Abstract
We describe a new species, Gyrodactylus ginestrae n. sp., a parasite of the big-scale sand smelt (Atherina boyeri) from the Black Sea. This is the third monogenean species known from this fish host, found at 70% prevalence, but at relatively low abundance (1.9), on fish gills and fins. The new species is, both morphologically and genetically, most similar to G. salinae, which parasitizes the killifish Aphanius fasciatus (Cyprinodontidae) in the Mediterranean region. These species differ in the size of the haptoral hard parts and the number of small spines of the male copulatory organ. For molecular characterization, the internal transcribed spacer 1 (ITS1), 5.8S rRNA gene, and the internal transcribed spacer 2 (ITS2) were sequenced, completed by a fragment of the COII gene, thereby representing the first molecularly characterized gyrodactylid species from the Black Sea. Phylogenetic reconstruction based on the ITS1–5.8S–ITS2 sequence data indicated the position of G. ginestrae n. sp. in the marine “rugiensis” group of G. (Paranephrotus) and G. (Neonephrotus) subgenera which is part of the monophyletic “long ITS1” group. Taking into account the similarity of G. ginestrae n. sp. to several monogeneans from the Atlantic and Mediterranean regions, we suggest the Boreal-Atlantic origin of this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Atheriniformes Rosen, 1964 includes small inshore fish inhabiting shallow waters (Quignard and Pras 1986), forming together with the orders Cyprinodontiformes Berg, 1940 and Beloniformes Berg, 1940 the Superorder Atherinomorphae (= Atherinomorpha sensu Greenwood et al., 1966) (Betancur-R et al. 2017). Of the five species of the genus Atherina currently known, three inhabit European marine and brackish waters (Quignard and Pras 1986), the sand smelt (Atherina presbyter Cuvier, 1829), the Mediterranean sand smelt (Atherina hepsetus L., 1758), and the big-scale sand smelt (Atherina boyeri Risso, 1810). Recent molecular data from Mediterranean populations reveal the existence of three forms of A. boyeri (lagoon/freshwater and two exclusively marine), indicating that A. boyeri is a species complex, but formal description has yet to be performed (Francisco et al. 2011).
The big-scale sand smelt, A. boyeri sensu lato, is a small demersal fish with its range in the Eastern Atlantic and throughout the Mediterranean and Black Seas (Froese and Pauly 2019). It inhabits marine and brackish/freshwater environments, corresponding to the different forms mentioned above (Francisco et al. 2011). The brackish/freshwater forms inhabit lower reaches of rivers and some lakes, where it has established permanent freshwater populations (Kottelat and Freyhof 2007). Recently, it has actively spread into the range of fresh waters of the Black Sea basin (Gençoğlu et al. 2017; Kvach and Kutsokon 2017).
The Black Sea is a part of the Mediterranean region, inhabited by Boreo-Atlantic and Mediterranean fauna, as well as by relict Ponto-Caspian fauna (Zaitsev and Mamaev 1997; Zaitsev 1998). This water body, including also the Sea of Azov (just a gulf of the Black Sea from an oceanographic view), is a distinctive region because of its low salinity ranging around 18‰ (Zenkevich 1963). Part of the Black Sea fish fauna is considered as having Mediterranean origin, penetrated the Black Sea about 7000 years ago (Zaitsev and Mamaev 1997). Traditionally, Mediterranean taxa, such as Atherina spp., Syngnathus spp., and Pomatoschistus spp., are assigned to this group (Zaitsev and Mamaev 1997).
The monogenean fauna of Black Sea fishes consists of at least 40 species (Gaevskaya and Dmitrieva 1997; Sarabeev et al. 2013). Among them, two species have been reported from the big-scale sand smelt: Gyrodactylus alviga Gaevskaya & Dmitrieva, 1997 and Gyrodactylus atherinae Bychowsky, 1933 (Roman 1956; Gaevskaya and Dmitrieva 1997; Kvach and Drobiniak 2017). While G. alviga is known from a wide range of fish species across taxonomically distant orders, G. atherinae appears specific to the big-scale sand smelt, recorded in the offshore northern Black Sea (Gerasev and Dmitrieva 2004) and the Caspian Sea (Semenova et al. 2007). Despite extensive surveys, neither of these parasites has ever been registered in big-scale sand smelt in the other parts of its host range such as the Adriatic and Tyrrhenian Seas or the Sea of Marmara (Sasal et al. 1997; Çolak 2013; Culurgioni et al. 2014; Radujković and Šundić 2014). Moreover, all known monogenean parasites of the species of Atherina have been described inside the Ponto-Caspian zone, from A. boyeri of Black Sea origin.
In the northwestern Black Sea, the specimens identified as G. alviga were found on fins of A. boyeri (Kvach and Drobiniak 2017), but illustrations were not provided; therefore, the species identification needs confirmation. The aim of our study was to provide a morphological description of the new Gyrodactylus species, complemented with molecular data (ITS rDNA and mitochondrial COII sequences). Molecular characterization of G. ginestrae n. sp. was performed by using both rDNA and mitochondrial markers. The combination of mtDNA and rDNA markers is commonly used on platyhelminths for phylogenetic and phylogeographic studies or for species identification. First, we used the fragment of nuclear ribosomal spacers regions (ITS1–5.8S–ITS2), which represents the most often used and highly effective molecular marker for species description and inferring phylogenetic relationships in the Gyrodactylidae (Ziętara et al. 2002; Matějusová et al. 2003; Paladini et al. 2011a). According to study of Bueno-Silva and Boeger (2014), a fragment of the cytochrome oxidase II (COII) gene was chosen as an additional molecular marker for barcoding of viviparous gyrodactylids. In addition, we analyzed the phylogenetic position of Gyrodactylus ginestrae n. sp. within a group of selected gyrodactylids mainly from the Mediterranean region and phylogenetically related fish hosts.
