Abstract
Cell differentiation depends on the proper and sequential expression of key genes required for morphogenesis. Several aspects of control are required for this which include: chromatin modifications, DNA methylation, correct amount of particular transcription factors, proper nuclear arrangement, etc. During the last few years the homeobox transcription factor WUSCHEL (WUS) has been shown to cause dedifferentiation when expressed on somatic cells followed by a production of new stem cells that can lead to somatic embryogenesis or organogenesis. We found that expression of WUS in coffee plants can induce calli formation as well as a 400% increase somatic embryo production. The results show that transgenic expression of the transcription factor WUS can be useful to increase somatic embryogenesis in heterologous systems. However, a critical developmental stage and additional hormonal requirements are required for the induction of embryogenesis by WUS in Coffea canephora.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of plant development has revealed many of the players relevant to the maintenance and differentiation of cells required for specialized tissues. Somatic embryogenesis has been a useful tool for understanding many of the genes and pathways involved in development. One particular field of interest during the last few years has been the study of the mechanisms that regulate cell identity and maintenance of undifferentiated cells in plant meristems (Schoof et al. 2000; Gallois et al. 2004; Lenhard and Laux 2003).
The higher plant shoot meristem is a dynamic structure whose maintenance depends on the coordination of two antagonistic processes, organ initiation and self renewal of the stem cell population. The homeobox gene WUSCHEL (WUS) is required to specify stem cell identity. WUS was originally identified as a central regulator of shoot and floral meristems in Arabidopsis (Mayer et al. 1998) where it is expressed in a small group of cells, and is required to maintain the overlying stem cells undifferentiated. In Arabidopsis WUS has been found to be sufficient to ectopically induce stem cells (Schoof et al. 2000; Gallois et al. 2004; Lenhard and Laux 2003, Zuo et al. 2002). Recent studies indicate that WUS can act both as a transcriptional activator of the floral gene AGAMOUS and as a transcriptional repressor of cytokinin response genes (Kieffer et al. 2006; Leibfried et al. 2005; Lohmann et al. 2001), suggesting that its molecular function is modified upon the developmental context. Mutations in WUS result in the misspecification of stem cells and premature termination of shoot and floral meristems after a few organs have been formed (Laux et al. 1996). CLV genes promote the progression of meristem cells toward organ initiation. Mutations in any of these genes result in delayed organ initiation, leading to an accumulation of meristem cells and increase in the size of the shoot meristem dome (Hamada et al. 2000).
The balance between the rate of cell division and differentiation is crucial for the ordered transition zones in the meristem. In Arabidopsis, WUS, CLV, and SHOOT MERISTEMLES (STM) have been implicated in the maintenance of undifferentiated cells in the meristem (Che et al. 2007; Sablowski 2007). STM is tough to prevent premature recruitment of cells into differentiation pathways, while WUS is required to keep the pool of stem cells in the central zone (Scofield et al. 2007). The combined effect of ectopic WUS and STM have an additive effect on cell division, expression of the central zone and initiation of organ primordial in Arabidopsis (Scofield et al. 2007).
Loss of WUS function leads to differentiation of stem cells and meristem termination. By contrast, ectopic WUS has been shown to induce somatic embryogenesis in some cases (Zuo et al. 2002) and in others to induce ectopic stem cell identity. During wild type development, WUS expression is initiated in four inner apical cells of the 16 cell embryo after several asymmetric divisions WUS expression is confined to the center of the developing shoot meristem. Asymmetric expression of WUS is established during or rapidly after cell division (Laux et al. 1996). Mature embryos express WUS in a small cell group underneath the two outermost cell layers. CLV 3 functions as a mobile intercellular signal in the shoot apical meristem that spreads laterally from the stem cells and acts both on their neighbors and on the stem cells themselves to repress WUS transcription (Muller et al. 2006). Furthermore, WUS has been shown to be part of a WOX family of proteins. The WOX proteins maintain a common regulatory sequence between them. The function still unknown in the majority of the WOX members however it is known that they are expresses asymmetrically and may be involved in the differentiation process.
