Abstract
Thailand is the origin of about 1, 300 tropical orchid species and 180–190 genera. Deforestation and over-collection of wild Thai orchids for trade has placed orchid species at a risk of extinction. Therefore, the conservation as well as sustainable use is urgently needed to conserve orchids by various means. The genera Paphiopedilum and Dendrobium cruentum are listed in Appendix I of CITES. At the Department of Plant Science, Faculty of Science, Mahidol University, various methods of cryopreservation and conservation of Thai orchid species have been carried out. For cryopreservation, recent methods were used, namely, vitrification (dehydration in PVS2 solution, consisted of 30% glycerol, 15% ethylene glycol and 15% dimethyl sulfoxide, prepared in modified Vacin and Went liquid medium), encapsulation-dehydration (encapsulation in calcium alginate beads followed by air-drying in a laminar air-flow cabinet), encapsulation-vitrification (encapsulation in calcium alginate beads followed by dehydration in PVS2 solution), droplet-vitrification (fast freezing from small drops of PVS2 solution on aluminium strip) and cryo-plate (a combination of encapsulation and droplet on very fast freezing aluminium plate) dehydrated with silica gel and drying beads. Application of these methods in seeds was successful in Dendrobium chrysotoxum (99%, vitrification), D. cruentum (32%, vitrification), D. draconis (95%, vitrification), D. hercoglossum (80%, encapsulation-vitrification), Doritis pulcherrima (62%, vitrification), Rhynchostylis coelestis (85%, vitrification), Vanda coerulea (67%, vitrification), as well as in protocorms of D. cruentum (33%, vitrification; 27%, encapsulation-dehydration), D. cariniferum (15%, encapsulation-vitrification), Grammaytophyllum speciosum (14%, encapsulation-vitrification), Rhynchostylis gigantea (19%, vitrification), V. coerulea (40%, encapsulation-dehydration), Seidenfadenia mitrata (67%, vitrification) and Arundina graminifolia (76% and 74%, cryo-plate dehydrated with drying beads and silica gel, respectively; 33% droplet-vitrification; 64% encapsulation-dehydration with drying beads or silica gel). Cryopreserved seeds and protocorms were able to develop into normal seedlings. These techniques appear to be promising for the cryopreservation of some Thai orchid germplasm.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitrification
- Encapsulation-dehydration
- Encapsulation-vitrification
- Droplet-vitrification
- Cryo-plate
- Drying beads
- Silica gel
1 Introduction
Thailand is the origin of about 1, 300 tropical orchid species and 178 genera. Many Thai orchid species have good horticultural characteristics and are used as parents for breeding, making Thailand the No.1 orchid exporter. Climate change, deforestation (habitat destruction), and over-collection of wild Thai orchids for trade has placed Thai orchids at a risk of extinction. Therefore, conservation, social awareness and consciousness, as well as sustainable use are urgently needed to conserve orchids by various means (Thammasiri 2008). At the Department of Plant Science, Faculty of Science, Mahidol University, various methods of ex situ conservation of Thai orchid species have been carried out, namely, cryopreservation, seed stores under Orchid Seed Stores For Sustainable Use (OSSSU) project and micropropagation.
2 Cryopreservation Technology
After meeting and discussing with Professor Akira Sakai at the International Workshop on In Vitro Conservation of Plant Genetic Resources, 4–6 July 1995 in Kuala Lumpur, Malaysia, he came to demonstrate vitrification-based methods for plant cryopreservation at my department. A little later, I started doing research on cryopreservation of jackfruit embryonic axes which was very successful and novel (Thammasiri 1999). I then shifted my interest in cryopreservation research into orchids for which I am the pioneer. I published the first paper on Doritis pulcherrima, a wild Thai orchid, on seed cryopreservation by vitrification with 62% (Thammasiri 2000). It was also the first paper on seed cryopreservation by vitrification [dehydration in PVS2 solution, consisting of 30% (w/v) glycerol, 15% (w/v) ethylene glycol and 15% (w/v) dimethyl sulfoxide, prepared in modified Vacin and Went liquid medium].
