Abstract
Protoplasts were isolated from hypocotyls of 5-day-old seedlings of five doubled-haploid (DH) lines of cauliflower after enzymatic digestion in 0.1% macerozyme R-10 and 1.0% cellulose R-10. Shoot regeneration was achieved in four of the five DH lines. Protoplast yield and frequency of cell division and shoot regeneration varied among experiments and DH lines. Sequence-related amplified polymorphism (SRAP) analysis on all the regenerated plants of each DH line indicated that their genetic compositions were homologous with their mother plants, but few regenerated plants of DH lines no. 3 and 5 were detected with changes in ploidy levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassica oleracea is an extremely diverse species which includes several important vegetable crops including broccoli, brussels sprouts, cauliflower, various types of cabbage, kohlrabi and kale (Hansen et al. 1999). Worldwide cauliflower production is estimated at 5.3 million tonnes making it an economically important vegetable (Hodgkin 1995). Traditional breeding of this crop has led to significant improvements in crop quality and productivity. However, plant breeding programmes are limited in terms of availability of suitable genes in the germplasm base. The application of new technologies, such as somatic hybridization and genetic transformation, to cauliflower varieties would pave the way for the generation of new cultivars possessing more agriculturally and economically viable genetic traits. An efficient plant regeneration system from protoplast has proved a very useful technique for crop genetic improvement programs, a prerequisite for somatic hybridization and genetic transformation by direct DNA uptake.
Techniques for protoplast isolation and culture in Brassica oleracea var. botrytis have been reported (Vatsya and Bhaskaran 1982; Jourdan et al. 1990; Walters and Earle 1990; Veera et al. 2009) and somatic hybridizations between cauliflower and other species have also been studied but only for a few genotypes of minor or no commercial importance (Jourdan et al. 1989; Yarrow et al. 1990; Walters et al. 1992; Navrátilová et al. 1997; Sheng et al. 2008; Scholze et al. 2010). Moreover, these investigators used mainly F1 cultivars and such material is genetically uniform, but it is often difficult to analyze the progeny owing to heterozygosity in the background. An alternative is doubled haploid (DH) lines produced by microspore culture, which are also uniform and show complete homozygosity in their background (Tsukazaki et al. 2002). Therefore, the improved DH lines can be directly used as parents for the production of F1 hybrids. For breeding, it is more necessary and significative to regenerate plants from different genotypes of DH lines.
The objectives of this study were to analyze protoplast yields derived from in vitro-grown hypocotyls of five DH lines of cauliflower with high breeding value, establish conditions for protoplast culture and shoot regeneration, and determine genetic fidelity of regenerated plants by SRAP analysis.
Materials and methods
Plant material
For protoplast isolation, a total of five DH lines of cauliflower (DH1-5) produced by microspore culture in our previous study (Gu et al. 2007) were selected, which have desirable attributes but need to be further improved.
Isolation and purification of protoplasts
Protoplasts were aseptically isolated from hypocotyls of 5-day-old seedlings grown in the dark at 25°C on hormone free Murashige and Skoog basal medium (MS medium, Murashige and Skoog 1962). Hypocotyls (500 mg) were cut into small segments and incubated for 2 h with 5 ml of a preplasmolytic solution (54.6 g/l Sorbitol, 7.4 g/l CaCl2·2H2O, pH 5.6–5.8) followed by incubation in 5 ml of a cell wall degrading enzyme solution (Table 1) containing 0.1% macerozyme R-10 and 1.0% cellulose R-10. Following overnight incubation (15 h) at 24°C in the dark, the solution of protoplast-enzyme mixtures was filtered through a 200 μm nylon mesh into a centrifuge tube. The filtrate was then over-layered with 2.5 ml washing solution (18.4 g/l CaCl2·2H2O, 9.0 g/l NaCl, 1.0 g/l Glucose, 0.3 g/l KCl, pH 5.6–5.8). Following centrifugation (1,200 rpm for 10 min), intact protoplasts, floating at the interface of the two solutions, were collected and washed twice by re-suspending in the washing solution (same solution as described above), and then centrifuged for 4 min at 800 rpm. Protoplast viability was measured with fluorescein diacetate (Widholm 1972).
