Abstract
An efficient protocol for cryopreservation of protocorm like bodies (PLBs) of Dendrobium nobile, based on encapsulation–dehydration (ED) and encapsulation–vitrification (EV), was established. In both cryogenic procedures, PLBs were initially osmoprotected with a mixture of 0.4 M sucrose and 2 M glycerol, incorporated in the encapsulation matrix [comprising 3% (w/v) sodium alginate and 0.1 M CaCl2]. Out of the two methods, EV resulted in higher survival (78.1%) and regrowth (75.9%) than ED (53.3 and 50.2% respectively). Incorporation of 0.4 M sucrose and 2 M glycerol in the encapsulation matrix resulted in higher survival percentage after cryopreservation. In both the cases (ED and EV), shoots regenerated from cryopreserved PLBs with an intermediary PLB formation. Regenerated shoots were successfully rooted in the medium containing 1.5 mg/l Indole-3 butyric acid. Successful acclimatization of plantlets was obtained in the compost containing brick pieces and charcoal chunks (1:1) + a top layer of moss with a maximum survivability (82%). EV method proved to be most appropriate way to cryopreserve the PLBs of D. nobile. Regenerated plantlets showed normal morphology as that of control plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dendrobium is one of the largest genus in the family Orchidaceae having numerous species including one of the most popular orchid Dendrobium nobile Lindl. It is an epiphyte and grows naturally in the states of North-east India, China and Nepal. Apart from its ornamental value, it is highly used in the Chinese herbal drug industry for its medicinal properties. The Taiwan people used its stem for treating night sweat, kidney disorder and to cure impotence. In China it is used as a tonic and strengthening medicine. The stems are used to alleviate thirst, calm restlessness, accelerate convalescence and reduce dryness of the mouth (Faria and Illg 1995). In India, the Khasi community of Meghalaya used the stem paste of this orchid externally on injuries and to set fractured bone (Hynniewta and Kumar 2008). Besides, D. nobile is well known in the pharmaceutical industry, as number of compounds including alkaloids (Suzuki et al. 1973), sesquiterpene glycosides (Zhao et al. 2001) are reported. Hu (1970) reported the antibiotic and possible anticancer properties of D. nobile. But in the current scenario the commercial exploitation of this orchid species has considerably depleted its population in wild hence needs conservation.
Due to fast growing nature of D. nobile, conservation of its germplasm through tissue culture requires frequent maintenance. Regular subcultures also increase the risk of tissue loss due to adverse culture conditions and somaclonal variation (Khoddamzadeh et al. 2010). To alleviate problems associated with culture maintenance, cryopreservation technique has been developed for the long-term conservation of several valuable germplasms. Cryopreservation offers long-term storage capability, high genetic stability along with minimal storage space and maintenance requirements of germplasm (Engelmann 1997).
Various approaches of cryopreservation such as vitrification, encapsulation–dehydration, encapsulation–vitrification and air-drying have been developed and used with varying degrees of success to preserve diverse species of plants (Xue et al. 2008; Hazubska-Przybyl et al. 2010; Hua and Rong 2010; Peng-Fei et al. 2011). Cryopreservation of seeds, shoot tips, protocorms and protocorm-like bodies (PLBs) of many orchids has been successfully attempted for short and long-term conservation. However, vitrification and air-drying methods have resulted in both low and slow rates of regrowth of plantlets in case of orchids (Bian et al. 2002), whereas encapsulation–dehydration and encapsulation–vitrification are comparatively more appropriate methods for orchid cryopreservation with higher success rate (Yin and Hong 2009; Subramaniam et al. 2011).
Encapsulated or artificial seeds have been reported as having advantages over non-encapsulated explants (Das et al. 2011) hence have wider applications for germplasm storage in cryopreservation studies. Though encapsulation–vitrification and encapsulation–dehydration are the most widely applicable methods of germplasm storage (Hirai and Sakai 1999) however, Khoddamzadeh et al. (2011) reported that these methods can be used mainly for cryopreservation of shoot-tips and only few PLBs (small vegetative parts of orchids that develop into whole plants). Limited number of studies has been reported on cryopreservation of protocorms and PLBs of Dendrobes (Chen et al. 2001; Lurswijidjarus and Thammasiri 2004; Pornchuti and Thammasiri 2008; Yin and Hong 2009; Anthony et al. 2010; Subramaniam et al. 2011; Pouzi et al. 2011). Though there is a single report on the cryopreservation of protocorms of D. nobile (Vendrame and Faria 2011), the emphasis was only given to the use of phloroglucinol in regrowth medium. There are no reports on the cryopreservation of PLBs of D. nobile using encapsulation–dehydration and encapsulation–vitrification method. Therefore, our present study represents the successful and efficient protocol for cryopreservation of D. nobile using PLBs as explant.
