Abstract
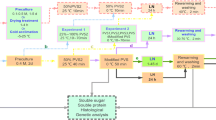
Protocorm-like bodies (PLBs) of Dendrobium candidum Wall. ex Lindl., orchid, were successfully cryopreserved using an encapsulation vitrification method. PLBs were precultured in liquid Murashige and Skoog (MS) medium containing 0.2 mg l−1 α-naphthalene acetic acid and 0.5 mg l−1 6-benzyladenine enriched with 0.75 M sucrose, and grown under continuous light (36 μmol m−2 s−1) at 25 ± 1°C for 5 days. PLBs were osmoprotected with a mixture of 2 M glycerol and 1 M sucrose for 80 min at 25°C and dripped in a 0.5 M CaCl2 solution containing 0.5 M sucrose at 25 ± 1°C and left for 15 min to form Ca-alginate beads (about 4 mm in diameter). Then, these were dehydrated with a plant vitrification solution 2 (PVS2) consisting of 30% (w/v) glycerol, 15% (w/v) ethylene glycol, and 15% (w/v) dimethyl sulfoxide in 0.5 M sucrose, pH 5.8, for 150 min at 0°C. Encapsulated and dehydrated PLBs were plunged directly into liquid nitrogen for 1 h. Cryopreserved PLBs were then rapidly re-warmed in a water bath at 40°C for 3 min and then washed with MS medium containing 1.2 M sucrose for three times at 10 min intervals. Within 60 days, plantlets with the cryopreserved PLBs developed normal shoots and roots, and without any observed morphological abnormalities, were obtained. The survival rate of encapsulated-vitrified PLBs was above 85%. Thus, this encapsulation-vitrification method was deemed promising for cryopreservation of PLBs of D. candidum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Dendrobium (Orchidaceae) is highly evolved and diversified, consisting of more than 1,100 plant species world-wide, and its major distribution regions range from Southeast Asia to New Guinea and Australia (Puchooa 2004; Xu et al. 2006). Dendrobium candidum Wall ex Lindl. is one of the most popular orchids in South and Southeast Asia, and valued for its attractive flowers and medicinal uses (Zhao et al. 2007; Shiau et al. 2005). Dried stems are used in traditional Chinese or folk medicine as Yin tonic to nourish the stomach, promote secretion of body fluids, prevent the development of cataracts, relieve throat inflammation and fatigue, reduce peripheral vascular obstruction, and enhance immunity (Bao et al. 2001). Due to its continuous collection to meet the increasing demands for medicinal and other health products, natural habitats of D. candidum have been largely destroyed and their fauna and flora are under danger of extinction (Yang et al. 2006). Populations of D. candidum are on the decline due to low rates of natural propagation and over-exploitation. Therefore, efforts to preserve and increase the population stands of this species are critical. Traditionally, D. candidum genetic resources are preserved as whole plants either in field repositories or maintained in greenhouses (Wang and Liu 2006). For both means, preserved plants are subject to risk of loss caused by biological and climatic hazards as well as due to human error by mislabeling samples (Vaillant et al. 2005). In addition, traditional maintenance of plant material is highly costly (Wu and Tang 2005).
Cryopreservation offers long-term storage capability, high stability of phenotypic and genotypic characters, minimal storage space and maintenance requirements, and deemed ideal for long-term storage of germplasm (Engelmann 1997). In the last two decades (since 1990), cryopreservation has been widely explored as an alternative for germplasm preservation. Various approaches, such as encapsulation-vitrification have been developed and used with varying degrees of success to preserve diverse species of plants (Xue et al. 2008). Cryopreservation of seeds (Wang et al. 1998), protoplasm (Chen 2000; Chen et al. 2001), protocorms (Wang et al. 1998), and protocorm-like-bodies (PLBs; Bian et al. 2002) of D. candidum by either vitrification or air-drying has been successful. However, these two methods have resulted in both low and slow rates of regrowth of plantlets (Wang et al. 1998; Chen 2000; Chen et al. 2001; Bian et al. 2002). Thus, developing an alternative protocol for long-term preservation of D. candidum is valuable for germplasm conservation, breeding programs, and the orchid floricultural industry.