Considering the function of WUS we decided to test the effect of over expressing WUS as a heterologous protein in Coffea canephora. The results show that transgenic lines that over expressed WUS lead to an altered increase growth and improved the production of somatic embryos. Histological analysis showed the development of meristem like structures, from which some produce somatic embryos after 4 weeks. Moreover, a 400% increase in the induction of somatic embryogenesis was observed from the transform WUS plants
Materials and methods
Plant growth and culture conditions
Plantlets of Coffea canephora cv. Robusta were grown in Magenta® boxes containing 40 ml of MS medium, supplemented with thiamine (11.86 μM), myo-inositol (0.550 μM), L-cysteine (0.158 μM), sucrose (87.64 mM), α-naphthalen acetic acid (0.54 μM), kinetin (2.32 μM) and Gelrite® (0.25%; w/v); pH was adjusted to 5.8 before autoclaving (20 min, 110°C). The plantlets were cultured under a 16 h light/8 h dark photoperiod at 25 ± 2°C. Grown plants were transfer to a test ground in normal soil after 6 months of green house adjustment.
Evaluation of somatic embryo production
For somatic embryo induction, leaf segments were excised with a 1 cm diameter cork borer and cultured in the Yasuda medium (Yasuda et al. 1985) modified as follows: 0.784 mM CaCl2, 5.15 mM NH4NO3, 4.7 mM KNO3, 0.624 mM KH2PO4, 0.5 mM Na2MoO4, 0.375 mM MgSO4, 50 μM H3BO3, 40 μM MnSO4·7H2O, 0.2 μM CuSO4, 15 μM ZnSO4, 75.53 μM FeSO4, 74.95 μM Na2EDTA, 4.86 μM pyridoxin, 8.12 μM nicotinic acid, 29.6 μM thiamine–HCl, 550 μM myo-inositol, 87.64 mM sucrose, 2.5 μM 6-benzil amino purine and the 5 μM 17-β-estradiol dissolved in DMSO; pH was adjusted to 5.8 and Gelrite (0.25% w/v) was used as gelling agent. Cultures were incubated under darkness or 16 h light/8 h dark photoperiod with a light intensity of 50 μmol m−2 s−1 at 25 ± 2°C (Quiroz-Figueroa et al. 2002). For the evaluation quantification of the number of embryos per explants was carried out and the number of embryos per explant from wild-type plants was set at 100%.
Plasmid binary constructs
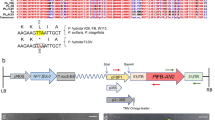
The binary vector generated, pER10 W-35SRed, contains the DsRFP reporter gene under the 35S constitutive and the gene WUSCHEL under an estradiol inducible transcriptional control (Zuo et al. 2002). The vector was generated by subcloning the gene DsRFP from pRSET B-RED that contain the DsRFP in EcoRI–BamHI (donated by Meredith Gould, Ens Baja California). DsRFP gene was removed from pRSET B-RED by digesting the plasmid with XbaI, followed by a fill in reaction with Klenow fragment and subsequent HindIII restriction digestion. The plasmid pCD contains a 35S promoter sequence (Gallie et al. 1989) and the plasmid was digested with SalI followed by a fill in reaction with Klenow fragment and a subsequent HindIII restriction digestion. The DsRed containing DNA was ligated into the digested pCD plasmid with T4 ligase for 8 h at 15°C. The 35S-DsRFP DNA fragment was obtained by digesting SmaI and SacI from the new vector named pCD-35SRed. These DNA was subcloned into pER10 W donated by Chua NH (Rochefeller University) by digesting pER10 W with SpeI. The ligation reaction was carried out after fill-in of the 5′ overhangs with DNA polymerse I (Klenow fragment, Invitrogene Life Technologies). pGFP binary vector was a gift from Tomas Gonzales.