Materials and methods
Induction of protocorm like bodies
The experiment was performed using nodal segments (0.5–1.0 cm) with one axillary bud excised from the 4-month old in vitro-grown seedlings. Nodes were inoculated on ½-strength MS medium (Murashige and Skoog 1962) with 2% sucrose, 0.7% agar supplemented with equimolar concentrations of KN and NAA (1.0 mg/l). The pH of the medium was adjusted to 5.7 before autoclaving at 121°C for 20 min. All the cultures were maintained in the culture room at 25 ± 2°C, under 50 μmol m−2 s−1 light intensity and 12 h photoperiod. Seventy percent of the nodal explants induced PLBs and were transferred for multiplication to a semisolid ½-strength MS basal medium with 2% sucrose, 0.7% agar.
Encapsulation–dehydration
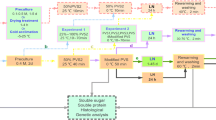
PLBs measuring 0.1–0.2 cm in diameter (obtained after 3-subculture) were excised, and used for cryopreservation by both encapsulation–dehydration (ED) as well as encapsulation–vitrification (EV) methods. Encapsulation was carried out with suspension of PLBs in calcium-free ½-strength MS medium supplemented with 3% (w/v) sodium alginate, 0.4 M sucrose and 2 M glycerol (pH 5.7). The PLBs were dropped into 0.1 M CaCl2 solution (prepared in ½-strength MS medium) containing 0.4 M sucrose and 2 M glycerol (pH 5.7). After 20 min, calcium alginate beads (about 4 mm in diameter) containing single PLB were recovered. Beads were then precultured on ½-strength MS liquid medium supplemented with different concentrations of sucrose (0.3, 0.5, 0.7 M) for 1/2/3 days keeping on a rotary shaker at 98 rpm (25°C). Subsequently pretreated beads were dehydrated gradually for 1–8 h under laminar airflow hood under aseptic conditions. At 1 h interval dehydrated beads were placed in 2.0 ml cryovials and directly plunged into liquid nitrogen, and stored for 1 day. Dehydrated samples without liquid nitrogen (LN) treatment were considered as control.
Determination of bead water content
Five independent samples of 10 beads were weighed at different times during the dehydration treatment and then placed in hot air oven (85°C) for 24 h for dry weight. The water content was calculated on a fresh weight (FW) basis using the following formula.
Encapsulation–vitrification
For EV experiment, encapsulated PLBs were precultured in ½-strength MS liquid medium supplemented with 0.5 M of sucrose for 2 days (as proven best in ED method). Encapsulated PLBs were rapidly surface-dried by plating them on a sterilized filter paper and loaded in a loading solution (2.0 M glycerol and 0.4 M sucrose) in a 100 ml Erlenmeyer flask, for various durations (0–120 min) at 25°C for investigating the effect of loading solution on osmotic tolerance of precultured PLBs to plant vitrification solution 2 (PVS2). The PVS2 solution consisted of 30% (w/v) glycerol, 15% (w/v) ethylene glycol, and 15% (w/v) dimethyl sulfoxide (DMSO) and 0.4 M sucrose in ½-strength MS medium (pH 5.7). The beads were directly dehydrated with PVS2 at either 25 or 0°C for various time periods (5–145 min) prior to their direct immersion in LN for 1 h. Samples dehydrated with PVS2 and without LN treatment were considered as control in this case.
Thawing and post-culture
The cryotubes containing encapsulated PLBs were rapidly thawed in water bath at 38 ± 2°C for 2 min. Thawed beads were directly cultured on the regrowth medium [½-strength MS medium + 2.0% (w/v) sucrose + 0.6% (w/v) agar + 1.0 mg/l BAP + 0.1 mg/l NAA with pH 5.7] in case of ED method. For EV method, the PVS2 solution was drained and gradually replaced with 1.2 M sucrose solution prepared in liquid ½-strength MS medium (unloading solution), and kept for 20 min at room temperature. The beads were then surface-dried by blotting on sterilized filter paper and cultured on the regrowth medium, and kept in dark for 1 day. Cultures were then transferred to fresh regrowth medium and incubated in the dark for 5 days, under dim light (5 μmol m−2 s−1) for 1 week, and then transferred to the light intensity (36 μmol m−2 s−1) provided by white cool fluorescent tubes with a 12 h photoperiod at 25 ± 2°C.