Later my post-graduate and doctoral students studied recent methods, namely, vitrification (dehydration in PVS2 solution), encapsulation-dehydration (encapsulation in calcium alginate beads followed by air-drying in a laminar air-flow cabinet), encapsulation-vitrification (encapsulation in calcium alginate beads followed by dehydration in PVS2 solution) and droplet-vitrification (fast freezing from small drops of PVS2 solution with plant materials inside on a 7 × 20 mm sterile aluminum foil strip). Application of these methods on seeds was successful in many Thai orchid species. Cryopreserved seeds and protocorms were able to develop into normal seedlings. These methods appear to be promising techniques for cryopreservation of many Thai orchid species.
Thammasiri (2002) presented “Preservation of Seeds of Some Thai Orchid Species by Vitrification” at the 16th World Orchid Conference. Dendrobium chrysotoxum, D. draconis, Doritis pulcherrima and Rhynchostylis coelestis had 99%, 95%, 62% and 85% germination, respectively, after seed cryopreservation by vitrification. Other Thai orchid seeds were later successfully cryopreserved, such as in D. cruentum (32% by vitrification) (Kagawa 2006), Vanda coerulea (67% by vitrification) (Thammasiri and Soamkul 2007), D. hercoglossum (80% by encapsulation-vitrification) as well as in protocorms of D. cruentum (33% by vitrification and 27% by encapsulation-dehydration) (Kagawa 2006), D. cariniferum (15% by encapsulation-vitrification) (Pornchuti and Thammasiri 2008), Vanda coerulea (40% by encapsulation-dehydration) (Jitsopakul et al. 2008) and Seidenfadenia mitrata (67% by vitrification).
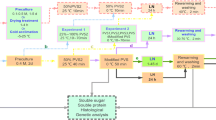
Sopalun et al. (2010a, b) studied three vitrification-based methods, namely, droplet-vitrification, encapsulation-dehydration and encapsulation-vitrification, for cryopreservation of protocorms of Grammatophyllum speciosum, known as “Tiger orchid” or “Giant orchid”. Protocorms, 0.1 cm in diameter, developed from 2-month-old germinating seeds were used. For droplet-vitrification (Fig. 1.1), protocorms were precultured on filter paper soaked in half strength Murashige and Skoog medium (½MS) containing 0.4 M sucrose at 25 ± 2 °C for 2 days, followed by soaking in loading solution (2 M glycerol and 0.4 M sucrose in ½MS liquid medium) for 20 min and then dehydrated with PVS2 solution in ½MS liquid medium containing 0.4 M sucrose at pH 5.7 for 30 min. For encapsulation-dehydration (Fig. 1.2), encapsulated protocorms were precultured in ½MS liquid medium containing 0.4M sucrose on a shaker (110 rpm) at 25 ± 2 °C for 2 days, followed by soaking in the same loading solution for 20 min and then exposed to a sterile air-flow at 2.5 inches/water column from the laminar air-flow cabinet for 8 h. For encapsulation-vitrification (Fig.1.3), encapsulated protocorms were precultured in ½MS liquid medium containing 0.4 M sucrose for 1 or 2 days, followed by soaking in the same loading solution for 20 min and then dehydrated with PVS2 solution for 60 min. For all three methods, preculturing with 0.4 M sucrose for 2 days resulted in a significant induction of dehydration and freezing tolerance. The cryopreservation results showed the highest protocorm regrowth after droplet-vitrification (38%), followed by encapsulation-dehydration (24%) and encapsulation-vitrification (14%). Plantlets developed from these three methods did not show any abnormal characteristics or ploidy level change when investigated by flow cytometry.
Cordova II and Thammasiri (2016) developed the cryo-plate method using silica gel or drying beads for dehydration (Fig. 1.4). Protocorms were placed in the preculture solution consisting of 0.7 M sucrose on a shaker (110 rpm) at 25 + 3 °C for 1 day. After that, protocorms were placed one by one in the wells which filled before with the alginate solution containing 2% (w/v) sodium alginate in calcium-free 1/2 MS basal medium with 0.4 M sucrose. The cryo-plates were hardened for 20 min by slowly dispensing the calcium chloride solution containing 0.1 M calcium chloride in 1/2 MS basal medium with 0.4 M sucrose. Then the cryo-plates were surface dried using sterile filter paper, placed in Petri dishes containing silica gel or drying beads in a laminar air-flow cabinet. Cryo-plates were dehydrated for 5 h until 25% moisture content was achieved. Dehydrated cryo-plates were placed in 2 ml cryotubes and plunged directly into liquid nitrogen for 1 day. Cryo-plates were removed from cryotubes and warmed in unloading solution (1.2 M sucrose solution) for 20 min. Protocorms were then removed from the cryo-plate and placed on 1/2 MS agar medium for regrowth. Growth conditions were conducted using 16 h light at 25 + 3 °C.
