Abstract
Gyrodactylus jennyae n. sp. is described from the body surface and mouthparts of tadpoles of the bullfrog Rana catesbeiana Shaw imported presumably from Missouri, USA, into a federal government facility in Moncton, New Brunswick, Canada. Its morphology resembles most closely that of G. chologastris Mizelle, Whittaker & McDougal, 1969 described from two amblyopsids (blind cave fishes) in Kentucky and North Carolina. Both species have long slender hamuli, a ventral bar with a relatively long membrane and small anterolateral processes, a cirrus with two rows of small spines and marginal hooks with a well-developed sickle heel and short handle. The two species differ morphologically; G. jennyae has a marginal hook sickle with a more pronounced heel than that found in G. chologastris. A BLAST search using a 945 base pair sequence that included the nuclear ribosomal DNA internal transcribed spacers 1 and 2 and the 5.8S rRNA gene from G. jennyae n. sp. showed that the overall similarity with other Gyrodactylus sequences on GenBank was relatively low. The ITS1 region was similar to that of G. misgurni Ling, 1962; however, no ITS2 and 5.8S rRNA sequences are available for that species. A separate search using 5.8S sequences revealed that G. markakulensis Gvosdev, 1950 and G. laevis Malmberg, 1957 were the closest to G. jennyae (1 and 2 bp differences, respectively). These species are parasites of cyprinids (or their predators) and are similar to G. jennyae and G. chologastris in having a double row of small hooks on the cirrus and overall similar morphologies of the haptoral hard parts. There are now five species of Gyrodactylus described exclusively from amphibians and this appears to have involved at least three separate host-switches from fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Gyrodactylus Nordmann, 1832 primarily parasitise fishes, with 450 nominal species being reported from hosts in freshwater and marine habitats worldwide (Bakke et al., 2007). Four species, G. ambystomae Mizelle, Kritsky & McDonald, 1969, G. aurorae Mizelle, Kritsky & McDonald, 1969, G. ensatus Mizelle, Kritsky & Bury, 1968 and G. catesbeiana Wootton, Ryan, Demaree & Critchfield, 1993 have been described from frogs and salamanders in North America (Tables 1, 2). Several more recent accounts of gyrodactylids found on larval amphibians surveyed in Canada and the USA could reveal additional species upon their identification beyond the level of the subgenus Gyrodactylus (Table 1) (Crawshaw, 1997; Dodd et al., 2004; Gunzburger et al., 2005; Green & Dodd, 2007). One species, G. arcuatus Bychowsky, 1933, was reported on Hyla arboria (L.) tadpoles from the Danube delta, Romania (Tables 1, 2) (Volgar-Pastukhova, 1959; Vojtkova, 1989; R. Bray, personal communication), but its type host is the three-spined stickleback (Gasterosteus aculeatus L.) (Geets et al., 1999), which suggests that the infected tadpoles were acting as transient hosts (Prudhoe & Bray, 1982). The present study describes a new species of Gyrodactylus from the body surface of captive bullfrogs Rana catesbeiana Shaw imported into Canada from a commercial frog farm believed to be located in Missouri, USA.
Materials and methods
The new species was first found on tadpoles in April, 2007 at a federal government research facility in Montreal, Quebec, which had obtained the amphibians from a stock of tadpoles housed by a federal government research facility in Moncton, New Brunswick. These tadpoles originally came from a commercial frog farm located in the southern USA, believed to be in Missouri, via an international commercial distributor. Following the discovery of the parasite, more tadpoles were ordered from the same distributor the following year and they also arrived infected, suggesting that the farm stock was likely the original source of the parasite.
In Montreal, the tadpoles were held in filtered, aerated and dechlorinated water, at a density of 1 tadpole/litre, and exposed to a 16 h–8 h light–dark cycle and a temperature of 21 (±1.3)°C. Clinical symptoms, including skin erosion, scoliosis, lethargy and emaciation, first appeared 5 weeks after their arrival at the Montreal facility and microscopical inspection showed that they were infected with a species of Gyrodactylus.