Evaluation of PLB survival and plant regeneration
Percentage survival and regrowth of beads were recorded after 60 and 90 days of culture respectively. The encapsulated PLBs were considered to have survived with emergence of PLBs from the beads, whereas emergence of shoots (leaves, nodes and internodes without roots) was considered as regeneration.
Rooting and hardening
All the regenerated plantlets with well developed shoots were cultured in 15 × 150 mm glass test tubes containing 10 ml of semisolid [½-strength MS basal medium with 2.0% (w/v) sucrose, 0.6% (w/v) agar and different concentrations of NAA and IBA (0.05, 0.1, 0.5 and 1.0 mg/l)]. The culture tubes were incubated at 25 ± 2°C under cool fluorescent light at 36 μmol m−2 s−1 with 12 h photoperiod for 2 months. Well rooted plantlets were transferred to plastic pots containing brick pieces and charcoal chunks (1:1) + a top layer of moss, and kept in the departmental green house. The plantlets were initially covered with a polythene sheet for 30 days to maintain high humidity (90%) and irrigated three times a week. The survivability percentage of plantlets was calculated after 90 days.
Experimental design and statistical analysis
For all cryopreservation experiments, 50 beads were used per treatment and each treatment repeated three times. Rooting experiments were carried out with 20 replicates. All data were subjected to analysis of variance (one way ANOVA) and significance (P < 0.05) was determined with Duncan’s multiple range test. Statistical tests were performed by the help of SPSS statistical package version 15.0 (SPSS Inc., Chicago, USA).
Results
Encapsulation–dehydration
Effect of dehydration by air-drying on water content and survival after cryopreservation
Preculture played a vital role in survival of cryopreserved PLBs. All the PLBs without preculture treatment died after cryopreservation. Changes in the water content of the precultured beads and survival of cryopreserved PLBs are presented in Figs. 1 and 2. The initial water content of the precultured beads was 87.8% on a fresh weight basis (after preculture in 0.5 M sucrose for 2 days). Water content decreased to 28.3% after 5 h of dehydration under laminar airflow hood and was recorded to be zero after 8 h of dehydration. Low percentage survival (3.1%) of cryopreserved PLBs was detected after 3 h of dehydration when bead water content reached 51.4%. Highest survival (53.3%) of cryopreserved encapsulated PLBs was obtained after 5 h of dehydration with reduced bead water content (28.3%) (Figs. 1, 2) and gradually decreased (37.4%) with 6 h of dehydration with bead water content of 16.7%. No survival was obtained after 7 h of dehydration with 11.3% water content in the bead.
Effect of liquid nitrogen on survival
Percentage survival of encapsulated beads precultured with 0.3 M sucrose in the medium for 2 days and dehydrated for different periods of time was higher in control than the cryopreserved samples. However, encapsulated PLBs precultured with 0.5 M sucrose for 2 days and air dried for 5 h or 6 h under laminar flow hood showed better percentage of survival than control. Maximum percentage survival (53.3%) of cryopreserved PLBs was recorded when pretreated with 0.5 M sucrose for 2 days and further dehydrated in the laminar flow hood for 5 h (Fig. 2). However in the same pretreatment for the same duration and with increase in dehydration time by an hour, a decrease in percentage survival was recorded. Similarly, with increase in sucrose concentration in the pretreatment culture for the same period, no survival of encapsulated PLBs after cryopreservation was observed.
Encapsulation–vitrification
Effect of loading solution, vitrification time and temperature on survival
Survival was markedly affected by loading time (Fig. 3). Without treatment with loading solution, PLBs failed to survive freezing in LN. Loading time of 60 min was found to be best for survival of PLBs (70.1%). Both duration of dehydration with PVS2 and exposure temperature significantly (P < 0.05) influenced percentage survival of encapsulated PLBs. Survival of cryopreserved PLBs increased rapidly with increasing dehydration periods both at 25°C and 0°C. However, maximum frequency of survival (78.1%) was achieved at 0°C with 115 min of dehydration compared with the frequency survival (70.1%) at 25°C with 85 min of dehydration (Fig. 4). In case of non-cryopreserved PLBs, survival rate gradually decreased and no survival was obtained after 75 and 95 min of dehydration with PVS2 at 25°C and at 0°C respectively (Fig. 4).