Material and methods
Specimen collection
The fish were sampled in the Gulf of Odessa (46.409640, 30.762071), Black Sea, Ukraine, using a dipnet in April 2017. In total, 20 individuals of the big-scale sand smelt were transported alive in aerated cans to the laboratory of the Odessa Center of Southern Scientific Research Institute of Marine Fisheries and Oceanography, where the fish are dissected for monogenean parasites within the 2 days after capture (Kvach et al. 2016).
Each fish was measured before dissection (standard length, SL, to the nearest 1 mm), with mean ± S.D. of 7.5 ± 0.8 cm and range 6.5–9.2 cm. The fins, skin, and gills were examined for monogeneans using a stereomicroscope Crystal-45 (Konus, Italy). Collected parasites were mounted in glycerine-ammonium-picrate for morphological study (Malmberg 1957). Holotype and paratype specimens were dehydrated in ethanol and mounted in Canada Balsam for museum deposition. A subsample of collected specimens was cut into two parts: posterior and anterior. The posterior part was mounted in glycerine-ammonium-picrate as described above, and the anterior part was preserved in 96% ethanol for further molecular analysis. In total, 22 specimens were collected; out of them, 16 specimens were subjected to morphological and 11 to molecular analyses, while 8 specimens were used both for morphology (only haptor) and molecular analysis.
Parasite individuals were characterized according to the shape and size of the haptoral hard parts (hamuli, connective bars, and marginal hooks) using a light microscope (Olympus BX51) equipped with a phase contrast and differential interference contrast. Drawings of haptoral hard components were made with the aid of a drawing attachment and phase-contrast optics. Measurements were obtained using the digital image analysis package MicroImage 4.0 for Windows (Olympus Optical co., Hamburg, Germany). All measurements are presented in micrometers. Nine morphological characters of the hamuli, ventral, and dorsal bars, along with seven characters of marginal hooks (MH), were measured according to Shinn et al. (2004), supplemented by length and width of the whole body, haptor, and male copulatory organ (MCO) (Table 1).
The parasitological indices follow Bush et al. (1997): prevalence (P, %), mean intensity (MI), intensity range (IR, as minimum–maximum), mean abundance (A). Standard deviation (sd) was calculated for means.
DNA extraction, amplification, and sequencing
Anterior parts of eleven specimens of Gyrodactylus ginestrae n. sp. collected from A. boyeri were placed individually in a 1.5 ml Eppendorf tube with 95% ethanol for genomic DNA extraction. Total genomic DNA of each individual was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the protocol for purification of total DNA from animal tissues. In order to make comparisons with other Gyrodactylus species, we amplified and sequenced widely used markers in gyrodactylids phylogenetics, comprising the 3′ end of the 18S rRNA gene, internal transcribed spacer 1 (ITS1), the 5.8S rRNA gene, the internal transcribed spacer 2 (ITS2), and the 5´end of the 28S rRNA gene. The primer pairs ITS1A (5′-GTAACAAGGTTTCCGTAGGTG-3′) and ITS2 (5′-TCCTCCGCTTAGTGATA- 3′) (Matějusová et al. 2001) were used. The amplification reaction was performed in a final volume of 25 μl, containing of 1xPCR buffer (Fermentas), 1.5 mM MgCl2, 200 μM of each dNTP, 0.5 μM primer, 1 μl of DNA, and 1.5 UTaq Polymerase (Fermentas). The PCR was carried out in the Mastercycler ep gradient S (Eppendorf) using the following steps: an initial denaturation at 96 °C for 3 min, followed by 39 cycles of denaturation at 95 °C for 50 s, annealing at 52 °C for 50 s and extension at 72 °C for 50 s, and a final elongation at 72 °C for 7 min. A fragment of the COII gene was amplified using degenerated primers cox2F (5′-TACAYAYCGCCCGTCAAYYTCG-3′) and cox2R (5′-AATAMWKATWGGCATRWAAGARTG-3′) following the conditions described in Bueno-Silva and Boeger (2014). All PCR products were electrophoresed on 1.5% agarose gels strained with Good View (SBS Genetech, Bratislava, Slovakia) and then were purified using ExoSAP-IT™ (Affymetrix Inc., Santa Clara, USA), following the manufacturer’s protocol. The purified PCR products were sequenced directly in both directions using the same primers as in the amplification reaction. Moreover, the internal primer ITSR3A (5′-GAGCCGAGTGATCCACC-3′) (Matějusová et al. 2001) complementary to sequence at the 5′ end of 5.8S gene was used for sequencing of ITS. Sequencing was carried out using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystem by Thermo Fisher Scientific, Prague, Czech Republic) and an Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems). The DNA sequences were assembled and edited using Sequencer software (Gene Codes Corp., Ann Arbor, MI, USA). The sequences were subjected to a BLAST search (Altschul et al. 1997) against GenBank for species identification. The uncorrected p-distances were computed in MEGA X (Kumar et al. 2018). The newly obtained sequences of Gyrodactylus ginestrae n. sp. were deposited in GenBank (ITS rDNA: MK550602; COII: MN061575–MN061581).
To provide phylogenetic comparison, a sample of the Ponto-Caspian monogenean, Gyrodactylus proterorhini Ergens, 1967, was collected from a western tubenose goby, Proterorhinus semilunaris (Heckel, 1837), samples inside the Ponto-Caspian zoogeographic zone, in the Danube River near Vidin, Bulgaria (ITS rDNA: MK584285). Gyrodactylus proterorhini was originally described from Proterorhinus marmoratus from the Middle Danube (Ergens 1967). That time, P. semilunaris was considered as a junior synonym of P. marmoratus. Recently, P. semilunaris is re-erected as a valid species with the type locality in the Middle Danube (Neilson and Stepien 2009), while P. marmoratus is absent in the fresh waters, but present only in the Black Sea.