For morphological studies, live specimens were frozen and then fixed in 10% formalin. Specimens were stained briefly in Masson’s trichrome and mounted in a 50% solution of glycerine for clearing and study. The holotype and paratype specimen slides were soaked overnight in tap-water to remove the glycerine, dehydrated in ethanol, cleared in xylene and mounted in Canada balsam. The descriptive terminology follows You et al. (2008). Measurements are presented in micrometres for the holotype, followed in parentheses by the mean ± SD, the range and the number of specific measurements taken from 10 additional paratype specimens. The holotype of G. chologastris Mizelle, Whittaker & McDougal, 1969 (USNM Accession Number 70461) was examined for comparative purposes because, morphologically, this was the most similar species.
For genomic DNA extraction, seven individual specimens were briefly air-dried to remove the ethanol, placed in 5 μl water and stored at −20°C. For three specimens, the haptor was separated from the body before drying, enabling simultaneous morphological and molecular analyses of these individuals. The body was placed in 5 μl of milli-Q water, while the haptor was mounted in ammonium picrate glycerine, as described by Malmberg (1970). All specimens were digested by the addition of 5 μl of lysis solution consisting of 1× PCR buffer (Eurogentec), 0.45% (v/v) Tween 20, 0.45% (v/v) NP 40 and 60 μg/ml of proteinase K (Sigma). The samples were incubated at 65°C for 25 min, followed by 10 min at 95°C to inactivate the proteinase. The primer pairs ITS1A (5′-GTAACAAGGTTTCCGTAGGTG-3′) and ITS2 (5′-TCCTCCGCTTAGTGATA-3′) (Matějusová et al., 2001) were used to amplify a fragment spanning the 3′ end of the 18S rRNA gene, the internal transcribed spacer 1 (ITS1), the 5.8S rRNA gene, ITS2 and the 5′ end of the 28S rRNA gene. The amplification reactions (20 μl) consisted of 1× PCR buffer (Eurogentec), 1.5 mM MgCl2 (Eurogentec), 200 μM of each dNTP (Amersham Pharmacia Biotech, Sweden), 1 μM of each primer (Eurogentec), 2 μl lysate, 1 unit Taq polymerase (Eurogentec) and milli-Q water. The mixtures were heated for 4 min at 96°C and subjected to 35 cycles of 1 min at 95°C, 1 min at 50°C and 2 min at 72°C, followed by final extension at 72°C for 7 min. The PCR products were visualised using ethidium bromide on a 1.2% agarose gel. The products were then purified by means of GFX columns according to the manufacturer’s instructions (Amersham Pharmacia). Both DNA strands were sequenced using a Big Dye Chemistry Cycle Sequencing Kit (version 1.1) in a 3130 DNA Analyzer (Applied Biosystems). The PCR primers and 2 internal primers, ITS1R (5′-ATTTGCGTTCGAGAGACCG-3′) and ITS2F (5′-TGGTGGATCACTCGGCTCA-3′) (Ziętara & Lumme, 2002), were used for sequencing.
We first compared sequences of specimens obtained from the bullfrog tadpoles. These were identical. We then searched for similar sequences among other species of Gyrodactylus in Genbank using BLAST (available at www.ncbi.nih.gov/BLAST/). Where possible, the entire ITS1-5.8S-ITS2 sequences were compared. Unfortunately, only ITS1 or ITS2 sequences exist for certain species, so these were compared separately. We also compared the 5.8S sequences separately. Previous work has shown that the 5.8S sequences are useful in distinguishing between the six subgenera of Gyrodactylus, as proposed by Malmberg (1970; see Ziętara et al., 2002; Huyse et al., 2003). This classification into subgenera is based on characteristics of the excretory system studied in living animals (Malmberg, 1970). The selected sequences were downloaded and aligned in Clustal W implemented in MEGA 4 (Tamura et al., 2007).