Protoplast culture
Culture in liquid medium
Isolated protoplasts were cultured at a density of 5 × 105 protoplasts ml−1 in liquid medium 1/2 (Table 1) containing 0.2 mg l−1 naphthaleneacetic acid (NAA), 0.2 mg l−1 benzyladenine (BA) and 0.5 mg l−1 2, 4-Dichlorophenoxyacetic acid (2, 4-D) in a total volume of 2 ml in 3 cm Petri dishes without agitation. The plates were sealed with parafilm and cultured in darkness at 25°C. When normal cell divisions were observed, 400 μl of 2/2 dilution medium (Table 1) was added to each dish every 3 days. Suspensions with colonies consisting of more than 20 cells were transferred to solidified 3/10 medium (Table 1) for callus growth under dim lighting (2.99 lmol m−2 s−1). Calli that reached a size of 2–3 mm were transferred to shoot inducing medium and subcultured once every 2 weeks.
Nurse culture
Protoplasts of tuber mustard were employed as the nurse cells in the present study, which had been reported with positive effect in the protoplasts culture of Brassica species (Chen et al. 2004). Purified protoplasts of cauliflower were resuspended in 1/2 medium at a density of 2 × 105 ml−1 and 1 ml of this mixture was quickly mixed with an equal volume of the 1/2 agarose medium (1/2 medium with 2 g/l agarose) to give evenly dispersed protoplasts. Then, 2 ml liquid medium (MS medium contained 0.5 mg l−1 BA, 1 mg l−1 2,4-D, 0.5mgl−1 NAA, 0.4 M mannitol) with protoplasts of tuber mustard at a density of 1 × 105 ml−1 was added onto the agarose medium containing cauliflower protoplasts. After 2 weeks of culture in darkness at 25°C, the liquid medium was removed and the plates were transferred under dim lighting (2.99 lmol m−2 s−1) for callus growth.
Shoot regeneration
Calli were transferred to shoot regeneration medium when they reached 2–3 mm in size. For shoot regeneration, various media (based on MS-medium, with 30 g l−1 sucrose and 8.0 g l−1 plant agar) including different plant growth regulator (PGR) combinations and concentrations were evaluated. Calli were cultured at 24°C in the light (16 h photoperiod, 25–30 μmol m−2 s−1). Shoot regeneration was evaluated after 10 weeks of culture by recording the number of calli with shoots.
Acclimatization
Regenerated plants from each DH line were transferred to the greenhouse to monitor their growth. Plants were transferred to soil (peat substrate) at 22–24°C and kept at 100% humidity for 2 weeks. After 4–6 weeks, they were transferred to 9 cm pots, and grown under normal greenhouse conditions.
Flow cytometry
Healthy young leaves, 0.5 cm2, were chopped with a razor blade in 0.6 ml ice-cold buffer (100 ml deionized water, 0.1 mol/l citric acid, 0.5 g Tween 20) and 2.4 ml propidium iodide (PI) solution was then added. The suspension was filtered through a mesh, and after 5 min, these were analyzed using a BD flow cytometer (FACSCalibur, BD Biosciences, USA). All regenerated plants were tested for ploidy changes by flow cytometry with three replications.
Regenerated plants analyzed by PCR-SRAP
Total genomic DNA was isolated from leaf tissue collected in bulk from regenerated plants as described using the cetyltrimethylammonium bromide (CTAB) method (Paterson et al. 1993). DNA concentration was quantified by visual comparison to λDNA standards on ethidium bromide-stained agarose gels.
Among the 50 pairs of primers tested, 10 primer combinations (Table 2) showed clear and reproducible bands were selected for amplification of the template DNA to detect variation. PCR amplification was performed following Sheng et al. (2008). The PCR products were electrophoresed in 6% polyacrylamide denatured gels, ran at 100 W for 2 h, and the banding patterns were visualized using silver staining as described by Panaud et al. (1996). The gel was photographed after being dried at room temperature.