Coffee plantlets and embryos transformation
Agrobacterium tumefaciens strain C58C1 was used for the transformation of coffee plantlets and embryos. Bacterial selection for this strain was carried out with 100 mg 1−1 of rifampicin and 100 mg 1−1 of espectromicin. Transformation of coffee plants and embryos was done as previously described (Canche-Moo et al. 2006). 30 days after transformation fluorescent embryos were selected under DsRed florescent light in a Leica MZFL III stereoscope.
Histological analysis
The selected tissue fragments were fixed in FAA at room temperature for 16 h. Afterwards, they were dehydrated in ethanol series (50%, 70%, 96%, 100%, 100%, 100%), and embedded with JB-4 embedding kit® (Polysciences, Inc.). Serial 10 μm thick sections were cut, transferred to polylysine-coated slides and stained with 0.02% toludine-blue for 5–10 min at room temperature. Pictures were taken with white or fluorescent light. On a Leica MZFL III stereoscope to be able to cover the entire length of the embryos with an excitation wavelength of 546/10 nm and filters D600/40.
Genomic DNA extraction and PCR
Coffee leaves (100 mg) were used for genomic DNA extraction using 600 μl DNA extraction buffer (100 mM Tris–HCl (pH 8), 50 mM EDTA, 2% SDS, 2% β-mercaptoethanol), following the protocol described by Lin et al. (2001).
PCR were carried out by using the Invitrogene DNA polymerase in the presence of 1 mM Mg2SO4. The primers used to check the transformed plants were: 5′-ACATATGGAGCCGCCACAG-3′ and 5′-GGAACGTTCTAGTTCAGACGTAGC-3′. The amplification conditions were 95°C for 2 min; 28 cycles of 95°C for 1 min, 56°C for 1 min and 72°C for 1 min and a final extension step at 72°C for 10 min.
Southern blot analysis
Wild type and selected transformed plant Genomic DNA (5 and 50 μg) were digested with EcoR1 restriction enzyme or HindIII, separated on a 0.7% agarose gel and blotted onto charged nylon membranes (Hybond N+, Amersham Pharmacia Biotech, England). For the probe labeling and hybridization we used the Gene Images Alkphos direct labeling and detection system (Amersham Biosciences, USA) kit according with the manufacturer's instructions. The hybridization was carried on at 65°C in 2 M urea, 0.1% SDS, 150 mM NaCl. A 850 pb probe was used which contains the gene sequence of WUSCHEL. The probe was amplified by PCR from the pER10 W-35SRed as template. Utilizing the same primers and conditions used to check transformed plants.
RNA extraction
Total RNA was obtained from 300 mg of coffee leaves following the protocol described by Kieffer and collaborators was used 300 mg of coffee leaves using extraction buffer (0.1 M Tris–HCl (pH 8.0), 0.25 mM EDTA; 2 M NaCl; 2% CTAB (w/v); 0.5% spermidina (w/v) and 2% β-mercaptoethanol) pre-warmed at 65°C, purified with chloroform/isoamylialcohol and was treated with DNase I (Invitrogen) as described elsewhere (Kiefer et al. 2000).
RT-PCR
cDNA synthesis was done with 1 μg of total RNA using SuperScript™ III Two-Step RT-PCR System with Platinum® Taq DNA polymerase (Invitrogen), following manufacturer’s instructions. First strand synthesis was carried out with wus R1 5′-ATCGCCTCCACATTCTTCTT-3′. The cycling conditions for PCRs were 95°C for 2 min; 40 cycles of 95°C for 1 min, 56°C for 1 min and 72°C for 1 min and a final extension step at 72°C for 10 min. The primers used were: wusNdeI 5′-ACATATGGAGCCGCCACAG-3′ and wus R1 5′-ATCGCCTCCACATTCTTCTT-3′. The RT-PCR products were resolved on 1.5% (w/v) agarose gels stained with ethidium bromide. RT-PCR products were separated on 1.5% agarose gels and transferred to nylon membranes (Hybond-N+, Amersham Pharmacia Biosciences) according to the manufacturer’s instructions. Following transfer, DNA was fixed to the membranes via UV crosslinking (120 mJ). Probe labelling and hybridization were realized with Gene Images Alkaphos direct labelling and detection system (Amersham Biosciences) kit. The hybridization was carried on at 55°C according with the manufacturer’s instructions.