The encapsulation-vitrification method has been reported as having advantages over traditional cryopreservation methods, and may have wider applications for germplasm storage (Hirai and Sakai 1999); however, it has been mainly used for cryopreservation of shoot-tips and only few PLBs (Engelmann 1997). PLBs of orchids are small organs that readily develop into whole plants. Although there are limited number of studies on cryopreservation of protocorms and PLBs of few orchids (Nikishina et al. 2007; Thammasiri 2000; Na and Kondo 1996; Ishikawa et al. 1997), there are no reports on the use of encapsulation-vitrification of D. candidum. Therefore, this study was initiated to evaluate the use of encapsulation-vitrification of PLBs of D. candidum as a means for efficient storage of this important species of orchids.
Materials and methods
Plant material
Mature capsules of D. candidum cv. Heijie, originating from the Yunnan province (China), were collected from greenhouse-grown plants at the Han Hai Ecological Development Co., Ltd. (Jiangxi province, China). These were washed with tap water, surface-sterilized in 70% ethanol for 30 s, 0.1% (w/v) HgCl2 solution for 30 min, and rinsed thoroughly three times with sterile-distilled water. Sterilized capsules were dried and then dissected longitudinally. Seeds were incubated in 100 ml conical glass flasks containing 40 ml of Murashige and Skoog (MS; Murashige and Skoog 1962) medium with 30 g l−1 sucrose and 7.5 g l−1 agar (Polyicynene, Zhentai Horticultural Facilities Company, Beijing, China). The pH of the medium was adjusted to 5.8 prior to autoclaving at 121°C for 20 min. A total of 5 seeds per flask were used, and 20 flasks were maintained in a controlled environment culture room at 25 ± 1°C and 80% relative humidity, and grown under 16 h photoperiod provided by white-fluorescent tubes (36 μmol m−2 s−1 light intensity). Nodal stem segments, about 0.5 cm in length, were excised from 25 day-old seedlings, approximately 4–6 cm in height, and used as explants to induce PLBs. Explants were placed in flasks containing MS solid medium, 30 g l−1 sucrose, 7.5 g l−1 agar, and supplemented with 5 mg l−1 6-benzyladenine (BA) and 0.5 mg l−1 α-naphthaleneacetic acid (NAA) as described previously by Luo et al. (2008) and Zhao et al. (2008). After 10 days, 62% of stem segments began to developed PLBs. Freshly formed PLBs were transferred to a fresh solidified MS medium but containing 4 g l−1 BA and 0.2 mg l−1 NAA for proliferation (Qin 2008). Following two passages (30 days each), PLBs, 0.2–0.3 cm in diameter, were then excised, and used for cryopreservation by encapsulation-vitrification.
Encapsulation-vitrification
For preculture, 10 PLBs were transferred to 2 ml cryotubes (screw-cap polypropylene ampoules) (MajorBio Technologies Co., Shanghai, China) containing 1.8 ml of liquid MS medium supplemented with 0.5 g l−1 BA and 0.2 mg l−1 NAA, and enriched with increasing concentrations of 0, 0.25, 0.5, 0.75, and 1.0 M sucrose. A total of 129 tubes were used, and these were grown under continuous light (36 μmol m−2 s−1) at 25 ± 1°C for 5 days. At the same time, The effect of preculture time on survival of cryopreserved D. candidum PLBs was also studied: PLBs were suspended in a fresh liquid MS medium containing 0.75 M sucrose, and maintained under continuous light (36 μmol m−2 s−1) at 25 ± 1°C for 0–7 days.
Precultured PLBs were then suspended in a fresh MS medium supplemented with 2% (w/v) sodium alginate solution supplemented with 0.5 M sucrose. Using a sterile pipette, 0.5 M CaCl2 solution containing 0.5 M sucrose was added in drips at 25 ± 1°C for 15 min, according to Hirai and Sakai (1999), to form Ca-alginate beads (about 4 mm in diameter), and each bead contained a single PLB.