Phylogenetic analyses based on the ITS1–5.8S–ITS2 rDNA
To determine the phylogenetic relationships of Gyrodactylus ginestrae n. sp. with other species of Gyrodactylus, the phylogenetic analysis based on the ITS1–5.8S–ITS2 rDNA sequences were conducted using maximum likelihood (ML) and Bayesian inference (BI) methods. A total of 19 selected Gyrodactylus species collected mainly from fish hosts in the Mediterranean and Atlantic regions and/or from phylogenetically related hosts (Cyprinodontiformes) (for details, see Table 1) was included into phylogenetic reconstruction. Three monogenean species, Diplogyrodactylus martini, Gyrodactyloides bychowskii, and Macrogyrodactylus heterobranchi, were used as outgroup. Sequences were aligned in MAFFT v. 7 (Katoh et al. 2019) and optimized manually in BioEdit (Hall 1999). The alignment was trimmed using trimAl v1.3. (Capella-Gutiérrez et al. 2009). The best fitting substitution model of evolution was determined using the software JModeltest v2.2.1 (Darriba et al. 2012) based on the Bayesian Information Criterion (BIC). ML analysis was conducted using the program IQ-TREE (Nguyen et al. 2015) as implemented in W-IQ-TREE (Trifinopoulos et al. 2016) under the General Time Reversible (GTR) model with gamma distribution (+ G) and invariable sites (+ I) and four gamma-rate categories. Nodal support was assessed through 10,000 ultrafast bootstrap (UFBoot) (Minh et al. 2013) and 1000 Shimodaira-Hasegava-like approximate likelihood ratio test (SH-aLRT) (Guindon et al. 2010) replicates. BI analysis was performed in MrBayes 3.2.1 (Ronquist et al. 2012) under the GTR+I+G model. Four simultaneous chains (one cold and three heated) of Markov chain Monte Carlo (MCMC) algorithm was run twice for 10 million generations. Tree topologies were sampled every 100 generations, whereby the first 25% of trees from each run were discarded as burn-in. The remaining trees were used to construct majority-rule consensus trees and determine the Bayesian posterior probability (BPP) for each clade. The trees were visualized and edited in FigTree ver. 1.4.3. (Rambaut 2017).
Results
Gyrodactylid parasites collected from 20 big-scale sand smelt were observed on fins and gills, with prevalence of 70% (14 fish infected), mean intensity of 2.7 ± 2.1, intensity range 1–8, and abundance of 1.9. All specimens represented a morphologically similar species which did not correspond to any other Gyrodactylus species known from the Black Sea/Mediterranean region. Comparative measurements for the new species, other two gyrodactylids known from big-scale sand smelt, G. atherinae collected near Karadag (Crimea, northeastern Black Sea), reported by Gerasev and Dmitrieva (2004), and G. alviga collected near Sevastopol (Crimea, northwestern Black Sea) (Gaevskaya and Dmitrieva 1997), and with morphologically and genetically similar species, Gyrodactylus salinae, collected from Aphanius fasciatus (Cyprinodontidae) in Cervia Saline, Adriatic Sea (Paladini et al. 2011b), are presented in Table 2.
Family Gyrodactylidae Cobbold, 1864
Genus Gyrodactylus von Nordmann, 1832
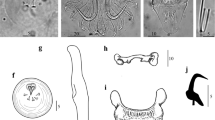
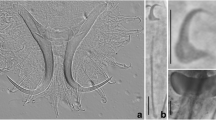
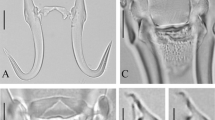
Gyrodactylus ginestrae n. sp. (Figs. 1 and 2)
Type host and locality: Atherina boyeri, Gulf of Odessa (46.409640, 30.762071), Black Sea, Ukraine
Site on the host: fins, gills
Type specimens: Holotype and one paratype (acc. No. IPCAS M-701) are deposited in the helminthological collection at the Institute of Parasitology, Academy of Sciences of the Czech Republic, České Budějovice.
Material examined: 16 flattened specimens (morphology), 11 ethanol preserved specimens (DNA analysis)
DNA reference sequences:
The 1204 bp sequence encoding partial 18S (17 bp), complete ITS1 (613 bp), 5.8S (157 bp), ITS2 (401 bp), and partial 28S (16 bp) is deposited in GenBank under accession No. MK550602. The partial COII sequences of 511 bp are deposited in GenBank under accession numbers (MN061575–MN061581).
Etymology: The specific epithet has a root after Ginestra, the medieval Byzantine name of the region around today’s Odessa, SW Ukraine.