Results
Whole coffee plantlets transformed with WUS show unorganized calli formation
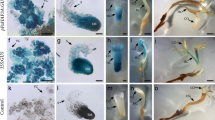
Previous works have shown that WUS overexpression may generate de novo organ or embryo formation from fully differentiated organs (Zuo et al. 2002; Gallois et al. 2004). This led to the hypothesis that induced WUS over expression could be of particular interest for recalcitrant species from which no efficient in vitro multiplication protocols are yet available. However as a first step we decided to test this hypothesis introducing WUS from Arabidopsis thaliana into C. canephora for which the conditions for transformation and somatic embryogenesis had already been set in our laboratory (Canche-Moo et al. 2006). The first transformations of plantlets with Agrobacterium containing the pERW-35SRED plasmid lead to calli formation around the plant shoot when grown in the presence of 17-β-estradiol. However these calli failed to form any type of embryos or new shoots, as can be seen in Fig. 1. Moreover, calli would readily form on transformed leaves that had been cut as can be seen in Fig. 1c. Calli formation could be observed around the edge of the incisions but these calli did not developed further. Cell cultures obtained from this kind of calli were treated with or without 17β-estradiol to induce further morphogenesis; however we failed to obtain any organized structures from this tissues. This initial set of experiments showed that somatic embryo formation could not be induced by the expression of WUS from whole plants. We therefore decided to carry out the transformation essays with pERW-35SRED using somatic embryos assuming they would contain a greater degree of potentially embryogenic cells. We took advantage of the red fluorescent protein expressed from the plasmid to differentiate between transformed and nontransformed embryos as previously published (Canche-Moo et al. 2006). After 80 days of culture in media supplemented with 5 mM 17-β-estradiol, 90% of the transformed somatic embryos increased up to 350% in size (Fig. 1e, f). This was not observed in the wild type somatic embryos or transformed embryos expressing GFP (Fig. 1d and data not shown) under the same conditions. Ten percent of the transformed embryos showed somatic embryo formation as can be seen in Fig. 1e. The red fluorescent embryos were selected as shown in Fig. 2 after 30 days of transformation. Nontransformed embryos showed a weak red fluorescence as compared with transformed embryos. Furthermore, histological dissections of 80 days embryo showed a homogeneous fluorescence as compared to wild type embryos (Fig. 2c, d).
Transient transformation of Coffea canephora plantlets. Plantlets transformed with an GFP expressing vector (a). WUS expression system was used in plantlets of Coffea canephora. A month after transformation with pERW-35SRED, some plantlets developed calli around the shoot or in the infected stems (b) and previously incised leaves grown on MS supplemented with estradiol (c). Wild-type typical somatic embryo (d). (e) and (f) show transient transform embryos with pER10 W-35SRed. 5 mm bars were place to compare the size of embryos
Selection transformed somatic embryos of Coffea canephora with pER10 W-35SRed. The embryos were maintained in Yasuda medium (supplemented with 17-β-estradiol) for 30 days. Wild-type typical somatic embryos were compared to transformed embryos (A under fluorescent light or B under visible light). Histological dissection of an 80 day old embryos is shown. (D under fluorescent light or E under visible light)
Embryos seen in Fig. 1f showed altered structures that resemble ectopic meristem center formation on the cotyledons (Fig. 3a). Transversal dissection of the embryos showed a multitude of dense nuclei cells around the leaves (Fig. 3b) from which some developed into somatic embryos (Fig. 3c). However, the majority of the embryos did not develop into plants. The hypocotyls usually got swollen and leaf development, when observed, was compromised by several distortions and new growths that did not continue to develop (results not shown). In addition to lateral organs, adventitious shoots or embryo-like structures could also form (Fig. 3c). All vegetative embryo tissues from the transform plants could produce shoot-like organs or embryo-like structures, but their further development was impaired. These observations show that continuous over expression of WUS as a regulatory protein results in malformations and alterations in growth. However, we took advantage of the inducible system to provide a transitory signal, in this case we tested WUS as a trigger for the genes involved in the developmental switch to embryogenesis.