The effect of a load solution containing 2 M glycerol and 1 M sucrose on osmotic tolerance of precultured PLBs to PVS2 was investigated to improve the efficiency of this protocol. Encapsulated PLBs were rapidly surface-dried by plating them on a cellulose tissue (Shanghai Regal Biotechnology Company, Shanghai, China), osmoprotected with the loading solution in a 100 ml Erlenmeyer flask, for various durations ranging from 0 to 120 min, with occasional shaking, at 25°C. Then, these were directly dehydrated with a plant vitrification solution 2 (PVS2; Sakai et al. 1990) at either 25°C or 0°C for various time periods (0, 30, 60, 90, 120, 150, 180, 210, and 240 min) prior to their direct immersion in LN for 1 h. The PVS2 solution consisted of 30% (w/v) glycerol, 15% (w/v) ethylene glycol, and 15% (w/v) dimethyl sulfoxide in 0.5 M sucrose (pH 5.8). A ratio of a single bead per 1 ml PVS2 was used. Following dehydration, beads were rapidly surface-dried by blotting them onto cellulose tissue. Then, 10 beads were transferred into 2 ml cryotubes, and immersed directly in LN for 1 h.
Cryotubes were removed from LN, rapidly re-warmed in a water bath at 40°C for 3 min, and then rinsed three times, 10 min per rinse, with MS medium containing 1.2 M sucrose. Thawed and washed beads were incubated in 50 mm Petri dishes containing 15 ml of solid MS medium containing 30 g l−1 sucrose, 7.5 g l−1 agar, and supplemented with 0.5 mg l−1 BA and 0.2 mg l−1 NAA, designated as post-culture medium (Qin 2008). These were maintained under controlled environment conditions, either for 5 day dark period and then transferred to light conditions or under continuous light of 16 h photoperiod with a light intensity of 36 μmol m−2 s−1 provided by white cool fluorescent tubes.
PLB survival and plant regeneration
Cryopreserved PLBs were extracted from alginate beads, and both cryopreserved and control (non-cryopreserved) PLBs were incubated in 60 mm Petri plates containing MS medium, as previously described, supplemented with 0.5 mg l−1 BA and NAA 0.2 mg l−1 (regeneration medium; Qin 2008). Cultures were maintained in darkness for 5 days, and then transferred to light conditions, as described above, at 25 ± 1°C and 75% relative humidity. Developing shoots from all PLBs were then transferred to MS medium, as described above, supplemented with 2 mg l−1 NAA for rooting (rooting medium; Qin 2008). Frequency of PLB survival was determined 40 days post-culture, and based on the total number of PLBs developing normal shoots, 1.5 cm in length, with healthy green color. After 60 days of culture, phenotypic data were recorded for length of plantlets, internode length, number of fully-expanded leaves, and number of roots per plantlet. All regenerated plantlets were transferred to soil, and established under greenhouse conditions. The above protocol was later used to cryopreserve PLBs of D. candidum cv. Ruanjiao.
Statistical analysis
For all experiments, at least 25 PLBs were used per treatment, and three replications per treatment. All experiments were repeated three times. All data were subjected to ANOVA, and means were subjected to the Independent-sample t test. All percent data were transformed to square root values, and then subjected to ANOVA. Duncan’s multiple range test was conducted for mean comparisons of data collected following plantlet growth using P < 0.05.
Results
Effect of preculture time and sucrose concentration in preculture medium on survival
All PLBs that were not precultured did not survive cryopreservation and died (Fig. 1). For those PLBs precultured in liquid MS medium containing 0.2 mg l−1 NAA and 4 g l−1 BAP and 0.75 M sucrose for 1–7 days showed increased frequency of survival. In particular PLBs precultured for 5 days showed the highest frequency of survival (87.6%) following cryopreservation (Fig. 1). Survival of cryopreserved PLBs significantly increased with increasing sucrose concentrations in the preculture medium reaching an optimal level (80.1–90.3%) in the presence of 0.75 M sucrose (Fig. 2). Therefore, a 5-day preculture treatment period combined with 0.75 M sucrose in the preculture medium was subsequently used in all remaining experiments.
Effect of loading solution on survival
Encapsulated PLBs treated with 2 M glycerol and 1 M sucrose prior to dehydration exhibited higher frequency of survival (Fig. 3). Among all loading durations tested, a loading time of 80 min resulted in optimum survival (87.1%) of cryopreserved PLBs.