Gyrodactylus jennyae n. sp.
Type-host: Rana catesbeiana Shaw (Anura: Ranidae), bullfrog larvae.
Type-locality: Unknown, but believed to be a bullfrog farm in Missouri, USA.
Site: Body surface, mainly on the head, around the oral region and at the base of the tail.
Type-material: The holotype and paratype slides have been deposited in the Harold Manter Laboratory of Parasitology (Accession numbers HWML 49087), The University of Nebraska, Lincoln, Nebraska, USA.
Etymology: This helminth is named for Dr Jenny Cook, a long time supporter of parasitology research and expert on immigration patterns into New Brunswick, Canada.
Description (Figs. 1–3)
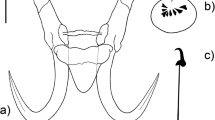
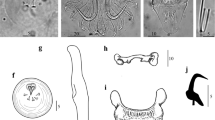
Coverslip-flattened specimens 508 (461 ± 63.5, 360–540, 11) long, 104 (114 ± 21.0, 92.5–166, 11) wide at mid-body. Prohaptor with distinct spike sensilla. Pharynx large, 46.4 (52.4 ± 8.0; 45–63, 6) long. Intestinal caeca typically containing large amounts of black pigment granules. Cirrus bulb small, 15 (16.4 ± 1.2, 15–17, 3) in diameter, with single large spine and 2 rows of smaller spines. Haptor oval in ventral view, 72 (73.7 ± 9.3, 62–87, 7) long, 95 (68.0 ± 17.3, 53–95, 5) wide. Hamuli slender, 62 (60.1 ± 2.5, 55.5–63.5, 10) long; root 24.1 (21.4 ± 1.8, 19.5–24.0, 7); shaft 43.5 (45.5 ± 2.2, 43–48, 7); point 31.8 (29.5 ± 2.5, 26–33, 7). Ventral bar sub-rectangular, 18 (18.1 ± 1.3, 16–20, 7) wide, 7.7 (6.5 ± 1.6, 4.5–8.5, 7) long medially; ventral bar membrane expanded distally, 16.7 (16.8 ± 1.8, 13.5–18.5, 6) long; ventral bar anterolateral processes 2.0 (1.9 ± 0.4, 1.5–2.0, 5) long. Dorsal bar simple, 1.8 (1.75 ± 1.8, 13.5–18.5, 6) long medially, 10.9 (12.6 ± 2.5, 11–15, 2) wide. Marginal hook 23.4 (23.1 ± 0.4, 22.5–23.5, 5) long; handle 14.5 (15.8 ± 0.9, 14.5–16.5, 4) long; sickle with prominent heel, length 8.4 (8.4 ± 0.8, 8–9.5, 4), distal width 2.7 (2.8 ± 0.5, 2.5–3.5, 4), proximal width 3.2 (3.5 ± 0.4, 3–4, 4); filament 15.1 (12.4 ± 2.4, 10.5–15.5, 4).
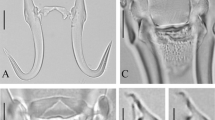
Wholemount of Gyrodactylus jennyae n. sp. in ventral view showing: the accumulation of host pigment granules within the intestinal caeca (A); the cirrus with the double row of small hooks (B); a ventral view of the haptor with the relatively thin hamuli (C); and the ventral bar with a characteristic sub-rectangular membrane (D). Scale-bars: A, 100 μm, B–D, 10 μm
Comments
The morphological features of G. jennyae n. sp. (Figs. 1–3) place it within the subgenus Gyrodactylus and the G. elegans species-group of Malmberg (1970). Morphologically G. jennyae most closely resembles G. chologastris Mizelle, Whittaker & McDougal, 1969 from blind cave fish Chologaster agassizi (Putman) in Kentucky and C. cornutus (Agassiz) in North Carolina. Both species of parasite have a cirrus armed with small spines in multiple rows, long slender hamuli, a ventral bar with a relatively long membrane and small anterolateral processes, and marginal hooks with a well-developed sickle heel and a short handle. All of these characters are consistent with the subgenus Gyrodactylus. G. jennyae has a marginal hook sickle with a much more pronounced heel than G. chologastris, as confirmed by our examination of the holotype slide of G. chologastris. Unfortunately, no sequence data are available for this species.