Regeneration of plantlets from cryopreserved PLBs
In the current study regeneration from both control and cryopreserved PLBs underwent an intermediary PLB formation. All the surviving PLBs first started dividing into multiple PLBs and gave rise to plantlets (Fig. 7e, f). Plantlets started developing after 35 days of culture in the regrowth medium (Fig. 7g, h). In case of encapsulation–dehydration maximum regeneration 50.2% (Fig. 5) was obtained after cryopreservation where as in case of encapsulation–vitrification it was 75.9% at 0°C (Fig. 6). All the regenerated plantlets with well developed shoots were cultured for rooting. Table 1 represents the effect of different auxins for induction of roots. The result revealed that though roots were induced in all the plantlets treated with auxins but the maximum number of roots (11 ± 0.14) was recorded in the medium supplemented with 1.5 mg/l IBA (Table 1; Fig. 7i). All the concentrations of NAA supplemented in the medium were found to be less significant in root induction compared to plantlets treated with IBA.
Regeneration of plantlets from cryopreserved PLBs of Dendrobium nobile through encapsulation–dehydration and encapsulation–vitrification. a D. nobile plant blooming in the departmental glass house. b PLBs encapsulated in alginate beads (Bar = 1 cm). c Close view of an encapsulated PLB (Bar = 1 mm). d Dehydrated beads after 5 h of dehydration. e and f Multiplication of PLBs after cryopreservation in regrowth medium in case of EV (Bar = 1 cm) and ED (Bar = 1 mm) respectively. g and h Regeneration of plantlets from cryopreserved PLBs in ED and EV respectively (Bar = 1 cm). i Complete plantlet with well developed shoot and roots in ½-strength MS medium containing 1.5 mg/l IBA (Bar = 1 cm). j Plantlets established under green house condition (Bar = 10 cm)
Successful acclimatization of plantlets was obtained in brick pieces and charcoal chunks (1:1) + a top layer of moss. Plantlets were successfully acclimatized (82%) in the green house conditions after 90 days hardening (Fig. 7j). Morphologically, both the cryopreserved and the controlled plantlets were similar.
Discussions
Encapsulation–dehydration
Effects of pretreatment
Encapsulation and preculture followed by dehydration resulted in higher survival and regrowth of PLBs after cryopreservation. Preculture of explants in suitable medium helps to increase tolerance to dehydration and subsequent freezing in LN. The addition of sucrose in the preculture media helps in osmoprotection by stabilizing cellular membranes and maintaining turgor (Valentovie et al. 2006). Higher concentrations of osmoticum protect the plant from desiccation injury. In the present study, it was found that all the PLBs withstood a sucrose concentration as high as 0.7 M when precultured after encapsulation. However increase of sucrose concentration in the preculture medium resulted in decrease of percentage survival of the encapsulated PLBs. This might have been due to sucrose creating harmful osmotic stress in the treated explants promoting excessive dehydration of the PLBs and hence incurring toxicity. It has long been reported that preculture duration influences high survival of different explants after cryopreservation in orchids (Ishikawa et al. 1997; Maneerattanarungroj et al. 2007). However, the survival percentage of cryopreserved protocorms was reduced to 15%, when protocorms of Dendrobium virgineum were precultured in a modified (Vacin and Went 1949) liquid medium supplemented with 0.3 M sucrose for 3 days (Pornchuti and Thammasiri 2008). Similarly, low regrowth (13.33%) was observed in encapsulated shoot tips of Dendrobium Walter Oumae that were precultured with 0.3 M sucrose in agar medium for 2 days (Lurswijidjarus and Thammasiri 2004). Our results with D. nobile PLBs reveal that application of a correct preculture duration (2 days) and concentration of sucrose (0.5 M) is essential for maximum survivability. Therefore, all these different studies suggest that different orchid species exhibit varying levels of tolerance to high sucrose concentrations.
Effect of dehydration
The control of water content of plant samples before freezing is the key factor in developing successful cryoprotection protocols (Zhang et al. 2001). The water content of the encapsulated beads was removed by both osmotic dehydration and sterile air-flow. Earlier studies suggested that if the cells are not sufficiently dehydrated, intracellular ice will be formed resulting in cryoinjury during cold storage in liquid nitrogen and if over-dehydrated, the osmotic stress can be damaging (Bian et al. 2002). In the present study, maximum survival of PLBs (53.3%) was achieved after cryopreservation when precultured beads were dehydrated for 5 h with reduced water content of 28.3% and gradually decreased (37.4%) with 6 h of dehydration with bead water content of 16.7%. As the water content of the beads containing PLBs decreased, the survival rates after cryopreservation increased whereas survival of encapsulated non-cryopreserved PLBs declined with increased dehydration (Fig. 2). This may be due to the osmotic shock created due to overdehydration (Maruyama et al. 1998). Similarly, Jitsopakul et al. (2007) reported that the regrowth rate of non-cryopreserved and cryopreserved protocorms of Vanda coerulea depend on the water content of the precultured beads during dehydration. The optimal water content of alginate beads as well as survival is dependent to a large extent on different plant species and explants (Suzuki et al. 1998; Gonzalez-Arnao et al. 2000; Padro et al. 2011). Therefore in order to achieve the highest survival of a particular cultivar of a given plant species, the optimum water content of encapsulated explants should be carefully determined before any application.