Results
Protoplast isolation and culture
Protoplasts were successfully isolated from hypocotyls of all five DH lines of cauliflower. Protoplast yield varied between 0.3–5.2 × 106 protoplasts per gram fresh weight in the presence of different enzyme combinations (Table 3). The protoplast yield significantly increased with increasing enzyme concentration, but protoplast viability was significantly diminished when beyond ER2. Therefore, the enzyme combinations ER2 was the optimum for protoplast production with the highest protoplast yield and viability.
Effect of genotype and culture system on cell division
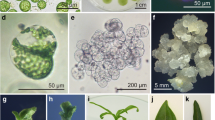
Five DH lines were compared to evaluate efficiency of protoplast culture under two culture conditions, liquid culture and nurse culture. For all genotypes, protoplast-derived cells began to divide after 3–4 days. Overall, significant differences between the two culture systems were observed in the five DH lines and nurse culture resulted in much higher frequency of cell division after 6 days of culture (Fig. 1), which is 5–63% in liquid medium but 32–92% for them employed by nurse culture. DH line 5 showed the highest frequency of cell division (92%) in nurse culture. However, DH line 1 cultured in liquid medium showed the lowest frequency of cell division (5%) but in nurse culture, the frequency improved to be 32%. In general, frequency of cell division for all five DH lines varied not only between genotypes, but also between different culture systems of protoplasts of the same genotype.
Effects of PGRs on shoot regeneration of different DH lines
Nearly all protoplast-derived cells, that underwent first divisions, developed further into micro-colonies of 2–3 mm in diameter 5–7 weeks following isolation. These were then transferred to differentiation media for shoot induction (Fig. 2). Significant differences in frequencies of shoot regeneration were observed among the different genotypes and PGR combinations and concentrations (Fig. 3). Shoot regeneration was achieved for four DH lines (except DH2). In the PGR combinations of 6-BA+ IAA and ZT +IAA, ZT was more effective for shoot induction in cauliflower (Fig. 3). The highest frequency of shoot regeneration (83% for DH line 3) was obtained on medium containing 1.0 mg l−1 ZT and 0.2 mg l−1 IAA (Figs. 3, 4) and this PGR combination also resulted in the highest frequency of shoot regeneration for DH line 1 and 5 (61 and 49%, respectively). For DH line 4, shoots could only regenerated on the medium containing 1.0 mg l−1 ZT, but the frequency was low (<10%).
Morphological, ploidy level and SRAP analysis
A total of 162 plants from different colonies originating from protoplasts of DH lines 1, 3, 4, 5 were successfully transferred to the greenhouse (Table 4). For DH lines 1 and 4, the ploidy levels of the regenerated plants were all diploid, which were consistent with their mother plants. However, two regenerated plants of DH line 3 and three plants originating from protoplasts of DH line 5 showed higher ploidy levels (Table 4; Fig. 5), which were generally stunted with shrunken leaves.
Histograms of nuclear DNA contents obtained by flow cytometric analysis. a DH5 with diploid level; b a regenerated plant of DH5 with tetraploid level; c a regenerated plant of DH5 with hexaploid level. Ordinate: Number of nuclei; Abscissa: PI fluorescence. Fluorescence intensity is proportional to nuclear DNA quantity and the position of the dominant peak (arrow indication) reflects the ploidy level
SRAP analysis was produced on all the regenerated plants of each DH line. Among the ten pairs of primers selected, each primer combination generated a unique set of amplification products ranging in size from 100 to 1,000 bp. The band patterns of the regenerated plants from protoplasts of each DH line were homologous with their mother plants, which indicated their genetic homogeneity. Figure 6 showed two representative amplified band patterns produced by primer combinations of Me1 + Em13 and Me9 + Em8.
