-F, Sang W-G (2007c) Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels. Acta Oecologica 31:40–47
Goldberg D (1987) Neighborhood competition in an old field plant community. Ecology 68:1211–1223
Goodger JQD, Gleadow RM, Woodrow IE (2006) Growth cost and ontogenetic expression patterns of defence in cyanogenic Eucalyptus spp. Trees Struct Funct 20:757–765
Griffin KL (1994) Calorimetric estimates of CC and their use in ecological studies. Funct Ecol 8:551–562
Grotkopp E, Rejmánek M, Rost TL (2002) Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat 159:396–419
Grotkopp E, Rejmánek M (2007) High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. Am J Bot 94:526–532
Harvey PH, Purvis A (1991) Comparative methods for explaining adaptations. Nature 351:619–624
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23:188–261
Loomis RS (1997) Commentary on the utility of nitrogen in leaves. Proc Natl Acad Sci USA 94:13378–13379
Loustau D, Beahim M, Gaudillère JP, Dreyer E (1999) Photosynthetic responses to phosphorous nutrition in two-year-old maritime pine seedlings. Tree Physiol 19:707–715
Mack RN (1996) Predicting the identity and fate of plant invaders, emergent and emerging approaches. Biol Conserv 78:107–121
McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am J Bot 89:1431–1438
Nagel JM, Griffin KL (2001) Construction cost and invasive potential: Comparing Lythrum salicaria (Lythraceae) with co-occurring native species along pond banks. Am J Bot 88:2252–2258
Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Niinemets Ü, Valladares F, Ceulemans R (2003) Leaf-level phenotypic variability and plasticity of invasive Rhododendron ponticum and non-invasive Ilex aquifolium co-occurring at two contrasting European sites. Plant Cell Environ 26:941–956
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425
Pattison RR, Goldstein G, Ares A (1998) Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rain-forest species. Oecologia 117:449–459
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. BioScience 50:53–65
Poorter H, Villar R (1997) The fate of acquired carbon in plants: chemical composition and construction costs. In: Bazzaz FA, Grace J (eds) Plant resource allocation. Academic Press, New York, pp 39–72
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116:26–37
Radford IJ, Cousens RD (2000) Invasiveness and comparative life-history traits of exotic and indigenous Senecio species in Australian. Oecologia 125:531–542
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reichard SH, White P (2001) Horticulture as a pathway of invasive plant introductions in the United States. BioScience 51:103–113
Schieving F, Poorter H (1999) Carbon gain in a multisoecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytol 143:201–211
Shipley B (2006) Net assimilation rate, specific leaf area and leaf mass ratio: which is most closely correlated with relative growth rate? A meta-analysis. Funct Ecol 20:565–574
Smith MD, Knapp AK (2001) Physiological and morphological traits of exotic, invasive exotic and native species in tallgrass prairie. Int J Plant Sci 162:785–792
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Tsialtas JT, Kassioumi M, Veresoglou DS (2002) Leaf construction cost of the most abundant species in an upland grassland area of northern Greece. Russ J Plant Physiol 49:360–363
Williamson M, Fitter A (1996) The varying success of invaders. Ecology 77:1661–1666
Xu C-Y, Griffin KL, Schuster WSF (2007) Leaf phenology and seasonal variation of photosynthesis of invasive Berberis thunbergii (Japanese barberry) and two co-occurring native understory shrubs in a northeastern United States deciduous forest. Oecologia 154:11–12
Xu H-G, Ding H, Li M-Y, Qiang S, Guo J-Y, Han Z-M, Huang Z-G, Sun H-Y, He S-P, Wu H-R, Wan F-H (2006) The distribution and economic losses of alien species invasion to China. Biol Invasions 8:1459–1500
Acknowledgments
The authors are grateful to Prof. Dorothea Bartels and two anonymous reviewers for their valuable comments on an earlier version of the manuscript. This study was funded by the Project of the National Natural Science Foundation of China (30670394), the Applied Basic Study Project of Yunnan Province and the Key Project of Knowledge Innovation Engineering of Chinese Academy of Sciences (KSCX1-SW-13-0X-0X).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, YL., Fu, GL. & Zheng, YL. Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta 228, 383–390 (2008). https://doi.org/10.1007/s00425-008-0732-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0732-2