Encapsulation–vitrification
Vitrification refers to the physical process by which a highly concentrated aqueous solution solidifies into a glassy solid at sufficiently low temperatures without crystallization (Hong et al. 2009). Thus, it is essential to enhance the dehydration tolerance of plant tissues to the vitrification solution. In the present study, before dehydrating with PVS2 the encapsulated PLBs were pretreated with 0.5 M sucrose for 2 days, as it was observed to promote maximum survivability of cryopreserved PLBs in the earlier experiment using ED method. For cryopreservation protocols, direct exposure to the vitrification solution is harmful due to either osmotic stress or chemical toxicity which has been described as a major hindrance to cryopreservation by vitrification (Matsumoto et al. 1994). However, osmotolerance is rarely achieved by preculture with sucrose alone. Therefore, a loading treatment with a load solution containing various amounts of sucrose and glycerol is commonly used (Xue et al. 2008). Loading treatment can be done either by mixing the loading solution in the alginate matrix as in case of Dendrobium cariniferum Rchb. f. or incubating the encapsulated beads in the loading solution for various time duration before treating with PVS2 as in case of Dendrobium candidum PLBs (Yin and Hong 2009). In the present study PLBs of D. nobile were treated in both the ways with loading solution. It was observed that there was a higher chance of survival when PLBs were encapsulated in alginate matrix along with a loading solution including 0.4 M sucrose and 2.0 M glycerol followed by treatment with same concentration of loading solution for about 60 min. Following incubation in a load solution containing 2.0 M glycerol and 0.4 M sucrose for 15 min at 25°C, 60% regeneration frequency of zygotic embryos of the Japanese terrestrial orchid, B. striata, resulted (Ishikawa et al. 1997). In case of D. candidum, loading treatment of a mixture of 2.0 M glycerol and 1.0 M sucrose enhanced survival and regeneration of PLBs. Highest survival percentage of cryopreserved PLBs of D. nobile was obtained when encapsulated PLBs were treated for 80 min with a loading solution prior to dehydration. Similar results were obtained in case of cryopreservation protocorms of Dendrobium cariniferum Rchb. f. by EV method (Pornchuti and Thammasiri 2008). The reason of applying loading solution in the bead is to avoid the intracellular ice formation as the PLBs remain in direct contact with the loading solution. But as the alginate bead itself contains a major amount of water which may result in extra cellular ice crystals formation, a further treatment with loading solution for different duration is preferable.
Incubation period and temperature of the vitrification solution are two important factors affecting survival of cryopreserved plant tissues. Overexposure of plant tissues to the vitrification solution may lead to chemical toxicity and excessive osmotic stress. The optimal exposure time for PVS2 varies with plant species and depends on the temperature during exposure (Hong et al. 2009). In earlier reports the optimal exposure time to PVS2 at 25 ± 2°C varied for different orchid species (Thammasiri 2000; Pornchuti and Thammasiri 2008; Yin and Hong 2009). However, dehydration at 0°C also yields higher survival, with the incubation time largely extended, thus allowing for greater flexibility in handling large numbers of samples at the same time (Wang et al. 2002). In the present study a significance increase in survivability was noticed when PLBs were dehydrated in PVS2 solution at 0°C with a frequency of survival 78.1% for 115 min as compared to 25°C with percentage survival of 70.1% for 85 min. These findings are similar to those results reported by Yin and Hong (2009), in case of Dendrobium candidum where survival percentage of cryopreserved PLBs treated with PVS2 increased from 76.2% following dehydration at 25°C for 120 min to 89.4% following dehydration at 0˚ C for 150 min. Interestingly, protocorms of Dendrobium cariniferum treated with PVS2 and subjected to cryopreservation by encapsulation–vitrification exhibited lower survival frequency of 15% (Thammasiri 2008).