The molecular data further supports that G. jennyae n. sp. fits the G. elegans species-group of Malmberg (1970). The amplified rRNA fragment was 945 bp long and consisted of the 3′ end of the SSU gene (34 bp), the ITS1 spacer (383 bp), the 5.8S gene (157 bp), the ITS2 (319 bp) and the 5′ end of the LSU gene (52 bp). The sequences of all seven specimens of G. jennyae were identical (GenBank Accession No. EU678357). Based on BLASTN searches on GenBank (Altschul et al., 1997), the ITS1 appeared most similar to that of G. misgurni Ling, 1962 (host: Misgurnus anguillicaudatus Cantor). Unfortunately, only ITS1 is available for G. misgurni. We aligned these sequences with Clustal W as implemented in Mega 4.0 (Tamura et al., 2007) and calculated the p-distance (15%). As all other BLAST hits were too distant, a reliable alignment was not possible and phylogenetic reconstruction was not attempted. The 5.8S sequence of the G. jennyae subunit was most similar to G. markakulensis Gvosdev, 1950 (host: Gobio gobio L.; 1 bp difference) and G. laevis Malmberg, 1957 (host: Phoxinus phoxinus L.; 2 bp difference). Comparisons with G. prostae Ergens, 1963, G. phoxini Malmberg 1957, G. magnificus Malmberg, 1957, G. neili Leblanc, Hansen, Burt & Cone, 2006, G. elegans Nordmann, 1832 and G. carassii Malmberg, 1957 revealed differences of 3 bp. These species are all members of the subgenus Gyrodactylus. The ITS2 sequences proved too difficult to align reliably, but BLASTN searches showed maximum identity of 70–80% with these last six species.
Pathology
Bullfrog tadpoles were the host of this parasite (Fig. 4). Like most monopisthocotylean monogeneans, G. jennyae n. sp. was observed to be an epidermal forager and possibly also ingests dermal tissue of the host (Cable et al., 1997; Bakke et al., 2007).
During the early stages of infection, the parasites were concentrated around the hosts’ eyes and tail base, but, over time, G. jennyae could be observed throughout the entire body surface of the tadpoles, as well as on their oral papillae and inside their buccal cavity (Nieto et al., 2007). In heavy infections, tadpoles hosted hundreds of parasites, in one case carrying 104 worms around the oral region alone. After a period of time, the infected areas of the tadpoles’ skin became paler in colour. Thus, the most prominent clinical symptoms of infection were the development of a characteristic light-coloured ‘facial mask’ (Fig. 4A) and a ‘patchy’ tail base. Lethargy and emaciation (Fig. 4B) followed simultaneously. A 10% mortality rate occurred in tadpoles within 2 weeks of the onset of symptoms.
Discussion
To date, there are 16 known cases of amphibian infection with Gyrodactylus spp., all but one of which were found in North America (Table 1). The majority of infections were observed in California and Florida and in only seven cases were the parasites identified to species. All but two cases involve tadpoles of anurans, with bullfrogs being the most common hosts, followed by southern leopard frogs.