Histological analysis of 40-day-old embryos maintained in Yasuda medium in the presence of 5 μM 17-β-estradiol. (a) Apical portion from a pERW-35SRED transformed somatic embryo, showing possible ectopic meristem-like structures in the cotyledonal section of the embryo is shown by the arrow. (b) Cross-section of the cotyledonear structure from a transformed embryo. The arrows showed highly dense structure from which some develop into secondary somatic embryos. (c) Somatic embryo showing the meristem portion
We tested on the 10% embryos that showed normal secondary embryo formation and developed cyclic somatic embryogenesis for 6 months as can be seen in Fig. 4. The essays were always carried out in parallel with a wild type plant to verify that the specific growing conditions were identical to those of the pERW-35SRED transformed embryos, as can be seen on the left of each panel. The long production of embryos can be attributed to the chemical stability of 17-β-estradiol under these conditions. The newly formed somatic embryos were grown on MS media for 16 months into well developed normal plantlets as seen on Fig. 4. From the plantlets obtained, only those showing effective transformation with pERW-35SRED were selected for further study. The above data seem to corroborate that WUS, in addition to its meristem function described previously (Laux et al. 1996; Mayer et al. 1998), it can also plays a critical role in promoting or maintaining embryonic potential as seen by Zuo et al. (2002).
Overexpression of WUS induces secondary embryogenesis in somatic embryos of Coffea canephora. (a–d) Wild type coffee somatic embryo is shown at the left side of the panel, and the somatic embryo transformed with pERW-35SRED forming calli is shown at the right. (a) after 10 days. (b) after 30 days. (c) 2 Months, (d) 6 Months. (e) Coffee plants 16 months old completely regenerated from the secondary somatic embryos. All the treatments were maintained in a medium containing MS + ANA + Kinetina (a–e) +5 μM 17-β-estradiol (a–d)
Roots from transformed plants grown on 17-β-estradiol supplemented media develop meristem like structures
Transformed plants with pERW-35SRED obtained as described above were transferred from MS + NAA + kinetin medium to the same MEDIUM supplemented with 17-β-estradiol. Roots were excised and submitted to histological observation. The root tips were often enlarged to give rise to lateral root primordial-like structures or embryo-like structures, which were unable to develop further, as seen in Fig. 5. However, after 2 months the roots swell into undifferentiated calli. Southern blot from transformed plants was used to verified insertion of the gene WUS in the selected plant and compared to a plant transformed with a GFP expression vector (Fig. 5d). High background is probably due to Wuschel related genes from Coffea. However a clear band was observed that was absent in the control plant. To verify the expression on WUS from the selected plant, RT-PCR was carried out. At 28 PCR cycles a difference in the expression was seen with or without estradiol (Fig. 5e). PCRs were carried out without a reverse transcription reactions to verify that amplifications were from mRNA and not from Genomic DNA contamination. The selected plant showed to expressed the WUS gene in the roots used for analysis.