Therefore, percentage survival by EV method could be attributed to genotypic differences as well as differences in incubation periods and temperatures of the vitrification solution.
Regeneration of plantlets
In this study, regrowth of control PLBs was initiated earlier and preceded faster than that of cryopreserved PLBs. These findings are consistent with those reported for Vitis vinifera L. cv. Red Globe (Wang et al. 2002). PLBs that have been subjected to cryostorage rarely display the characteristic of green colour that indicates viability. Greenish appearance indicates a quicker regrowth and higher viability whereas appearance of other colours, could be attributed to osmotic shock or unfavorable regrowth conditions (Moges et al. 2004). All the cryostored PLBs recovered in our study were initially light green when recovered from the liquid nitrogen and incubated in the dark, but underwent either bleaching or browning within 24 h of direct exposure to light (without dark incubation). Hence, a higher degree of PLBs viability can be obtained if the PLBs were incubated in the dark continuously for 5 days and then exposed to a cool fluorescent light. Similar observation has been reported in orchids as well as other plant species (Yin and Hong 2009, 2010; Padro et al. 2011; Sharaf et al. 2011), stating that this step is essential to reduce shock due to photo oxidation to the cryopreserved plant tissues. In particular, dark incubation for a short time following post-thawing enhanced survival and this was presumably attributed to damage repair of tissues that might take place during darkness.
Rooting and hardening of cryopreserved plantlets
Roots were induced in all the regenerated shoots treated with auxins. All the concentrations of NAA were found to be less significant for root induction as compared to IBA. Kim et al. (2003) reported IBA to be more effective than NAA in case of rhizogenesis. The effectiveness of IBA in rooting has been reported for medicinal orchids like Vanilla planifolia (Giridhar et al. 2001) and Cymbidium pendulum (Nongdam et al. 2006). Aktar et al. (2007) also reported maximum rooting in Dendrobium species in response to IBA (1.0 mg/l) which is quite similar to our findings wherein highest percentage as well as number of roots (100%; 11.00 ± 0.14) was obtained in the medium supplemented with 1.5 mg/l IBA.
Successful acclimatization of plantlets was obtained in the compost containing brick pieces and charcoal chunks (1:1) + a top layer of moss with maximum survivability (82%) under glass house conditions. Being epiphytic in nature, D. nobile needs a good growing substratum having the properties of maximum water holding capacity, porosity and drainage. Charcoal and brick pieces provide a good drainage and aeration to the roots, which is of prime importance to the orchid (Dohling et al. 2008). A layer of moss on the top of this substratum proved to be the best due to higher moisture retaining capacity.
Conclusion
Current studies on D. nobile showed an advantage of encapsulation–vitrification method with higher survival and regeneration rates than encapsulation–dehydration, However, there are few studies having experimental evidence for the cause of such differences between EV and ED. According to Wang et al. (2005), histological studies revealed that following EV, most cells in the meristems of raspberry survived freezing in liquid nitrogen, whereas massive structural damage was observed following ED. These differences might be directly reflected as differences in regrowth and regeneration of the cryopreserved explants. This study describes the efficient, simple protocol for cryopreservation of D. nobile germplasm which can also be widely applicable to different Dendrobium species. The EV procedure can be used to replace the previous procedures that require cold-hardening or slow-freezing of the stock plants and, thus, avoid the use of programmable freezers or expensive growth chambers, enabling accurate low-temperature treatments.