Morphological and molecular results place G. jennyae n. sp. within Malmberg’s (1970) subgenus Gyrodactylus and the G. elegans species-group (Table 2), which is typically found on cyprinid fishes in freshwaters of the northern hemisphere (Malmberg, 1970), implying that there has been a host-switch within the lineage from fish to amphibians. Host-switching is common among gyrodactylids (Bakke et al., 2002) and it may be an important mode of speciation (Bakke et al., 2007), despite strong barriers. When a switch is successful, the hyperviviparous reproduction used by gyrodactylids may lead to the evolution of a new species by way of genetic isolation on the host (Ziętara & Lumme, 2002). Such a host-switch amongst members of Gyrodactylus is thought to have occurred between cyprinids and piscivorous escocid predators (pike and pickerel), giving rise to G. neili Leblanc, Hansen, Burt & Cone, 2006 and G. fryi Cone & Dechtiar, 1984 (Cone & Dechtiar, 1984; Leblanc et al., 2006).
Two additional host-switches likely occurred to produce the other Gyrodactylus spp. that have been described exclusively from amphibians: one switch may have produced G. aurorae Mizelle, Kritsky & McDonald, 1969, G. ambystomae Mizelle, Kritsky & McDonald, 1969 and G. ensatus Mizelle, Kritsky & Bury, 1968, which are members of Malmberg’s (1970) subgenus Metanephrotus Malmberg, 1964 and the G. eucaliae species group (Table 2) described from various gasterosteid and centrarchid fishes. Another switch appears to have given rise to G. catesbeiana Wootton, Ryan, Demaree & Critchfield, 1993, which is of unknown subgenus placement, but which morphologically resembles G. stunkardi Kritsky & Mizelle, 1968 and G. spathulatus Mueller, 1936 (Wootton et al., 1993) from cyprinid and catostomid fishes. Thus, parasitism of amphibian larvae has resulted from at least three separate host-switches from fishes to amphibian tadpoles, and, along with the previous description of G. catesbeiana, the present study suggests that these have involved switches to bullfrog tadpoles at least twice.
The captive bullfrog tadpoles infected with G. jennyae n. sp. developed clinical symptoms, including emaciation and lethargy, before death, although these could not be attributed to the presence of the parasite due to the lack of an uninfected tadpole control group. The pale-coloured ‘facial masks’ and ‘patchy’ tail bases observed on the tadpoles, however, were possibly caused by the superficial grazing of the parasites. Indeed, the intestinal caeca of many of the parasites were filled with pigment granules, potentially of host integumental origin. On fish, it is believed that gyrodactylids secrete proteolytic enzymes onto the host epithelium and then ingest the mucus and partly-digested epithelial cells for further digestion and that, occasionally, the worms may ingest dermal cells of the fish, as well as their melanocytes (Bakke et al., 2007). In fact, Cable et al. (1997) confirmed that the pigment granules present in the gut of Macrogyrodactylus polypteri Malmberg, 1957 were of host origin. In amphibians, melanophores are present in both layers of the amphibian integument: they are individually scattered in the epidermis as melanocytes and form a chromatophore unit with other pigment cells in the dermis (Herman, 1992; Zug, 1993). Thus the decolourised regions that we observed on the tadpoles were potentially due to the destruction or ingestion of their melanophores by the parasites in one or both layers of the integument.
The mortality of tadpoles coinciding with gyrodactylid infections has only been observed on bullfrog tadpoles maintained in laboratory facilities (Crawshaw, 1997; Wootton et al., 1993; this study). Certainly, captive conditions can affect normal host-parasite relationships and lead to disease (Barber, 2007). But Gyrodactylus spp. have already gained notoriety for the widespread disease epidemics they have caused when they apparently spread among both cultured and wild fish populations, not to mention the enormous economic impacts and conservation threats that have ensued (Malmberg, 1993; Bakke et al., 2007). Thus the possibility exists that gyrodactylids could also cause extensive mortality among amphibian larvae in natural conditions, further adding to the suite of factors that are globally threatening the existence of these vertebrates (Stuart et al., 2004).
References
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Andrews, K. D., Lampley, R. L., Gillman, M. A., Corey, D. T., Ballard, S. R., Blasczyk, M. J., et al. (1992). Helminths of Rana catesbeiana in southern Illinois with a checklist of helminths in bullfrogs of North America. Transactions of the Illinois State Academy of Sciences, 85, 147–172.