Effects of dehydration and exposure temperature on survival
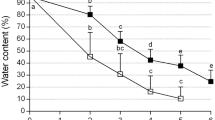
Both duration of dehydration and exposure temperature significantly (P < 0.05) influenced frequency of PLB survival. At 25°C, survival of cryopreserved PLBs increased rapidly with increasing dehydration periods. The highest frequency of survival (76.2%) was achieved following 120 min of dehydration (Fig. 4A). At 0°C, frequency of survival of cryopreserved PLBs gradually increased with increasing dehydration periods reaching maximal (89.4%) level at 150 min of dehydration (Fig. 4B).
Effects of culture conditions
When PLBs were cultured under continuous light conditions, frequency of survival was lower than those PLBs subjected to 5 day dark treatment prior to transfer to light conditions (Fig. 5). Consequently, 5 day dark treatment was used in subsequent experiments.
Phenotypic evaluation of regenerated plants derived from PLBs with and without encapsulation-vitrification treatment
Regrowth of control PLBs (without cryopreservation) initiated earlier (by 4 days) than those that of cryopreserved PLBs. Moreover, buds of plantlets derived from control PLBs grew after 7 days. Whereas, treated PLBs began to grow after 9 days, and buds grew after 13 days following transfer to regeneration medium. Therefore, a 5-day lag phase was observed in re-growth of cryopreserved PLBs. All control and treated plants grown for a period of 60 days were evaluated for various phenotypic characteristics. All phenotypic parameters measured, including length of plantlets, internode lengths, number of leaves, and number of roots were not significantly different between the two groups of plants (Fig. 6; P > 0.05).
Discussion
Vitrification refers to the physical process by which a highly concentrated aqueous solution solidifies into a glassy solid at sufficiently low temperatures without crystallization (Hong et al. 2009). Thus, it is essential to enhance the dehydration tolerance of plant tissues to the vitrification solution. Sucrose concentrations of 0.75–1.0 M have been widely reported to be most frequently used for cryopreservation techniques (Wang et al. 2002a). It has been reported that survival of cryopreserved cells of Medicago sativa precultured in 0.75 M sucrose was almost fivefold higher than that of those precultured in 0.25 M (Shibli et al. 2001). Therefore, preculture of plant tissues is an important step for the survival of cryopreserved cells, which may be related to the improvement of freezing tolerance. In this study, preculture of PLBs of orchid in 0.75 M sucrose was also found to be optimum for survival.
It has long been reported that preculture duration also influenced survival of cryopreserved plant tissues (Bouafia et al. 1996; Ishikawa et al. 1997). When potato shoot-tips were precultured in the presence of 1 M sucrose over 1–7 days, a preculture period of 2 days promoted the highest survival of cryopreserved shoot-tips (Bouafia et al. 1996). In this study, survival of cryopreserved PLBs increased to 87.6% when these were precultured in the presence of 0.75 M sucrose for 5 days. Previously, zygotic embryos of a Japanese terrestrial orchid (Bletilla striata) were precultured with 0.3 M sucrose for 3 days, and 50–60% of these embryos withstood preculture and developed into normal plantlets (Ishikawa et al. 1997). When protocorms of D. virgineum Rchb.f. were precultured in a semi-liquid New Dogashima (ND) medium with 0.25 M sucrose in darkness for 1 week and then transferred to a liquid medium containing 0.75 M sucrose for 2 days, a 49% survival frequency was observed (Maneerattanarungroj et al. 2007). However, when protocorms of D. virgineum were precultured in a modified Vacin and Went (1949) (VW) liquid medium supplemented with 0.3 M sucrose for 3 days, the survival rate of cryopreserved protocorms was only about 15% (Pornchuti and Thammasiri 2008). Similarly, low re-growth (13.33%) was observed from encapsulated shoot-tips of D. Walter Oumae that were previously precultured on 0.3 M sucrose agar medium for 2 days (Lurswijidjarus and Thammasiri 2004). All these findings suggested that different orchid species exhibited varying levels of tolerance to high sucrose concentrations.