Abbreviations
- ED:
-
Encapsulation–dehydration
- EV:
-
Encapsulation–vitrification
- PVS2:
-
Plant vitrification solution 2
- PLB:
-
Protocorm like bodies
- NAA:
-
α-Naphthaleneacetic acid
- IBA:
-
Indole-3 butyric acid
References
Aktar S, Nasiruddin KM, Huq H (2007) In vitro root formation in Dendrobium orchid plantlets with IBA. J Agric Rural Dev 5:48–51
Anthony JJJ, Keng CL, Rathinam X, Sinniah UR, Subramaniam S (2010) Preliminary study on cryopreservation of Dendrobium Bobby Messina protocorm-like bodies by vitrification. Afr J Biotechnol 9:7063–7070
Bian HW, Wang JH, Lin WQ, Han N, Zhu MY (2002) Accumulation of soluble sugars, heat-stable proteins and dehydrins in cryopreservation of protocorm-like bodies of Dendrobium candidum by the air-drying method. J Plant Physiol 159:113–1145
Chen Y, Wan JH, Huan CN (2001) Germplasm cryopreservation of Dendrobium candidum by vitrification. J Zhejiang Univ (Agric & Life Sci) 27:436–438
Das MC, Kumaria S, Tandon P (2011) Storage and high conversion frequency of encapsulated protocorm-like bodies of Cymbidium devonianum (orchid). J Hortic Sci Biotechnol 86:611–615
Dohling S, Kumaria S, Tandon P (2008) Optimization of nutrient requirements for asymbiotic seed germination of Dendrobium longicornu Lindl. and D. formosum Roxb. Proc Indian Natl Sci Acad 74:167–171
Engelmann F (1997) In vitro conservation methods. In: Callow CA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources. CAB International, Oxford, pp 119–161
Faria RT, Illg RD (1995) Propagação clonal de híbridos de Dendrobium nobile Lindl. In: Congresso Brasileiro de Floriculturae Plantas Ornamentais, 10. Campinas. Anais. Campinas (ed) SBF, pp 40–41
Giridhar P, Obul RB, Ravishankar GA (2001) Silver nitrate influences in vitro shoot multiplication and root formation in Vanilla planifolia. Androl Curr Sci 81:1166–1170
Gonzalez-Arnao MT, Engelmann F, Urra C, Morenza M, Rios A (2000) Cryopreservation of citrus apices using the encapsulation–dehydration technique. Cryo Lett 19:177–182
Hazubska-Przybył T, Chmielarz P, Michalak M, Bojarczuk K (2010) Cryopreservation of embryogenic tissues of Picea omorika (Serbian spruce). Plant Cell Tiss Org Cult 102:35–44
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of mint (Mentha spicata L.) by encapsulation-vitrification. Plant Cell Rep 19:150–155
Hong SR, Yin MH, Shao XH, Wang AP, Xu WH (2009) Cryopreservation of embryogenic callus of Dioscorea bulbifera by vitrification. Cryo Lett 30:64–75
Hu SY (1970) Dendrobium in Chinese medicine. Econ Bot 24:165–174
Hua YM, Rong HS (2010) A simple cryopreservation protocol of Dioscorea bulbifera L. embryogenic calli by encapsulation-vitrification. Plant Cell Tissue Org Cult 102:35–44
Hynniewta SR, Kumar Y (2008) Herbal remedies among the Khasi traditional healers and village folks in Meghalaya. Ind J Trad Knowl 7:581–586
Ishikawa K, Harata K, Mii M, Sakai A, Yoshimatsu K, Shimomura K (1997) Cryopreservation of zygotic embryos of a Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep 16:754–757
Jitsopakul N, Thammasiri K, Ishikawa K (2007) Cryopreservation of Vanda coerulea protocorms by encapsulation–dehydration method. In: 33rd Congress on Science and Technology of Thailand
Khoddamzadeh A, Sinniah U, Kadir MA, Kadzimin S, Mahmood M, Sreeramanan S (2010) Detection of somaclonal variation by random amplified polymorphic DNA analysis during micropropagation of Phalaenopsis bellina (Rchb.f.) Christenson. Afr J Biotechnol 9:6632–6639
Khoddamzadeh A, Sinniah U, Lynch P, Kadir MA, Kadzimin S, Mahmood M (2011) Cryopreservation of protocorm-like bodies (PLBs) of Phaleonopsis bellina (Rchb.f) Christenson by encapsulation-dehydration. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-9997-4
Kim YS, Hahn EJ, Yeung EC, Paek KY (2003) Lateral root development and saponin accumulation as affected by IBA or NAA in adventitious root cultures of Panax ginseng CA Meyer. In Vitro Cell Dev Biol 39:245–249
Lurswijidjarus W, Thammasiri K (2004) Cryopreservation of shoot tips of Dendrobium Walter Oumae by encapsulation-dehydration. Sci Asia 30:293–299
Maneerattanarungroj P, Bunnag S, Monthatong M (2007) In vitro conservation of Cleisostoma areitinum (Rchb.f.) Garay, rare Thai orchid species by an encapsulation-dehydration method. Asian J Plant Sci 6:1235–1240
Maruyama E, Ishii K, Kinoshita I (1998) Alginate encapsulation technique and cryogenic procedure for long term storage of the tropical forest tree Guazuma crinite Mart. In Vitro cultures. Japan Agric Res Q 32:301–309