Histological analysis of roots of Coffea canephora plants after 40 days exposure to 5 μM 17-β-estradiol. (a) Root from a wild type plant. (b) Longitudinal section of a pERW-35SRED transformed root showing an apparent increase of cell proliferation across the root. (c) Apparent lateral root primordial-like structure formation in a pERW-35SRED transformed root. Southern blot from a selected pERW-35SRED transformed plant was used to verify that WUS was introduced into the genome. Five and 50 μg of genomic DNA were loaded either for the control (GFP transformed plant) or WUS transformed plant. Genomic DNA was digested with Hind III or EcoRI for 5 hours at 37°C. RT-PCR was carried out as described in Materials and methods. RNA from a single either GFP transformed or WUS transformed plantlets was used. Plantlets were grown with our without 5 μM estradiol. RNA was treated with or without SuperScript™ III for 1 h
Embryogenic cultures obtained from transformed plants show an high increase in somatic embryo formation in the presence of 17-β-estradiol
We also carried out somatic embryogenesis induction with leaves from the pERW-35SRED transformed plantlets. Explants were excised with a cork borer from these leaves and were placed in Yasuda medium as described in materials and methods. This liquid medium allows normally a small number of somatic embryos to develop from wild type plants, due to its low cytokinin level. We found that pERW-35SRED transformed cultures showed a 400% ± 30% increase in somatic embryo formation as compared to cultures obtained from wild type leaf explants, as seen in Fig. 6a. These essays were repeated eight times to check for reproducibility. RNA extraction from transformed parent plants was tested for WUS expression via RT-PCR (see Fig. 6b). Total extracted RNA from selected parent transformed plants and wild type is shown in Fig. 6c. Therefore the increase in somatic embryo formation corresponds to expression of WUS in these tissues, which its known function is to keep cells undifferentiated.
Somatic embryogenesis induction in cultures from pERW-35SRED transformed leaves. Leaf explants either from normal wild type, pGFP transformed plants or from two selected pERW-35SRED transformed plants, were used as starting material for somatic embryogenesis induction. (a) Somatic embryo quantification after 1 month in liquid Yasuda medium supplemented with 5 μM 17-β-estradiol. (b) RT-PCR blotting to detect WUS expression. in leaves: 1: WUS positive control PCR product; 2 water; 3 wild type plant; 4 pERW-35SRED transformed plant line1; 5 pERW-35SRED Transformed plant line 2. (c) Total RNA extracted from leaves used for the RT-PCR. 1 Wild type plant;, 2 pERW-35SRED Transformed plant line 1; 3 pERW-35SRED Transformed plant line 2
Discussion
Unlike animals most plant organogenesis takes place postembryonically, allowing plants to modify their development in response to the environment. The shoot apical meristem (SAM) has the ability to maintain an organized structure while responding to intrinsic and extrinsic developmental signals. To accomplish this, a constant pool of slowly dividing undifferentiated stem cells is maintained within the central zone of the SAM (Kieffer et al. 2006). The proliferation of stem cells within the SAM must be exactly matched to the rate at which daughter cells differentiate (Baurle and Laux 2005; Carles and Fletcher 2003; Gross-Hardt et al. 2002).
Our observation that WUS is capable of promoting the vegetative-to-embryonic transition, and eventually somatic embryo formation, suggests that the homeodomain protein can play a critical role during embryogenesis, in addition to its function in meristem development. Furthermore this regulatory control appears to work well on a heterologous system such as Coffea plants. It is Presume that the highly restrictive expression of WUS hallmarks the putative embryonic organizing centre which, in turn, may give rise to stem cells during embryogenesis and later development. (Zou et al. 2002).