Discussion
Although plantlets were successfully regenerated from protoplasts of cauliflower in previous studies (Vatsya and Bhaskaran 1982; Jourdan et al. 1990; Walters and Earle 1990; Veera et al. 2009), their protocols varied greatly in either culture parameters or genotypes and mostly used leaves as explant material with low frequencies of shoot regeneration. In the present investigation, a new nurse culture method using protoplasts of tuber mustard as nurse cells was employed in the culture of cauliflower protoplasts for the first time and the highest frequency of cell division (92%) was obtained using this method. Moreover, plant regeneration from hypocotyls protoplasts of different DH lines of cauliflower was reproducibly achieved and the frequency of shoot regeneration is higher than previously reported for cauliflower, which gave a maximum regeneration rate of 72% (Veera et al. 2009).
Plant regeneration from protoplasts is influenced by many factors, including the methods of protoplast isolation, inoculation density, culture system, nurse cells, and medium compositions (Meyer et al. 2009; Eeckhaut et al. 2009; Castelblanque et al. 2010; Prange et al. 2010; Tiwari et al. 2010). The use of feeder layer-culture systems with protoplasts of other species as nurse cells provided a simple and reliable technique for enhancing cell division and plating efficiency of isolated protoplasts (Walters and Earle 1990; Karamian and Ebrahimzadeh 2001). Chen et al. (2004) reported the use of tuber mustard protoplasts as nurse cells, which was essential for sustained division and colony formation from red cabbage protoplasts. In the present study, nurse cells of tuber mustard protoplasts also had an enhancing effect on cauliflower protoplast division. By nurse culture, all five DH lines showed much higher cell division frequency compared to liquid culture, probably as a result of the release of growth factors by nurse cells into the surrounding medium, which stimulated the cell division of cauliflower. A similar stimulatory effect of nurse culture on cell division and plant regeneration from protoplasts has been reported for rice (Kyozuka et al. 1987; Li and Murai 1990) and maize (Petersen et al. 1992).
It was evident that there was no correlation between division efficiency and subsequent shoot differentiation in the present study (Figs. 1, 3). A similar phenomenon had been observed in B. oleracea (Robertson et al. 1988) and Petunia (Meyer et al. 2009). These results indicated that cell division and shoot differentiation are controlled by different developmental mechanisms.
The ploidy of protoplast-derived plants from each DH line were analyzed by flow cytometry, a well established and reliable method that is less time consuming than chromosome counting (Ulrich and Ulrich 1991; Mallón et al. 2010; Stancheva et al. 2011; Yang et al. 2010; Zhang et al. 2010). Changes in ploidy level were just observed in the regenerated plants of DH lines 3, 5 and the frequency were 3.5 and 2.8% respectively, which were much lower than the a previous study on protoplast culture of cauliflower with 15–25% tetraploidy in the regenerated plants (Hansen and Earle 1994). This may be attributed to the application of nurse culture, which inhibited the spontaneous fusion of diploid protoplasts.
SRAP is a new but very powerful molecular marker system, which was first reported by Li and Quiros (2001). Budak et al. (2004) compared the four marker systems in buffalograss and found the values of average discriminating power as: SRAP > SSR > ISSR > RAPD. Ferriol et al. (2003) reported that SRAP could provide more information and was more concordant with the genomic variations and the evolutionary history of the morphotypes than AFLP. In this study, SRAP amplification gave rise to a lot of polymorphic bands, leading to desirable discrimination ability. SRAP analysis indicated that plants regeneration from protoplast of cauliflower was homogeneous, although the morphology of few regenerated plantlets varied from mother plants. Variation of the morphology may be due to variation of the ploidy levels and the changing of the culture environments.
In summary, this study has demonstrated that protoplast isolation and culture up to the microcallus stage can be achieved for all the DH lines of cauliflower with breeding importance. At present, shoot regeneration is somewhat limiting, although this can be achieved for most DH lines used in this study. The protoplast regeneration system described herein will enable the pursuit of somatic hybridization and transformation by direct DNA uptake, which may result in the development and production of novel and popular cauliflower varieties.