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Moges AD, Shibli RA, Karam NS (2004) Cryopreservation of African Violet (Saintpaulia ionantha Wendl.) shoot-tips. In Vitro Cell Dev Biol Plant 40:389–395
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nongdam P, Nirmala C, Tewari R (2006) In vitro multiplication of Cymbidium pendulum orchids via embryo culture. Plant Cell Biotech Mol Biol 7:145–150
Padro MDA, Frattarelli A, Sgueglia A, Condello E, Damiano C, Caboni E (2011) Cryopreservation of white mulberry (Morus alba L.) by encapsulation-dehydration and vitrification. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-0017-5
Peng-Fei A, Lu LP, Song JJ (2011) Cryopreservation of in vitro-grown shoot-tips of Rabdosia rubescens by encapsulation-dehydration and evaluation of their genetic stability. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-0049-x
Pornchuti W, Thammasiri K (2008) Cryopreservation of protocorms of Dendrobium virgineum Rchb.f. Acta Hortic 788:63–68
Pouzi NZ, Rathinam X, James AJJ, Poobathy R, Subramaniam S (2011) Early investigation on cryopreservation of Dendrobium sonia-28 using encapsulation-dehydration with modified Evan blue assay. Afr J Biotechnol 10:3534–3539
Sharaf SA, Shibli RA, Kasrawi MA, Baghdadi SH (2011) Cryopreservation of wild Shih (Artemisia herba-alba Asso.) shoot tips by encapsulation-dehydration and encapsulation-vitrification. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-011-0054-0
Subramaniam S, Sinniah UR, Khoddamzadeh AA, Periasamy S, James JJ (2011) Fundamental concept of cryopreservation using Dendrobium sonia-17 protocorm-like bodies by encapsulation-dehydration technique. Afr J Biotechnol 10:3902–3907
Suzuki M, Hayakawa Y, Aoki K (1973) Stereochemistry of intermediates in the syntheses of Dendrobium alkaloids. Tetrahedron Lett 4:331–334
Suzuki M, Ishikawa M, Akihama T (1998) A novel preculture method for the induction of desiccation tolerance in gentian axillary buds for cryopreservation. Plant Sci 135:69–76
Thammasiri K (2000) Cryopreservation of seeds of a Thai orchid by vitrification. Cryo Lett 21:237–244
Thammasiri K (2008) Cryopreservation of some Thai orchid species. Acta Hortic 788:53–62
Vacin EF, Went FW (1949) Some pH changes in nutrient solutions. Bot Gaz 110:605–613
Valentovie P, Luxová M, Kolaroviè L, Ga š paríková O (2006) Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Env 52:186–191
Vendrame WA, Faria RT (2011) Phloroglucinol enhances recovery and survival of cryopreserved Dendrobium nobile protocorms. Sci Hortic 128:131–135
Wang QC, Gafny R, Sahar N, Sela I, Mawassi M, Tanne E, Perl A (2002) Cryopreservation of grapevine (Vitis vinifera L.) embryogenic cell suspensions and subsequent plant regeneration by encapsulation-dehydration. Plant Sci 162:551–558
Wang Q, Laamanen J, Uosukainen M, Valkonen JPT (2005) Cryopreservation of in vitro-grown shoot-tips of raspberry (Rubus idaeus L.) by encapsulation-vitrification and encapsulation-dehydration. Plant Cell Rep 24:280–288
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill. Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Organ Cult 92:251–260
Yin M, Hong S (2009) Cryopreservation of Dendrobium candidum Wall. Ex Lindl. protocorm-like bodies by encapsulation-vitrification. Plant Cell Tissue Organ Cult 98:179–185
Yin M, Hong S (2010) A simple cryopreservation protocol of Dioscorea bulbifera L. embryogenic calli by encapsulation–vitrification. Plant Cell Tissue Organ Cult 101:349–358
Zhang YX, Wang JH, Bian H, Zhu MY (2001) Pregrowth-desiccation: a simple and efficient procedure for the cryopreservation of rice (Oryza sativa L.) embryogenic suspension cells. Cryo Lett 22:221–228
Zhao W, Ye Q, Tan X, Jiang H, Li X, Chen K, Kinghorn AD (2001) Three new sesquiterpene glycosides from Dendrobium nobile with immunomodulatory activity. J Nat Prod 64:1196–1200
Acknowledgments
This research was financially supported by the Centre for Advanced studies in Botany, North-Eastern Hill University (NEHU), Shillong, India. The authors are also thankful to SAIF, NEHU for supply of liquid nitrogen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohanty, P., Das, M.C., Kumaria, S. et al. High-efficiency cryopreservation of the medicinal orchid Dendrobium nobile Lindl.. Plant Cell Tiss Organ Cult 109, 297–305 (2012). https://doi.org/10.1007/s11240-011-0095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-0095-4