The mechanism by which WUS prevents the differentiation of stem cells is unknown. Furthermore, its activity in dedifferentiating cells may prove unconventional as a way to reorganize heterochromatin to the level of stem cells when expressed in selected tissues (Zou et al. 2002). This activity is consistent with our results shown in Fig. 1 in which somatic cells dedifferentiates to form calli. Similar to the effect seen when abnormal added levels of auxins are added in plants (Golz 2006). In tissue that is not highly differentiated like that of somatic embryos the ectopic expression of WUS provided an abnormal growth in the majority of the embryos, this could be due to the effect of WUS on ARF(s) which has a direct influence upon its expression and mutations in ARF have shown abnormal growth in Arabidopsis. Figure 1 shows and increase in overall size of the embryos with several abnormalities that may lead to ectopic meristems seen in Figs. 2 and 3. Furthermore, low expression may lead to the formation of somatic embryogenesis in a few cells. This results are consistent with previous findings seen in Arabidopsis by Zou et al. (2002) and Xu et al. (2005) which may reflect how a ectopic expression of a transcription factor can lead to a an alteration in the development signals. Furthermore, the time and type of cells are crucial for induction into somatic embryogenesis. Previously Zou et al. (2002) had shown that root meristems could produce somatic embryos in Arabidopsis. Here we found that the best tissue for induction was during the first stages of somatic embryogenesis. This provided a cyclic somatic embryogenesis system. Moreover, when the embryos were put in a noninducing environment they show a proper development. Although roots did show abnormal development during induction and increase meristem formation along the roots, no embryos develop from this tissue. Mostly a higher number of roots formed from exposed plants (data not shown). Probably due to the similarities in function with WOX5 which controls root meristem maintenance and WUS expression may interfere with normal development in roots as can be seen in Fig. 5 which is consistent with previous observations (Imin et al. 2007; Nardmann et al. 2007). This is however the first time that expression of WUS has been carried out in a somatic embryogenesis induction system from which may provide a useful tool for propagation of difficult cultivars of plants. Figure 6 shows that transformed plants that overexpresed WUS showed a 400% ± 30% increase in somatic embryo formation as compared to cultures obtained from wild type leaf explants. Further research would be required to define if over expression of WUS in recalcitrant plants can lead to somatic embryogenesis. Additionally the regulatory pathway in which WUS may be able to act promoting somatic embryo formation in Arabidopsis seems to be conserved as it functions in a similar way in Coffea. However, unlike Arabidopsis that an induction of WUS was all that was necessary for somatic embryo formation or organogenesis (Zou et al. 2002; Xu et al. 2005). In Coffea canephora only an increase in number embryo was observed Fig. 6 and a small percentage of secondary embryos were obtained as seen in Fig. 1e. RT-PCR showed that WUS was also expressed in the absence of 17-β Estradiol. This leaking of transcription can be attributed to the location of the insertion of this gene in the genome (a euchromatin region) or a cross reactivity of the estrogen receptive portion interacting with an estrogen like molecule. This may explain the low difference in embryo formation, between the induced and non-induced transformed explants seen in Fig. 6a, b.
References
Baurle I, Laux T (2005) Regulation of WUSCHEL transcription in the stem cell niche of the Arabidopsis shoot meristem. Plant Cell 17:2271–2280. doi:10.1105/tpc.105.032623
Canche-Moo A, Ku-Gonzalez C, Burgeff C, Loyola-Vargas VM, Rodríguez-Zapata LC, Castaño E (2006) Genetic transformation of Coffea canephora by vacuum infiltration. Plant Cell Tiss Org Cult 84:373–377. doi:10.1007/s11240-005-9036-4
Carles CC, Fletcher JC (2003) Shoot apical meristem maintenace: the art of a dynamic balace. Trends Plant Sci 8(8):394–401. doi:10.1016/S1360-1385(03)00164-X