Morphological description
General morphology based on 3–16 specimens (measurements shown in Table 2): body small, elongate, with length 385 ± 63 (259–483) comprising prohaptor and opisthaptor and width 69 ± 11 (51–88) at midbody. Haptor circular, 61 ± 10 (44–76) long, 61 ± 11 (41–77) wide. Hamuli with proportionately short straight roots, total length 42 ± 1.6 (40–44), shaft length 28 ± 1.4 (26–30), root length 17 ± 0.9 (15–19), point length 20 ± 1.1 (18–22), and hamulus aperture angle of 35 ± 2 (31–38). Dorsal bar simple 1 ± 0.2 (1–2) long, 17 ± 1 (15–19) wide. Ventral bar 4.8 ± 0.5 (4.1–5.9) long, 19.8 ± 1.3 (17.5–21.9) wide, membrane triangular. Anterolateral processes of ventral bar less prominent, projecting laterally. Marginal hook total length 29 ± 1 (27–30), shaft length 23 ± 1 (21–24). Marginal hook sickles crescent-shaped, toe pointed, short; sickle length 5.7 ± 0.2 (5.3–6.0), 2.6 ± 0.2 (2.3–2.9) wide distally, 3.7 ± 0.2 (3.4–4.0) wide proximally, aperture 5.2 ± 0.3 (4.8–5.6); base with distinct rounded heel. MCO spherical, located laterally to pharynx, 17 ± 0.6 (16–17) long and 13 ± 1.1 (12–15) wide, observed on 4 specimens. MCO armed with 1 principal spine and 8 smaller spines in a single row.
Remarks
Gyrodactylus ginestrae n. sp. is larger than G. atherinae in total body length, all measurements of hamuli, ventral bar width and marginal hook total length, but smaller in marginal hook sickle length (Table 2). The hamuli of G. ginestrae are more robust than those of G. atherinae and G. alviga, the latter two (G. atherinae in particular) showing narrowing of the roots towards the distal edge (Fig. 3A). The marginal hook sickles of all three species compared are morphologically distinct, being more slender in G. atherinae compared to other species (Fig. 3B). The newly described species is the most morphologically similar to G. salinae, which is larger in total length, shaft and point lengths of hamuli (Fig. 3A), ventral bar width, marginal hook sickle length and distal width, and squarer heel (Fig. 3B) and it has larger number of small spines in MCO (Paladini et al. 2011b) compared to G. ginestrae n. sp.
Molecular characterization
The identical sequences of the ITS fragment were obtained from 4 specimens. The results of a BLASTn search (Altschul et al. 1997) of the ITS1–5.8S–ITS2 fragment revealed no identical hits with entries in GenBank (May, 2019). Gyrodactylus ginestrae n. sp. appeared most closely related to G. anguillae (91.81%, acc. No. AB063294) obtained from the European eel (Anguilla anguilla, Anguilliformes) in Spain and Gyrodactylus salinae (91.42%, acc. No. JF950559 from Aphanius fasciatus (Cyprinodontiformes) collected in Italy. When the 5.8S gene (157 bp) was submitted to a BLASTn search separately, it was found to be identical to several gyrodactylid species, namely, G. anguillae (AB063291), G. bubyri (KU355879), G. gracilihamatus (AF484531,32), G. hildae (FJ231869), G. jussii (AY061982), G. micropsi (AF328868), G. rugiensis (AF328870), and G. rugiensoides (AJ427414).
Seven different COII sequences were obtained from eight specimens of G. ginestrae n. sp. The results from the BLASTn search revealed no identical hits with entries in GenBank (May, 2019). Average intraspecific p-distance of COII sequences was 1%.
Phylogenetic analysis
The phylogenetic tree based on the ML analysis is shown in Fig. 4. The final ITS1–5.8S–ITS2 alignment constituted by 22 sequences was 841 bp long and comprised 569 variable sites. Both methods of phylogenetic reconstruction recovered identical phylogenetic relationships among the species studied, with several well-supported nodes. Gyrodactylus ginestrae n. sp. clusters with high bootstrap support (100% of bootstrap values, 1 of pp) in a group consisting of several marine Gyrodactylus species collected from Mediterranean areas and G. leptorhynchi infecting the bay pipefish Syngnathus leptorhynchus (Syngnathiformes), from the Pacific coast of North America. These Mediterranean Gyrodactylus species were isolated from various fish hosts (Anguilliformes, Cyprinodontiformes, Gobiiformes), and, except for G. salinae, share identical 5.8S sequence with G. ginestrae n. sp.
The maximum likelihood tree of selected Gyrodactylus species based on ITS1–5.8S–ITS2 rDNA sequences. Support values beside branches represented Shimodaira-Hasegawa-like approximate likelihood ratio test (SH-aLRT)/ultrafast bootstrap (UFBoot), both implemented in IQ-TREE/ Bayesian inference (posterior probability) implemented in MrBayes. Values < 0.90 for BI and < 70% for ML are indicated by dashes (–). The phylogram is rooted with Diplogyrodactylus martini, Gyrodactyloides bychowskii, and Macrogyrodactylus heterobranchi. Branch lengths indicate the expected number of substitutions per site
Discussion
Gyrodactylus ginestrae n. sp. is the fifteenth described gyrodactylid species from the Black Sea, the first one molecularly characterized, and the third Gyrodactylus parasitizing Atherina (see details in Lisitsyna and Miroshnichenko 2008). In contrast to another specific parasite of A. boyeri found only on gills, G. atherinae (Gerasev and Dmitrieva 2004), the newly described species has been located both on fins and gills. Both morphologically and genetically, it is most similar to G. salinae, which parasitizes the fins and skin (occasionally on the gills) of the killifish Aphanius fasciatus (Cyprinodontidae) from a hyperhaline lagoon in Italy (Paladini et al. 2011b), differing from G. ginestrae in length of haptoral hard parts and number of small spines of the male copulatory organ. Such similarity may reflect phylogenetic proximity between the host species, i.e., representatives of Atheriniformes and Cyprinodontiformes, both belonging to the Superorder Atherimonomorphae (Betancur-R et al. 2017). On the other hand, the newly described species differs from Gyrodactylus notatae King et al. 2009, a parasite of the Atlantic silverside Menidia menidia, an atheriniform marine fish from North America (Sargent et al. 2008). This may indicate the distinguishing of the G. ginestrae from the Western Atlantic group. It is plausible that the geographic origin of the hosts is more important than their taxonomic relations.