Bakke, T. A., Cable, J., & Harris, P. D. (2007). The biology of gyrodactylid monogeneans: The “Russian-doll killers”. Advances in Parasitology, 64, 161–376.
Bakke, T. A., Harris, P. D., & Cable, J. (2002). Host specificity dynamics: Observations on gyrodactylid monogeneans. International Journal for Parasitology, 31, 281–308.
Barber, I. (2007). Parasites, behaviour and welfare in fish. Applied Animal Behaviour Science, 104, 251–264.
Cable, J., Harris, P. D., & Tinsley, R. C. (1997). Melanin deposition in the gut of the monogenean Macrodactylus polypteri Malmberg 1957. International Journal for Parasitology, 27, 1323–1331.
Cone, D. K., & Dechtiar, A. O. (1984). Gyrodactylus fryi n sp. (Monogenea) from Esox masquinongy Mitchill in Ontario. Canadian Journal of Zoology, 62, 1089–1090.
Crawshaw, G. J. (1997). Disease in Canadian amphibian populations. Herpetological Conservation, 1, 258–270.
Dodd, K. C. Jr., Gunzburger, M. S., Barichivich, W. J., & Staiger, J. S. (2004). Southeast amphibian research and monitoring initiative (National Wildlife Refuges Summary Report for 2004). United States Geological Survey. Gainsville, FL: Florida Integrated Science Center. http://armi.usgs.gov/SEARMI2004Report.pdf.
Geets, A., Appleby, C., & Ollivier, F. (1999). Host-dependent and seasonal variation in opisthaptoral hard parts of Gyrodactylus cf. arcuatus from three Pomatoschistus spp. and G. arcuatus from Gasterosteus arculeatus: A multivariate approach. Parasitology, 119, 27–40.
Green, D. E., & Dodd, C. K., Jr. (2007). Presence of amphibian chytrid fungus Batrachochytrium dendrobatidis and other amphibian pathogens at warm-water fish hatcheries in southeastern North America. Herpetological Conservation and Biology, 2, 43–47.
Gunzburger, M. S., Dodd, C. K. Jr., Barichivich, W. J., & Staiger, J. S. (2005). Southeast amphibian research and monitoring initiative (2005 Annual Report). United States Geological Survey. Gainsville, FL: Florida Integrated Science Center. http://fl.biology.usgs.gov/armi/2005_Annual_Report/2005_annual_report.html.
Herman, C. A. (1992). Endocrinology. In M. E. Feder & W. W. Burggren (Eds.), Environmental physiology of the amphibians (pp. 40–54). Chicago: University of Chicago Press.
Huyse, T., Audenaert, V., & Volckaert, F. A. M. (2003). Speciation and host–parasite relationships in the parasite genus Gyrodactylus (Monogenea, Platyhelminthes) infecting gobies of the genus Pomatoschistus (Gobiidae, Teleostei). International Journal for Parasitology, 33, 1679–1689.
Leblanc, J., Hansen, H., Burt, M., & Cone, D. (2006). Gyrodactylus neili n. sp. (Monogenea: Gyrodactylidae), a parasite of chain pickerel Esox niger Lesueur (Esocidae) from freshwaters of New Brunswick, Canada. Systematic Parasitology, 65, 43–48.
Malmberg, G. (1970). The excretory systems and the marginal hooks as a basis for the systematics of Gyrodactylus (Trematoda, Monogenea). Arkiv För Zoologie, 23, 1–237.
Malmberg, G. (1993). Gyrodactilidae et gyrodactylose des salmonidae. Bulletin Français de la Pêche et de la Pisciculture, 328, 5–46.
Matějusová, I., Gelnar, M., McBeath, A. J. A., Collins, C. M., & Cunningham, C. O. (2001). Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). International Journal for Parasitology, 31, 738–745.
