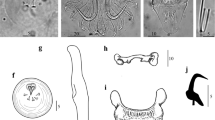
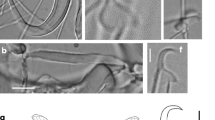
Abstract
Eight new species of Gyrodactylus are described from Poecilia mexicana, Poeciliopsis gracilis, Pseudoxiphophorus bimaculatus [syn. = Heterandria bimaculata], and Xiphophorus hellerii collected in the Nautla and La Antigua River Basins in Veracruz, and in the Tecolutla River Basin in Puebla, Mexico. Analyzing the morphology of the marginal hooks, Gyrodactylus pseudobullatarudis n. sp. and Gyrodactylus xtachuna n. sp. are both very similar to Gyrodactylus bullatarudis; Gyrodactylus takoke n. sp. resembles Gyrodactylus xalapensis; Gyrodactylus lhkahuili n. sp. is similar to Gyrodactylus jarocho; and both Gyrodactylus microdactylus n. sp. and Gyrodactylus actzu n. sp. are similar to Gyrodactylus poeciliae in that all three species possess extremely short shaft points. A hypothesis of the systematic relationships of the eight new Gyrodactylus species and some of the known gyrodactylids infecting poeciliids was constructed with sequences of the Internal Transcribed Spacers (ITS1 and ITS2) and the 5.8S ribosomal gene of the rRNA. Phylogenetic trees showed that the new and previously described species of Gyrodactylus infecting poeciliid fishes do not form a monophyletic assemblage. Trees also showed that the eight new species described morphologically correspond to well-supported monophyletic groups; and that morphologically similar species are also phylogenetically close. Additionally, we correct previous erroneous records of the presence of Gyrodactylus bullatarudis on wild Poecilia mexicana and Xiphophorus hellerii collected in Mexico, as re-examination of the original specimens indicated that these corresponded to Gyrodactylus pseudobullatarudis n. sp. (infecting Poecilia mexicana and Xiphophorus hellerii) and to Gyrodactylus xtachuna n. sp. (on Xiphophorus hellerii). Finally, given the widespread anthropogenic translocation of poeciliid fishes for the aquarium trade and mosquito control programs, as well as the existence of invasive, feral poeciliid populations worldwide, we discuss the possibility that gyrodactylid parasites could be introduced along with the fish hosts—this work provides taxonomic information to assess that possibility, as it describes parasites collected from poeciliid fishes within their native distribution range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Poeciliidae Garman, 1895 comprises three subfamilies (Poeciliinae, Procatopodinae, and Aplocheilichthyinae) of fish restricted to lowland fresh and brackish continental waters (Morales-Cazan and Albert 2012). Poeciliinae were originally distributed from southeastern USA to northeastern Argentina, also in Africa and Madagascar, and are one of the most dominant fish groups in Middle America and the West Indies (Miller 2005; Morales-Cazan and Albert 2012). In Mexico, 81 species of poeciliid fishes occur naturally (Miller 2005). Poeciliids have been extensively translocated, both with the aquarium trade of live-bearing fishes (including “guppies,” “mollies,” “platies,” and “swordtails”) and as part of mosquito control programs utilizing different Poecilia spp. and Gambusia spp.; and nowadays, introduced feral populations of poeciliids can be found in all continents except Antarctica (Pyke 2008). Poeciliid fishes have been shown to modify the ecological structure, function, and native species abundance of water bodies following invasion, and are considered “invasive species of concern” in the USA, Australia, and New Zealand (Holitzki et al. 2013). Invasive poeciliids have likewise been shown to exert negative ecological effects even in regions closer to their original distribution range: for instance, in the highlands of central Mexico where invasive guppies harass endemic, endangered, native goodeid fish (Valero et al. 2008).