In contrast to the wide use of the ITS region, COII has been sequenced only for a few species of Gyrodactylus (e.g., Bueno-Silva and Boeger 2014; Huyse et al. 2017; Vanhove et al. 2018; Xavier et al. 2015). Both markers display differences mainly in intra- and interspecific sequence variation due to their different molecular evolution. While there is little or no intraspecific variation observed for the ITS region in gyrodactylids (Cable et al. 1999; Vanhove et al. 2013; García-Vásquez et al. 2015; Tu et al. 2015), the intraspecific variation of COII sequences of Gyrodactylus varies from 0 to 3% (Bueno-Silva and Boeger 2014; Huyse et al. 2017). Differences in intraspecific variation of these two markers have also been revealed in the present study. The ITS sequence of G. ginestrae n. sp. displayed no intraspecific variation, in contrast to COII, where 1% of intraspecific variation was observed.
Phylogenetic reconstruction based on the ITS1–5.8S–ITS2 sequence data indicated the position of G. ginestrae n. sp. in the marine “rugiensis” group of G. (Paranephrotus) and G. (Neonephrotus) sub-genera which is part of the monophyletic “long ITS1” group. Ziętara et al. (2002) observed that the genetic differences of the 5.8S locus provide objective criteria to separate Gyrodactylus (sub) genera (Ziętara et al. 2002), as defined by (Malmberg 1970) on the basis of the excretory system. Each Gyrodactylus subgenus should possess a unique sequence of the 5.8S gene. The 5.8S fragment of all Gyrodactylus species of “rugiensis” group observed in the present study is identical, or in case of G. salinae, near-identical (BLASTn searches using the 5.8S fragment of G. salinae did not reveal any identical hits, and the highest similarity was observed with the members of this group). The uncorrected p-distance between species of this group varied from 0.06 to 12.62%, which corresponds to the genetic distance between Gyrodactylus species sharing identical 5.8S sequences (Huyse and Volckaert 2002; Paladini et al. 2011b).
Based on the length of ITS1 (613 bp), G. ginestrae n. sp. falls into the category of “long ITS1” group (535–688 bp) (Cable et al. 1999; Ziętara et al. 2002) (Fig. 3), similarly to species clustered based on the ITS region: a parasite of European eel, G. anguillae and the parasites of annual gobies of family Gobionellidae, G. bubyri, and G. rugiensis. On the other hand, the euryhaline species G. proterorhini, parasitizing Ponto-Caspian gobies, the only Gyrodactylus species of Ponto-Caspian origin analyzed, clustered with species of the “short ITS1” group.
The similarities between the Black Sea and Atlantic fauna have already been recorded, based on the phylogeny of cryptogonimid trematodes, such as Aphalloides coelomicola and Timoniella imbutiforme (Kvach et al. 2017, 2018). Both species are connected with the annual gobies in their life cycles. The gobionellid fishes of the genera Pomatoschistus and Knipowitschia appeared in the Sarmatian period (Middle Miocene) (Schwarzhans et al. 2017a). One of the species mentioned above, T. imbutiforme, is also a parasite of Atherina spp. (Maillard 1973; Kvach et al. 2018). In contrast to the cryptogonimids, gyrodactylids such as G. atherinae or the newly described species G. ginestrae n. sp. have not been recorded from the Mediterranean and Atlantic coasts of Europe, nor has, for example, the closely related G. bubyri. We consider both species as Boreal relict species in the Black Sea fauna, which are probably extinct in the rest of Europe, or, at least their presence needs confirmation. For example, G. bubyri, previously known only from the Ponto-Caspian region, has recently been registered in the Strymon River in Greece (Vanhove et al. 2014).
In the Early Miocene, the Eastern Parathetys was located where the Black Sea is now (Popov et al. 2004). This water body was inhabited by 5 extinct species of Atherina (Schwarzhans et al. 2017b). They probably were the source of modern Atherina populations in the Black Sea. The annual gobies, i.e., Knipowitschia and Pomatoschistus, inhabiting Tarkhanian Sea, appeared later, in the Middle Miocene (Schwarzhans et al. 2017a). But, the Ponto-Caspian fauna, represented by, for example, Proterorhinus gobies, appeared just after Carangian Crisis, during Konkian and Eastern Sarmatian Transgressions. We therefore consider that G. ginestrae n. sp. is related to the group of Boreal-Atlantic relict species, together with its host, A. boyeri. This group in the Black Sea fauna consists of the parasites of the Boreal-Atlantic relicts, such as Pomatoschistus and Knipowitschia including two digeneans, A. coelomicola and T. imbutiforme (Kvach et al. 2017, 2018). Another parasite of Knipowitschia, G. bubyri, appears to extend this group according to current data.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402. https://doi.org/10.1093/nar/25.17.3389
Barson M, Přikrylová I, Vanhove MPM, Huyse T (2010) Parasite hybridization in African Macrogyrodactylus spp. (Monogenea, Platyhelminthes) signals historical host distribution. Parasitology 137(10):1585–1595. https://doi.org/10.1017/S0031182010000302
Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G (2017) Phylogenetic classification of bony fishes. BMC Evol Biol 17:162. https://doi.org/10.1186/s12862-017-0958-3
Bruno DW, Collins CM, Cunningham CO, MacKenzie K (2001) Gyrodactyloides bychowskii (Monogenea: Gyrodactylidae) from sea-caged Atlantic salmon Salmo salar in Scotland: occurrence and ribosomal RNA sequence analysis. Dis Aquat Organ 45(3):191–196. https://doi.org/10.3354/dao045191
Bueno-Silva M, Boeger WA (2014) Neotropical Monogenoidea. 58. Three new species of Gyrodactylus (Gyrodactylidae) from Scleromystax spp. (Callichthyidae) and the proposal of COII gene as an additional fragment for barcoding gyrodactylids. Folia Parasitol 61(3):213–222. https://doi.org/10.14411/fp.2014.028
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.7939/R3J38KV04
Cable J, Harris PD, Tinsley RC, Lazarus CM (1999) Phylogenetic analysis of Gyrodactylus spp. (Platyhelminthes: Monogenea) using ribosomal DNA sequences. Can J Zool 77(9):1439–1449. https://doi.org/10.1139/z99-069
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25(15):1972–1973
Çolak SÖ (2013) The helminth community of the sand smelt (Atherina boyeri Risso, 1810) from Lake Iznik, Turkey. J Helminthol 87(2):129–134. https://doi.org/10.1017/S0022149X11000770
Cone DK, Appy R, Baggett L, King S, Gilmore S, Abbott C (2013) A new gyrodactylid (Monogenea) parasitizing bay pipefish (Syngnathus leptorhynchus) from the Pacific coast of North America. J Parasitol 99(2):183–188. https://doi.org/10.1645/GE-3224.1
Culurgioni J, Sabatini A, De Murtas R, Mattiucci S, Figus V (2014) Helminth parasites of fish and shellfish from the Santa Gilla Lagoon in southern Sardinia, Italy. J Helminthol 88:489–498. https://doi.org/10.1017/S0022149X13000461
Darriba D, Taboada GL, Doallo R, Posada D (2012) JModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Francisco SM, Congiu L, von der Heyden S, Almada VC (2011) Multilocus phylogenetic analysis of the genus Atherina (Pisces: Atherinidae). Mol Phylogen Evol 61:71–78. https://doi.org/10.1016/j.ympev.2011.06.002
Froese R, Pauly D (2019) FishBase. World Wide Web electronic publication, www.fishbase.org, version 08/2019
Gaevskaya AV, Dmitrieva EV (1997) Review of Black Sea monogenean fauna. Ekologiya Morya 46:7–17 [in Russian with English summary]
García-Vásquez A, Razo-Mendivil U, Rubio-Godoy M (2015) Morphological and molecular description of eight new species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from poeciliid fishes, collected in their natural distribution range in the Gulf of Mexico slope, Mexico. Parasitol Res 114(9):3337–3355. https://doi.org/10.1007/s00436-015-4559-z
Gençoğlu L, Kırankaya ŞG, Yoğurtçuoğlu B, Ekmekçi FG (2017) Feeding properties of the translocated marine fish sand smelt Atherina boyeri Risso, 1810 (Atherinidae) in a freshwater reservoir. Acta Zool Bulg Suppl 9:131–138
Gerasev PI, Dmitrieva EV (2004) A redescription of Gyrodactylus atherinae Bychowsky, 1933 based on the collection of В.E. Bychowsky of 1947 from Atherina boyeri pontica in the Black Sea. Parazitologiya 38(6):562–565 [in Russian with English summary]
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Harris PD, Cable J (2000) Gyrodactylus poeciliae n. sp. and G. milleri n. sp. (Monogenea: Gyrodactylidae) from Poecilia caucana (Steindachner) in Venezuela. Syst Parasitol 47:79–85. https://doi.org/10.1023/A:1006413804061
Hayward CJ, Iwashita M, Ogawa K, Ernst I (2001) New evidence that Gyrodactylus anguillae (Monogenea) is another invading pest of anguillid eels. Biol Invasions 3:417–424
Huyse T, Volckaert FAM (2002) Identification of a host-associated species complex using molecular and morphometric analyses, with the description of Gyrodactylus rugiensoides n. sp. (Gyrodactylidae, Monogenea). Int J Parasitol 32:907–919. https://doi.org/10.1016/S0020-7519(02)00026-7
Huyse T, Pampoulie C, Audenaert V, Volckaert FAM (2006) First report of Gyrodactylus spp. (Platyhelminthes: Monogenea) in the Western Mediterranean Sea: molecular and morphological descriptions. J Parasitol 92(4):682–690. https://doi.org/10.1645/GE-690R.1
Huyse T, Oeyen M, Larmuseau MHD, Volckaert FAM (2017) Co-phylogeographic study of the flatworm Gyrodactylus gondae and its goby host Pomatoschistus minutus. Parasitol Int 66:119–125. https://doi.org/10.1016/j.parint.2016.12.008
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. https://doi.org/10.1093/bib/bbx108
King SD, Forest JJH, Cone DK (2009) Description of Gyrodactylus notatae n. sp. (Monogenea: Gyrodactylidae) from Menidia menidia (L.) (Actinopterygii: Atherinidae) in Nova Scotia, Canada. Syst Parasitol 74(1):23–27. https://doi.org/10.1007/s11230-009-9185-7
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Publications Kottelat, Cornol and Freyhof, Berlin
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Kvach Y, Drobiniak O (2017) The parasites of the big-scale sand-smelt, Atherina boyeri Risso, 1810 (Actinopterygii: Atherinidae), in the North-Western Black. Sci Bull Uzhgorod Univ (Ser Biol) 42:38–43 [in Ukrainian with English summary]