Che P, Lall S, Howell SH (2007) Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta (Jun):21. doi:10.1007/s00425-007-0565-4
Gallie DR, Lucas WJ, Walbot V (1989) Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell 1:303–311
Gallois JL, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380. doi:10.1101/gad.291204
Golz JF (2006) Signalling between the shoot apical meristem and developing lateral organs. Plant Mol Biol 60(6):889–903. doi:10.1007/s11103-005-1270-y
Gross-Hardt R, Lenhard M, Laux T (2002) WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev 16:1129–1138. doi:10.1101/gad.225202
Hamada S, Onouchi H, Tanaka H, Kudo M, Liu YG, Shibata D et al (2000) Mutations in the WUSCHEL gene of Arabidopsis thaliana result in the development of shoots without juvenile leaves. Plant J 24:91–101. doi:10.1046/j.1365-313x.2000.00858.x
Imin N, Nizamidin M, Wu T, Rolfe BG (2007) Factors involved in root formation in Medicago truncatula. J Exp Bot 58(3):439–451. doi:10.1093/jxb/erl224
Kiefer E, Heller W, Ernst D (2000) A simple and efficient protocol for isolation of functional RNA from plant tissues rich in secondary metabolites. Plant Mol Biol Rep 18:33–39. doi:10.1007/BF02825291
Kieffer M, Stern Y, Cook H, Clerici E, Maulbetsch C, Laux T et al (2006) Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell 18:560–573. doi:10.1105/tpc.105.039107
Laux T, Mayer KF, Berger J, Jürgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96
Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M et al (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438:22–29
Lenhard M, Laux T (2003) Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130:3163–3173. doi:10.1242/dev.00525
Lin RC, Ding ZS, Li LB, Yu T (2001) A rapid and efficient DNA minipreparation suitable for screening transgenic plants. Plant Mol Biol Rep 19:379a–379e. doi:10.1007/BF02772839
Lohmann J, Hong R, Hobe M, Busch M, Parcy F, Simon R et al (2001) A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105(6):793–803. doi:10.1016/S0092-8674(01)00384-1
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95:805–815. doi:10.1016/S0092-8674(00)81703-1
Muller R, Borghi L, Kwiatkowska D, Laufs P, Simon R (2006) Dynamic and compensatory responses of Arabidopsis shoot and floral meristems to CLV3 signaling. Plant Cell 18(5):1188–1198. doi:10.1105/tpc.105.040444
Nardmann J, Zimmermann R, Durantini D, Kranz E, Werr W (2007) WOX gene phylogeny in Poaceae: a comparative approach addressing leaf and embryo development. Mol Biol Evol 24(11):2474–2484. doi:10.1093/molbev/msm182
Quiroz-Figueroa FR, Fuentes-Cerda CF, Rojas-Herrera R, Loyola-Vargas VM (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20:1141–1149. doi:10.1007/s00299-002-0464-x
Sablowski R (2007) Flowering and determinacy in Arabidopsis. J Exp Bot 58(5):899–907. doi:10.1093/jxb/erm002
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644. doi:10.1016/S0092-8674(00)80700-X
Scofield S, Dewitte W, Murray JA (2007) The KNOX gene SHOOT MERISTEMLESS is required for the development of reproductive meristematic tissues in Arabidopsis. Plant J 50(5):767–781. doi:10.1111/j.1365-313X.2007.03095.x
Xu YY, Wang XM, Li J, Li JH, Wu JS, Walker JC et al (2005) Activation of the WUS gene induces ectopic initiation of floral meristems on mature stem surface in Arabidopsis thaliana. Plant Mol Biol 57:773–784. doi:10.1007/s11103-005-0952-9
Yasuda T, Fujii Y, Yamaguchi T (1985) Embryogenic callus induction from Coffea arabica leaf explants by benzyladenine. Plant Cell Physiol 26:595–597
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to embryonic transition in Arabidopsis. Plant J 30(3):349–359. doi:10.1046/j.1365-313X.2002.01289.x
Acknowledgments
We thank to Fernando Contreras for her technical help and Luis Joel Figueroa from Accesolab for the contribution of reagents for this study. Dr. Castaño is partially funded by CONACYT grant 39731-z and CONACYT grant 056001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arroyo-Herrera, A., Ku Gonzalez, A., Canche Moo, R. et al. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tiss Organ Cult 94, 171–180 (2008). https://doi.org/10.1007/s11240-008-9401-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9401-1