Mizelle, J. D., Kritsky, D. C., & Bury, R. B. (1968). Studies on the monogenetic trematodes. XLI. Gyrodactylus ensatus sp. n., the first species of the genus described from Amphibia. Journal of Parasitology, 54, 281–282.
Mizelle, J. D., Kritsky, D. C., & McDougal, H. D. (1969). Studies on monogenetic trematodes. XLII. New species of Gyrodactylus from Amphibia. Journal of Parasitology, 55, 740–741.
Nieto, N. C., Camann, M. A., Foley, J. E., & Reiss, J. O. (2007). Disease associated with integumentary and cloacal parasites in tadpoles of northern red-legged frog Rana aurora aurora. Diseases of Aquatic Organisms, 78, 61–71.
Prudhoe, S., & Bray, R. A. (1982). Platyhelminth parasites of the Amphibia. Oxford: Oxford University Press, 217 pp.
Stuart, S. N., Chanson, J. S., Cox, N. A., Young, B. E., Rodrigues, A. S. L., Fischman, D. L., et al. (2004). Status and trends of amphibian declines and extinctions worldwide. Science, 306, 1783–1786.
Stunkard, H. W., & Dunihue, F. W. (1933). Gyrodactylus as a parasite of the tadpoles of Rana catesbeiana. Journal of Parasitology, 20, 137.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Vojtkova, L. (1989). The occurrence of the representatives of the class Monogenea in amphibians in Europe. Scripta Facultatis Scientarium Naturalium Universitatis Purkynianae Brunensis, 19, 331–338.
Volgar-Pastukhova, L. G. (1959). Parasitic fauna of the Anura in the Danube delta. In Y. I. Polyanksy (Ed.), Ecological parasitology (pp. 58–95). Leningrad: Leningradskogo Universiteta. (In Russian).
Wootton, D. M., Ryan, K. A., Demaree, R. S., & Critchfield, R. L. (1993). A new species of Gyrodactylus (Monogenea: Monopisthocotylea) on tadpoles of Rana catesbeiana from California, U.S.A. Transactions of the American Microscopical Society, 112, 230–233.
You, P., Easy, R. H., & Cone, D. K. (2008). Gyrodactylus parvae n. sp. (Monogenea) from the fins and body surface of Pseudorasbora parva (Cyprinidae) in central China. Comparative Parasitology, 75, 28–32.
Ziętara, M., Huyse, T., Lumme, J., & Volckaert, F. A. M. (2002). Deep divergence among subgenera of Gyrodactylus inferred from rDNA ITS region. Parasitology, 124, 39–52.
Ziętara, M. S., & Lumme, J. (2002). Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea, Gyrodactylidae). Evolution, 56, 2445–2458.
Zug, G. R. (1993). Herpetology: An introductory biology of amphibians and reptiles. San Diego, California: Academic Press, 527 pp.
Acknowledgements
This work was partly funded by the Natural Science and Engineering Research Council of Canada through Discovery Grants awarded to D.K.C. and J.D.M., an NSERC Postgraduate Scholarship (PGS M) awarded to L.P., and funds from the Pesticide Science Fund (PSF, Environment Canada) to D.J.M. and Bruce Pauli. T.H. is funded through a postdoctoral grant of the Research Foundation—Flanders (FWO-Vlaanderen). The authors thank Dr Eric Hoberg for arranging loan of museum material, Dr David E. Green for sharing field data and Dr Rodney Bray for sharing records. Ken Doe and Paula Jackman (Environment Canada) are gratefully acknowledged for providing the tadpoles, as is Bruce Pauli (Environment Canada) for coordinating the PSF project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paetow, L., Cone, D.K., Huyse, T. et al. Morphology and molecular taxonomy of Gyrodactylus jennyae n. sp. (Monogenea) from tadpoles of captive Rana catesbeiana Shaw (Anura), with a review of the species of Gyrodactylus Nordmann, 1832 parasitising amphibians. Syst Parasitol 73, 219–227 (2009). https://doi.org/10.1007/s11230-009-9183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-009-9183-9