Abbreviations
- BA:
-
6-Benzyladenine
- IAA:
-
Indole-3-acetic acid
- NAA:
-
Naphthaleneacetic acid
- 2, 4-D:
-
2, 4-Dichlorophenoxyacetic acid
- ZT:
-
Zeatin
- FW:
-
Fresh weight
References
Budak H, Shearman RC, Parmaksiz I, Dweikat I (2004) Comparative analysis of seeded and vegetative biotype buffalograsses based on phylogenetic relationship using ISSRs, SSRs, RAPDs, and SRAPs. Theor Appl Genet 109:280–288
Castelblanque L, García-Sogo B, Pineda B, Moreno V (2010) Efficient plant regeneration from protoplasts of Kalanchoe blossfeldiana via organogenesis. Plant Cell Tissue Organ Cult 100:107–112
Chen LP, Zhang MF, Xiao QB, Wu JG, Hirata Y (2004) Plant regeneration from hypocotyl protoplasts of red cabbage (Brassica oleracea) by using nurse cultures. Plant Cell Tissue Organ Cult 77:133–138
Eeckhaut T, Duquenne B, Laksmanan P, Huylenbroeck JV (2009) Development of microcolonies in protoplast culture of Spathiphyllum wallisii. Acta Hort 829:51–54
Ferriol M, Pico B, Nuez F (2003) Genetic diversity of a germplasm collection of Cucurbita pepo using SRAP and AFLP markers. Theor Appl Genet 107:271–282
Gu HH, Zhu DH, Yang JF, Rao LB, Zhang XH (2007) Obtaining doubled haploid plants of loose-curd cauliflowers (Brassica oleracea var. botrytis) by microspore culture. J Agric Biotech 15(2):301–305 (in Chinese with English abstract)
Hansen LN, Earle ED (1994) Regeneration of plants from protoplasts of rapid cycling Brassica oleracea L. Plant Cell Rep 13:335–339
Hansen LN, Ortiz R, Andersen SB (1999) Genetic analysis of protoplast regeneration ability in Brassica oleracea. Plant Cell Tissue Organ Cult 58:127–132
Hodgkin T (1995) Cabbages, kales etc. Brassica oleracea (Cruciferae). In: Smartt J, Simmonds NW (eds) Evolution of crop plants. Longman, London, pp 76–82
Jourdan PS, Earle ED, Mutschler MA (1989) Atrazine-resistant cauliflower obtained by somatic hybridization between Brassica oleracea and ATR-B. napus. Theor Appl Genet 78(2):271–279
Jourdan PS, Earle ED, Mutschler MA (1990) Improved protoplast culture and stability of cytoplasmic traits in plants regenerated from leaf protoplasts of cauliflower (Brassica oleracea ssp. botrytis). Plant Cell Tissue Organ Cult 21:227–236
Karamian R, Ebrahimzadeh H (2001) Plantlet regeneration from protoplast-derived embryogenic calli of Crocus cancellatus. Plant Cell Tissue Organ Cult 65:115–121
Kyozuka J, Hayashi Y, Shimamoto K (1987) High frequency plant regeneration from rice protoplasts by novel culture methods. Mol Gen Genet 206:408–412
Li ZJ, Murai N (1990) Efficient plant regeneration from rice protoplasts in general medium. Plant Cell Rep 9(4):216–220
Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 103:455–461
Mallón R, Oubińa JR, González ML (2010) In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flow cytometry and RAPD analysis. Plant Cell Tissue Organ Cult 101(1):31–39
Meyer L, Serek M, Winkelmann T (2009) Protoplast isolation and plant regeneration of different genotypes of Petunia and Calibrachoa. Plant Cell Tissue Organ Cult 99:27–34
Murashige T, Skoog F (1962) A revised media for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–479
Navrátilová B, Buzek J, Siroky J, Havranek P (1997) Construction of intergeneric somatic hybrids between Brassica oleracea and Armoracia rusticana. Biol Plantarum 39(4):531–541
Panaud O, Chen X, Mcouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Paterson AH, Curt LB, Wendel JF (1993) A rapid method for extraction of cotton (Gossypium spp.) genomic DNA for RFLP and PCR analysis. Plant Mol Biol Rep 11:112–127