For cryopreservation protocols involving vitrification, direct exposure to the vitrification solution is harmful due to either osmotic stress or chemical toxicity. However, osmotolerance is rarely achieved by preculture with sucrose alone. Therefore, a loading treatment with a load solution containing various amounts of sucrose and glycerol is commonly used (Xue et al. 2008). Although the precise mechanism by which the loading treatment induces osmotolerance to the vitrification solution remains unknown, the loading solution may minimize osmotic stress caused by severe dehydration (Jitsuyama et al. 1997). Following incubation in a load solution containing 2 M glycerol and 0.4 M sucrose for 15 min at 25°C, regeneration frequency of zygotic embryos of the Japanese terrestrial orchid, B. striata, amounted to about 60% (Ishikawa et al. 1997). In this study, a loading treatment of a mixture of 2 M glycerol and 1 M sucrose enhanced survival and regeneration of D. candidum PLBs. Moreover, the highest frequency of survival of cryopreserved PLBs was obtained when these were treated for 80 min with a loading solution prior to dehydration. This finding is in accordance with results of cryopreservation by encapsulation-vitrification of protocorms of D. cariniferum Rchb. f. (Pornchuti and Thammasiri 2008).
Incubation period and temperature of the vitrification solution are two important factors affecting survival of cryopreserved plant tissues (Hong et al. 2009). Over-exposure of plant tissues to the vitrification solution such as PVS2 may lead to chemical toxicity and excessive osmotic stress (Hong et al. 2009). The optimal exposure time for PVS2 varies with plant species and depends on the temperature during exposure (Hong et al. 2009). For example, it has been reported that the optimal exposure time to PVS2 at 25 ± 2°C for a Thai orchid (Doritis pulcherrima Lindl.) seeds is 50 min (Thammasiri 2000); while, that for D. cariniferum is 60 min (Pornchuti and Thammasiri 2008). Moreover, dehydration at 0°C also yields higher survival, and the incubation time is also largely extended, thus allowing for greater flexibility in handling large numbers of samples at the same time (Wang et al. 2002b). In this study, the frequency of survival of cryopreserved PLBs treated with PVS2 increased from 76.2% following dehydration at 25°C for 120 min to 89.4% following dehydration at 0°C for 150 min. These findings are similar to those results reported by Nishizawa et al. (1993). Whereas, zygotic embryos of the Japanese terrestrial orchid dehydrated with PVS2 solution for 3 h at 0°C have exhibited lower frequency of survival (about 60%; Ishikawa et al. 1997). Interestingly, protocorms of D. cariniferum treated with PVS2 and subjected to cryopreservation by encapsulation-vitrification exhibited even lower survival frequency (15%; Thammasiri 2008). Therefore, it is clear that responses of different orchid species to cryopreservation by encapsulation-vitrification are different and this could be attributed to genotypic differences as well as differences in incubation periods and temperatures of the vitrification solution.
In this study, regrowth of control PLBs was initiated earlier and proceeded faster than that of cryopreserved PLBs. This suggested that there was a 5-day lag phase in regrowth of cryopreserved PLBs. These findings were consistent with those reported for Vitis vinifera L. cv. Red Globe (Wang et al. 2002b).
Culture conditions following cryopreservation also influenced regeneration of plantlets from D. candidum PLBs. In particular, dark incubation for a short time following post-thawing enhanced survival. Similar observations have been noted with dormant shoot-tips of persimmon (Ai and Luo 2003) and apple buds (Liu and Wang 2002) subjected to cryopreservation, and this was presumably attributed to damage repair of tissues that might take place during darkness.
In this study, a simple and efficient method for cryopreservation of PLBs of D. candidum has been developed. This was also successfully used in cryopreservation of another genotype of D. candidum cv. Ruanjiao wherein an 83% regeneration frequency was obtained. To the best of our knowledge, this is the first report of successful cryopreservation of D. candidum PLBs by encapsulation-vitrification. With the optimized parameters, an 85–90% survival of cryopreserved PLBs was achieved. Moreover, plants regenerated from cryopreserved PLBs exhibited normal phenotypes and morphology to control plants.