Kvach Y, Kutsokon Y (2017) The non-indigenous fishes in the fauna of Ukraine: a potentia ad actum. BioInvasions Rec 6(3):269–279. https://doi.org/10.3391/bir.2017.6.3.13
Kvach Y, Ondračková M, Janáč M, Jurajda P (2016) Methodological issues affecting the study of fish parasites. I. Duration of live fish storage prior to dissection. Dis Aquat Organ 119(2):107–115. https://doi.org/10.3354/dao02990
Kvach Y, Bryjová A, Sasal P, Winkler HM (2017) A revision of the genus Aphalloides (Digenea: Cryptogonimidae), parasites of European brackish water fishes. Parasitol Res 116(7):1973–1980. https://doi.org/10.1007/s00436-017-5480-4
Kvach Y, Bryjová A, Sasal P, Winkler HM (2018) The taxonomic and phylogenetic status of digeneans from the genus Timoniella (Digenea: Cryptogonimidae) in the Black and Baltic Seas. J Helminthol 92(5):596–603. https://doi.org/10.1017/S0022149X1700075X
Lisitsyna OI, Miroshnichenko AI (2008) Catalog of helminthes of Ukraine. Acanthocephala. Monogenea, Akademperiodika, Kiev [in Russian]
Lumme J, Ziętara MS (2018) Horizontal transmission of the ectoparasite Gyrodactylus arcuatus (Monogenea: Gyrodactylidae) to the next generation of the three-spined stickleback Gasterosteus aculeatus. Folia Parasitol 65:006. https://doi.org/10.14411/fp.2018.006
Maillard C (1973) Etude du cycle évolutif du Trématode: Acanthostomum imbutiforme (Molin, 1859) Gohar, 1934, parasite de Morone labrax (Linné, 1758). Ann Parasitol hum comp 48:33–46
Malmberg G (1957) Om forekomsten av Gyrodactylus pa svenska fiskar. Skrifter Utgivna av Sodra Sveriges Fiskeriforening 1956:19–76
Malmberg G (1970) The excretory systems and the marginal hooks as a basis for the systematics of Gyrodactylus (Trematoda, Monogenea). Arkiv Zool 23:1–235
Matějusová I, Gelnar M, McBeath AJA, Collins CM, Cunningham CO (2001) Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). Int J Parasitol 31:738–745. https://doi.org/10.1016/S0020-7519(01)00176-X
Matějusová I, Gelnar M, Verneau O, Cunningham CO, Littlewood DTJ (2003) Molecular phylogenetic analysis of the genus Gyrodactylus (Platyhelminthes: Monogenea) inferred from rDNA ITS region: subgenera versus species groups. Parasitology 127(6):603–611. https://doi.org/10.1017/S0031182003004098
Minh BQ, Nguyen MAT, von Haeseler A (2013) Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol 30(5):1188–1195. https://doi.org/10.1093/molbev/mst024
Neilson ME, Stepien CA (2009) Evolution and phylogeography of the tubenose goby genus Proterorhinus (Gobiidae: Teleostei): evidence for new cryptic species. Biol J Linn Soc 96:664–684. https://doi.org/10.1111/j.1095-8312.2008.01135.x
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274. https://doi.org/10.1093/molbev/msu300
Paladini G, Cable J, Fioravanti ML, Faria PJ, Di Cave D, Shinn AP (2009) Gyrodactylus orecchiae sp. n. (Monogenea: Gyrodactylidae) from farmed populations of gilthead seabream (Sparus aurata) in the Adriatic Sea. Folia Parasitol 56(1):21–28
Paladini G, Hansen H, Fioravanti ML, Shinn AP (2011a) Gyrodactylus longipes n. sp. (Monogenea: Gyrodactylidae) from farmed gilthead seabream (Sparus aurata L.) from the Mediterranean. Parasitol Int 60(4):410–418. https://doi.org/10.1016/j.parint.2011.06.022
Paladini G, Huyse T, Shinn AP (2011b) Gyrodactylus salinae n. sp. (Platyhelminthes: Monogenea) infecting the south European toothcarp Aphanius fasciatus (Valenciennes) (Teleostei, Cyprinodontidae) from a hypersaline environment in Italy. Parasit Vectors 4:100. https://doi.org/10.1186/1756-3305-4-100
Popov SV, Rögl F, Rozanov AY, Steininger FF, Shcherba IG, Kovac M (2004) Lithological-paleogeographic maps of Paratethys. Courrier Forschungs-Institut Senckenberg 250:1–46
Přikrylová I, Matějusová I, Musilová N, Gelnar M, Harris PD (2009) A new gyrodactylid (Monogenea) genus on gray bichir, Polypterus senegalus (Polypteridae) from Senegal (West Africa). J Parasitol 95(3):555–560. https://doi.org/10.1645/GE-1652.1
Quignard J-P, Pras A (1986) Atherinidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (eds) Fishes of the North-eastern Atlantic and the Mediterranean, Vol. 3. UNESCO, Paris, pp 1207–1210
Radujković BM, Šundić D (2014) Parasitic flatworms (Platyhelminthes: Monogenea, Digenea, Cestoda) of fishes from the Adriatic Sea. Natura Montenegrina 13(1):7–280
Rambaut A (2017) FigTree-version 1.4.3, a graphical viewer of phylo-genetic trees. Available from http://tree.bio.ed.ac.uk/software/figtree/ (12-2018 accessed)
Roman E (1956) Noi contribuții la cunoaşterea fauneli de Monogenee din R.P.R. Communicarile Academiei RPR 6(1):133–144
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2:efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Sarabeev V, Rubtsova N, Yang T, Balbuena JA (2013) Taxonomic revision of the Atlantic and Pacific species of Ligophorus (Monogenea, Dactylogyridae) from mullets (Teleostei, Mugilidae) with the proposal of a new genus and description of four new species. Vestnik Zoologii Suppl 28:1–113