Petersen WL, Sule S, Armstrong CL (1992) Effect of nurse cultures on the production of macro-calli and fertile plants from maize embryogenic suspension culture protoplasts. Plant Cell Rep 10(12):591–594
Prange ANS, Serek M, Bartsch M, Winkelmann T (2010) Efficient and stable regeneration from protoplasts of Cyclamen coum Miller via somatic embryogenesis. Plant Cell Tissue Organ Cult 101(2):171–182
Robertson D, Earle E, Mutschler MA (1988) Increased totipotency of protoplasts from Brassica oleracea plants previously regenerated in tissue culture. Plant Cell Tissue Organ Cult 14:15–24
Scholze P, Krämer R, Ryschka U, Klocke E, Schumann G (2010) Somatic hybrids of vegetable brassicas as source for new resistances to fungal and virus diseases. Euphytica 176:1–14
Sheng XG, Liu F, Zhu YL, Zhao H, Zhang L, Chen B (2008) Production and analysis of intergeneric somatic hybrids between Brassica oleracea and Matthiola incana. Plant Cell Tissue Organ Cult 92:55–62
Stancheva N, Weber J, Schulze J, Alipieva K, Ludwig-Müller J, Haas C, Georgiev V, Bley T, Georgiev M (2011) Phytochemical and flow cytometric analyses of Devil’s claw cell cultures. Plant Cell Tissue Organ Cult 105(1):79–84
Tiwari JK, Sarkar D, Pandey SK, Gopal J, Kumar SR (2010) Molecular and morphological characterization of somatic hybrids between Solanum tuberosum L. and S. etuberosum Lindl. Plant Cell Tissue Organ Cult 103(2):175–187
Tsukazaki H, Kuginuki Y, Aida R, Suzuki T (2002) Agrobacterium-mediated transformation of a doubled haploid line of cabbage. Plant Cell Rep 21:257–262
Ulrich I, Ulrich W (1991) High-resolution flow cytometry of nuclear DNA in higher plants. Protoplasma 165:212–215
Vatsya B, Bhaskaran S (1982) Plant regeneration from cotyledonary protoplasts of cauliflower (Brassica oleracea L. var. botrytis). Protoplasma 113:161–163
Veera RN, Gregory DN, Philip JD, Trevor WS (2009) Regeneration from leaf explants and protoplasts of Brassica oleracea var. botrytis (cauliflower). Sci Hortic 119:330–334
Walters TW, Earle ED (1990) A simple, versatile feeder layer system for Brassica oleracea protoplast culture. Plant Cell Rep 9:316–319
Walters TW, Mutschler MA, Earle ED (1992) Protoplast fusion-derived Ogura male sterile cauliflower with cold tolerance. Plant Cell Rep 10(12):624–628
Widholm JM (1972) The use of fluorescein diacetate and phenosaphranine for determining the viability of cultured cells. Stain Technol 47:189–194
Yang JL, Seong ES, Kim MJ, Ghimire BK, Kang WH, Yu CY, Li CH (2010) Direct somatic embryogenesis from pericycle cells of broccoli (Brassica oleracea L. var. italica) root explants. Plant Cell Tissue Organ Cult 100(1):49–58
Yarrow SA, Burnett LA, Wildeman RP, Kemble RJ (1990) The transfer of ‘Polima’ cytoplasmic male sterility from oilseed rape (Brassica napus) to broccoli (B. oleracea) by protoplast fusion. Plant Cell Rep 9(4):185–188
Zhang QY, Luo FX, Guo FC, Liu L (2010) In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tissue Organ Cult 101(1):41–47
Acknowledgments
Special thanks is given to Professor Liu F. for initiating our protoplast culture research. This work was financially supported by Science and Technology Department of Zhejiang Province, and Zhejiang Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng, X., Zhao, Z., Yu, H. et al. Protoplast isolation and plant regeneration of different doubled haploid lines of cauliflower (Brassica oleracea var. botrytis). Plant Cell Tiss Organ Cult 107, 513–520 (2011). https://doi.org/10.1007/s11240-011-0002-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-0002-z