Abbreviations
- MS:
-
Tissue culture medium from Murashige and Skoog (1962)
- NAA:
-
α-Naphthalene acetic acid
- BA:
-
6-Benzyladenine
- PLBs:
-
Protocorm-like bodies
- LN:
-
Liquid nitrogen
- PVS2 :
-
Plant vitrification solution 2
References
Ai PF, Luo ZR (2003) Cryopreservation of dormant shoot-tips of persimmon by vitrification and plant regeneration. Sci Agric Sin 36:553–556
Bao XS, Shun QS, Chen LZ (2001) The medicinal plants of Dendrobium (Shi-Hu) in China, a coloured atlas. Fudan University Press, Shanghai
Bian HW, Wang JH, Lin WQ, Han N, Zhu MY (2002) Accumulation of soluble sugars, heat-stable proteins and dehydrins in cryopreservation of protocorm-like bodies of Dendrobium candidum by the air-drying method. J Plant Physiol 159:1139–1145. doi:10.1078/0176-1617-00824
Bouafia S, Jelti N, Lairy G, Blanc A, Bonnel E, Dereuddre J (1996) Cryopreserveation of potato shoot tips by encapsulation-dehydration. Potato Res 39:69–78. doi:10.1007/BF02358208
Chen Y (2000) Protoplasm of Dendrobium candidum cryopreserved by vitrification. J Wenzhou Teachers Coll (Nat Sci) 21:40–41
Chen Y, Wan JH, Huan CN (2001) Germplasm cryopreservation of Dendrobium candidum by vitrification. J Zhejiang Univ (Agric & Life Sci) 27:436–438
Engelmann F (1997) In vitro conservation methods. In: Callow CA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources. CAB International, Oxford, pp 119–161
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of mint (Mentha spicata L.) by encapsulation vitrification. Plant Cell Rep 19:150–155. doi:10.1007/s002990050725
Hong SR, Yin MH, Shao XH, Wang AP, Xu WH (2009) Cryopreservation of embryogenic callus of Dioscorea bulbifera by vitrification. Cryo Lett 30:64–75
Ishikawa K, Harata K, Mii M, Sakai A, Yoshimatsu K, Shimomura K (1997) Cryopreservation of zygotic embryos of a Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep 16:754–757. doi:10.1007/s002990050314
Jitsuyama Y, Suzuki T, Harada T, Fujikawa S (1997) Ultrastructural study on mechanism of increased freezing tolerance due to extracelluar glucose in cabbage leaf cells. Cryo Lett 18:33–44
Liu YG, Wang XY (2002) Study of cryopreservation technique of apple germplasm by vitrification. J Shandong Agric Univ (Nat Sci) 33:32–36
Luo JP, Wang Y, Zha XQ, Huang L (2008) Micropropagation of Dendrobium densiflorum Lindl. ex Wall. through protocorm-like bodies: effects of plant growth regulators and lanthanoids. Plant Cell Tissue Organ Cult 93:333–340. doi:10.1007/s11240-008-9381-1
Lurswijidjarus W, Thammasiri K (2004) Cryopreservation of shoot tips of Dendrobium Walter Oumae by encapsulation/dehydration. Sci Asia 30:293–299. doi:10.2306/scienceasia1513-1874.2004.30.293
Maneerattanarungroj P, Bunnag S, Monthatong M (2007) In vitro conservation of Cleisostoma areitinum (Rchb.f.) Garay, rare Thai orchid species by an encapsulation-dehydration method. Asian J Plant Sci 6:1235–1240. doi:10.3923/ajps.2007.1235.1240
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479. doi:10.1111/j.1399-3054.1962.tb08052.x
Na HY, Kondo K (1996) Cryopreservation of tissue cultured shoot primordia from shoot apices of cultured protocorms in Vanda pumila following ABA preculture and desiccation. Plant Sci 118:195–201. doi:10.1016/0168-9452(96)04438-X
Nikishina TV, Popova EV, Vakhrameeva MG, Varlygina TI, Kolomeitseva GL, Burov AV, Popovich EA, Shirokov AI, Shumilov VY, Popov AS (2007) Cryopreservation of seeds and protocorms of rare temperate orchids. Russ J Plant Physiol 54:121–127. doi:10.1134/S1021443707010189
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73. doi:10.1016/0168-9452(93)90189-7
Pornchuti W, Thammasiri K (2008) Cryopreservation of protocorms of Dendrobium virgineum Rchb.f. Acta Hortic 788:63–68