Sargent PS, Methven DA, Hooper RG, McKenzie CH (2008) A range extension of the Atlantic silverside, Menidia menidia, to coastal waters of southwestern Newfoundland. Can Field-Natur 122(4):338–344. https://doi.org/10.22621/cfn.v122i4.641
Sasal P, Morand S, Guégan J-F (1997) Determinants of parasite species richness in Mediterranean marine fishes. Mar Ecol Prog Ser 149:61–71. https://doi.org/10.3354/meps149061
Schwarzhans W, Ahnelt H, Carnevale G, Japundžić S, Bradić K, Bratishko A (2017a) Otoliths in situ from Sarmatian (Middle Miocene) fishes of the Paratethys. Part III: tales from the cradle of the Ponto-Caspian gobies. Swiss J Palaeontol 136:45–92. https://doi.org/10.1007/s13358-016-0120-7
Schwarzhans W, Carnevale G, Bannikov AF, Japundžić S, Bradić K (2017b) Otoliths in situ from Sarmatian (Middle Miocene) fishes of the Paratethys. Part I: Atherina suchovi Switchenska, 1973. Swiss J Palaeontol 136:7–17. https://doi.org/10.1007/s13358-015-0111-0
Semenova NN, Ivanov VP, Ivanov VM (2007) Parasite fauna and diseases of fishes of the Caspian Sea. ASTU Press, Astrakhan [in Russian with English summary]
Shinn AP, Hansen H, Olstad K, Bachmann L, Bakke TA (2004) The use of morphometric characters to discriminate specimens of laboratory-reared and wild populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol 51:239–252. https://doi.org/10.14411/fp.2004.029
Stoyanov B, Huyse T, Pankov P, Georgiev BB (2016) Morphological and molecular identification of Gyrodactylus bubyri Osmanov, 1965 (Monogenea: Gyrodactylidae) from Caucasian dwarf goby, Knipowitschia caucasica (Berg) (Actinopterygii: Gobionellidae) from a Black Sea lagoon. Parasitol Res 115(4):1617–1625. https://doi.org/10.1007/s00436-015-4899-8
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44(W1):W232–W235. https://doi.org/10.1093/nar/gkw256
Tu X, Ling F, Huang A, Wang G (2015) An infection of Gyrodactylus kobayashii Hukuda, 1940 (Monogenea) associated with the mortality of goldfish (Carassius auratus) from central China. Parasitol Res 114:737–745. https://doi.org/10.1007/s00436-014-4241-x
Vanhove MP, Tessens B, Schoelinck C, Jondelius U, Littlewood DT, Artois T, Huyse T (2013) Problematic barcoding in flatworms: a case-study on monogeneans and rhabdocoels (Platyhelminthes). ZooKeys 365:355–379. https://doi.org/10.3897/zookeys.365.5776
Vanhove MPM, Economou AN, Zogaris S, Giakoumi S, Zanella D, Volckaert FAM, Huyse T (2014) The Gyrodactylus (Monogenea, Gyrodactylidae) parasite fauna of freshwater sand gobies (Teleostei, Gobioidei) in their centre of endemism, with description of seven new species. Parasitol Res 113(2):653–668. https://doi.org/10.1007/s00436-013-3693-8
Vanhove MPM, Briscoe AG, Jorissen MWP, Littlewood DTJ, Huyse T (2018) The first next-generation sequencing approach to the mitochondrial phylogeny of African monogenean parasites (Platyhelminthes: Gyrodactylidae and Dactylogyridae). BMC Genomics 19:520. https://doi.org/10.1186/s12864-018-4893-5
Xavier R, Faria PJ, Paladini G, van Oosterhout C, Johnson M, Cable J (2015) Evidence for cryptic speciation in directly transmitted gyrodactylid parasites of Trinidadian guppies. PloS One 10(1):e0117096. https://doi.org/10.1371/journal.pone.0117096
Zaitsev YP (1998) The bluest in the World. UN Publication, New York [in Russian]
Zaitsev Y, Mamaev V (1997) Biological diversity in the Black Sea: a study of change and decline. UN Publication, New York
Zenkevich LA (1963) Biology of the seas of the USSR. Izdatelsto AN SSSR, Moskva [in Russian]
Ziętara MS, Lumme J (2002) Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea, Gyrodactylidae). Evol 56(12):2445–2458. https://doi.org/10.1111/j.0014-3820.2002.tb00170.x
Ziętara MS, Lumme J (2003) The crossroads of molecular, typological and biological species concepts: two new species of Gyrodactylus Nordmann, 1832 (Monogenea: Gyrodactylidae). Syst Parasitol 55:39–52. https://doi.org/10.1023/A:1023938415148
Ziętara MS, Huyse T, Lumme J, Volckaert FA (2002) Deep divergence among subgenera of Gyrodactylus inferred from rDNA ITS region. Parasitology 124(1):39–52. https://doi.org/10.1017/S0031182001008939
Ziętara MS, Rokicka M, Stojanovski S, Lumme J (2010) Introgression of distant mitochondria into the genome of Gyrodactylus salaris: nuclear and mitochondrial markers are necessary to identify parasite strains. Acta Parasitol 55(1):20–28. https://doi.org/10.2478/s11686-010-0016-4
Acknowledgments
We thank Dr. Rodney A. Bray for proofreading of the manuscript.
Funding
This study received financial support through Project No. P505/12/G112 of the European Centre of Ichthyoparasitology, Grant Agency of the Czech Republic—Centre of Excellence.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Stephen A. Bullard
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kvach, Y., Ondračková, M., Seifertová, M. et al. Gyrodactylus ginestrae n. sp. (Monogenea: Gyrodactylidae), a parasite of the big-scale sand smelt, Atherina boyeri Risso, 1810 (Actinopterygii: Atherinidae) from the Black Sea. Parasitol Res 118, 3315–3325 (2019). https://doi.org/10.1007/s00436-019-06483-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06483-8