Cryo-plate method dehydrated with silica gel or drying beads. (a) Protocorm development. (b) Preculture of protocorms in 1/2 MS liquid medium with 0.7 M sucrose for 1 day. (c) Pour the alginate solution containing 2% (w/v) sodium alginate in calcium-free 1/2 MS basal medium with 0.4 M sucrose in the wells. (d) Place the precultured protocorms in the wells one by one. (e) Pour the calcium chloride solution containing 0.1 M calcium chloride in 1/2 MS basal medium with 0.4 M sucrose. (f) Dehydration with 50 g silica gel. (g) Dehydration with 30 g drying beads. (h) Put each cryo-plate in a 2 ml cryotube. (i) Plunge 2 ml cryotubes into liquid nitrogen for 1 day. (j) Warming in 1.2 M sucrose solution for 20 min. (k) Plate on 1/2 MS agar medium. (l) Regrowth, (m) Regrowth after 60 days
For effect of the cryo-plate method, regrowth of control treatments dehydrated using silica gel was observed to be 90%. Regrowth of control treatments dehydrated using drying beads was observed to be 92.1%. In all other treatments, regrowth was observed to be 73.8% using silica gel for dehydration. Regrowth for all other treatments dehydrated using drying beads was observed to be 76.5%. Regrowth was observed in the second week of transfer to 1/2 MS media. Dehydration using silica gel or drying beads did not significantly affect regrowth rate. Protocorms dehydrated using silica gel or drying beads developed into normal plantlets.
References
Cordova LB II, Thammasiri K (2016) Cryopreservation on a cryo-plate of Arundina graminifolia protocorms, dehydrated with silica gel and drying beads. CryoLetters 37:68–76
Jitsopakul N, Thammasiri K, Ishikawa K (2008) Cryopreservation of Vanda coerulea protocorms by encapsulation-dehydration method. CryoLetters 29:253–260
Kagawa K (2006) Cryopreservation of Dendrobium cruentum Rchb. f. M.Sc. Thesis, Faculty of Graduate Studies, Mahidol University, Thailand
Pornchuti W, Thammasiri K (2008) Cryopreservation of protocorms of Dendrobium cariniferum Rchb. f. Acta Hortic 788:63–68
Sopalun K, Thammasiri K, Ishikawa K (2010a) Micropropagation of the Thai orchid Grammatophyllum speciosum Blume. Plant Cells Tissue Organ Cult 101:143–150
Sopalun K, Thammasiri K, Ishikawa K (2010b) Vitrification-based cryopreservation of Grammatophyllum speciosum protocorms. CryoLetters 31:347–357
Thammasiri K (1999) Cryopreservation of embryonic axes of jackfruit. Cryo-Letters 20:21–28
Thammasiri K (2000) Cryopreservation of seeds of a Thai orchid (Doritis pulcherrima Lindl.) by vitrification. CryoLetters 21:237–244
Thammasiri K (2002) Preservation of seeds of some Thai orchid species by vitrification. In: Proceedings of the 16th World Orchid Conference, pp 248–251
Thammasiri K (2008) Cryopreservation of some Thai orchid species. Acta Hortic 788:53–62
Thammasiri K, Soamkul L (2007) Cryopreservation of Vanda coerulea Griff. Ex Lindl. Seeds by vitrification. ScienceAsia 33:223–227
Acknowledgements
This research project was supported by the Faculty of Science, and the Faculty of Graduate Studies, Mahidol University, The Royal Golden Jubilee Ph.D. Program, The National Research Council and the Biodiversity-Based Economic Development Office (Public Organization).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Thammasiri, K. (2020). Cryopreservation Development of Some Endangered Thai Orchid Species. In: Khasim, S., Hegde, S., González-Arnao, M., Thammasiri, K. (eds) Orchid Biology: Recent Trends & Challenges . Springer, Singapore. https://doi.org/10.1007/978-981-32-9456-1_1
Download citation
DOI: https://doi.org/10.1007/978-981-32-9456-1_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9455-4
Online ISBN: 978-981-32-9456-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)