Puchooa D (2004) Comparison of different culture media for the in vitro culture of Dendrobium (Orchidaceae). Int J Agric Biol 6:884–888
Qin TH (2008) Rapid propagation of Dendrobium candidum Wall. ex Lindl. in vitro. Chin J Trop Agric 28:25–29
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33. doi:10.1007/BF00232130
Shiau Y-J, Nalawade SM, Hsia C-N, Mulabagal V, Tsay H-S (2005) In vitro propagation of the Chinese medicinal plant, Dendrobium candidum wall. ex lindl., from axenic nodal segments. In Vitro Cell Dev Biol Plant 41:666–670. doi:10.1079/IVP2005685
Shibli RA, Haagenson DM, Cunningham SM, Berg WK, Volenec JJ (2001) Cryopreservation of alfalfa (Medicago sativa L.) cells by encapsulation-dehydration. Plant Cell Rep 20:445–450. doi:10.1007/s002990100347
Thammasiri K (2000) Cryopreservation of seeds of a Thai orchid by vitrification. Cryo Lett 21:237–244
Thammasiri K (2008) Cryopreservation of some Thai orchid species. Acta Hortic 788:53–62
Vacin EF, Went FW (1949) Some pH changes in nutrient solutions. Bot Gaz 110:605–613
Vaillant V, Bade P, Constant C (2005) Photoperiod affects the growth and development of yam plantlets obtained by in vitro propagation. Biol Plant 49:355–359. doi:10.1007/s10535-005-0007-8
Wang Y, Liu Y (2006) Cryopreservation of ornamental plant germplasm. Plant Physiol Commun 42:559–566
Wang JH, Ge JG, Liu F, Bian HW, Huang CN (1998) Cryopreservation of seeds and protocorms of Dendrobium candidum. Cryo Lett 19:123–128
Wang QC, Batuman O, Li P, Bar-Joseph M, Gafny R (2002a) Cryopreservation of in vitro-grown shoot tips of ‘Troyer’ citrange [Poncirus trifoliata Raf. × Citrus sinensis (L.) Osbeck.] by encapsulation-dehydration. Plant Cell Rep 20:901–906. doi:10.1007/s00299-001-0425-9
Wang QC, Gafny R, Sahar N, Sela I, Mawassi M, Tanne E, Perl A (2002b) Cryopreservation of grapevine (Vitis vinifera L.) embryogenic cell suspensions and subsequent plant regeneration by encapsulation-dehydration. Plant Sci 162:551–558. doi:10.1016/S0168-9452(01)00594-5
Wu XM, Tang HR (2005) Research advances in cryopreservation of plant germplasm by encapsulation-vitrification method. Chin Bull Bot 22:238–245
Xu H, Wang ZT, Ding XY, Zhou KY, Xu LS (2006) Differentiation of Dendrobium species used as ‘‘Huangcao Shihu’’ by rDNA ITS sequence analysis. Planta Med 72:89–92. doi:10.1055/s-2005-916228
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Organ Cult 92:251–260. doi:10.1007/s11240-007-9329-x
Yang L, Wang ZT, Xu LS (2006) Simultaneous determination of phenols (bibenzyl, phenanthrene, and fluorenone) in Dendrobium species by high-performance liquid chromatography with diode array detection. J Chromatogr A 1104:230–237. doi:10.1016/j.chroma.2005.12.012
Zhao P, Wang W, Feng FS, Wu F, Yang ZQ, Wang WJ (2007) High-frequency shoot regeneration through transverse thin cell layer culture in Dendrobium candidum Wall Ex Lindl. Plant Cell Tissue Organ Cult 90:131–139. doi:10.1007/s11240-006-9181-4
Zhao P, Wu F, Feng FS, Wang WJ (2008) Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum Wall ex Lindl. In Vitro Cell Dev Biol Plant 44:178–185. doi:10.1007/s11627-007-9101-2
Acknowledgments
This study was supported by Science and Technology Foundation of the Education Department of Jiangxi province (No. GJJ09617) and key scientific and technological plan of Shangrao Normal College in 2009–2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, M., Hong, S. Cryopreservation of Dendrobium candidum Wall. ex Lindl. protocorm-like bodies by encapsulation-vitrification. Plant Cell Tiss Organ Cult 98, 179–185 (2009). https://doi.org/10.1007/s11240-009-9550-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9550-x