Monogeneans of the genus Gyrodactylus von Nordmann, 1832 are skin and gill parasites of marine and freshwater fishes, of which 450+ species are known (Harris et al. 2004; Shinn et al. 2011). Up to date, only 11 species of Gyrodactylus have been recorded from poeciliid fishes (Table 1); most of the fish hosts belong to the subfamily Poeciliinae, with only three species from the African subfamily Aplocheilichthyinae known so far to harbor the gyrodactylid Gyrodactylus cytophagus Paperna, 1968. The first gyrodactylid to be described from a poeciliid host was Gyrodactylus bullatarudis Turnbull, 1956 from guppies, Poecilia reticulata held in an aquarium in Canada; this parasite was subsequently recorded in several poeciliids, including wild fish within their native range, as well as feral and captive hosts—see Table 1. A further two gyrodactylids, Gyrodactylus rasini Lucký, 1973 and Gyrodactylus turnbulli Harris, 1986 were described from aquarium-held poeciliids (Xiphophorus hellerii and Poecilia reticulata, respectively); and Gyrodactylus gambusiae Rogers and Wellborn, 1965 was described from Gambusia affinis reared in a hatchery. The remaining six gyrodactylids were described from wild poeciliid fishes collected within their native distribution ranges: Gyrodactylus costaricensis Kritsky and Fritts, 1970 from Poecilia sphenops; Gyrodactylus jarocho Rubio-Godoy, Paladini, García-Vásquez and Shinn, 2010 from Xiphophorus hellerii; Gyrodactylus milleri Harris and Cable, 2000 from Poecilia caucana; Gyrodactylus pictae Cable, van Oosterhout, Barson and Harris, 2005 from Micropoecilia [syn. = Poecilia] picta; Gyrodactylus poeciliae Harris and Cable, 2000 from Poecilia caucana; and Gyrodactylus xalapensis Rubio-Godoy, Paladini, García-Vásquez and Shinn, 2010 from Pseudoxiphophorus bimaculatus [syn. = Heterandria bimaculata]. Among these parasites, Gyrodactylus bullatarudis exhibits low host specificity, having been recorded from six poeciliid host species from the genera Gambusia, Poecilia, Pseudoxiphophorus [syn. = Heterandria], and Xiphophorus; and also from Xiphophorus hybrids. Gyrodactylus turnbulli has been recovered from fish belonging to two host genera, Poecilia and Poeciliopsis. The remaining gyrodactylids infecting poeciliids have only been recorded from one host species each.
During surveys of the parasite fauna of wild fishes in different rivers flowing into the Gulf of Mexico, eight new species of Gyrodactylus were recovered from four species of poeciliids collected within their native distribution ranges: Poecilia mexicana Steindachner, Poeciliopsis gracilis (Heckel), Pseudoxiphophorus bimaculatus Heckel, and Xiphophorus hellerii Heckel. Morphological description of the parasites is complemented with molecular data: sequences of the internal transcribed spacer (ITS) region of the nuclear ribosomal RNA (rRNA) were used to assess the phylogenetic position of the new species, by comparing them with those ITS sequences of gyrodactylids infecting poeciliids available in GenBank. Molecular data not only provide additional taxonomic information for a group of morphologically similar parasites (Shinn et al. 2011), but also allow establishing the phylogenetic relationships of newly described species of Gyrodactylus in the Americas to better known taxa, such as European parasites (Gilmore et al. 2012; Kvach et al. 2014; Vanhove et al. 2014; Ziętara and Lumme 2002).
Materials and methods
Sample collection and preparation
Between May 2013 and May 2014, poeciliid fishes (Poecilia mexicana, Poeciliopsis gracilis, Pseudoxiphophorus bimaculatus, and Xiphophorus hellerii) were collected by electrofishing from several ponds of the La Antigua, Nautla, and Tecolutla River Basins in Mexico (Table 2). When referring to the two-spot livebearer, we use Pseudoxiphophorus bimaculatus instead of Heterandria bimaculata, following recent morphological (Morales-Cazan and Albert 2012) and molecular (Agorreta et al. 2013) revisions of the phylogenetic relationships of this host. Fish were anesthetized with 2-Phenoxyethanol (Sigma-Aldrich, St. Louis, MO) and then fixed in 95 % ethanol; gyrodactylids were removed using surgical needles and were processed individually. Haptors were excised using a scalpel and subjected to partial proteolytic digestion to remove tissue enclosing the haptoral armature following Rubio-Godoy et al. 2012. Digestion was arrested by the addition of a 50:50 formaldehyde/glycerine solution, and specimens were then coverslipped and sealed with nail varnish. Bodies were fixed in 95 % ethanol and stored at −20 °C, individually labeled for subsequent molecular analyses.
Morphometric analysis
The digested haptoral hard parts were studied on a Nikon Eclipse compound microscope using an oil immersion ×100 objective lens. Pictures were taken using imaging analysis software NIS Elements Version 4.0 for Nikon. Attachment hook measurements were taken on the images using the ImageJ 1.46r software. A total of 25 point-to-point measurements detailed by García-Vásquez et al. 2011 were made on each specimen (see Table 3). All measurements are given in micrometers, showing average ± standard deviation, and minima and maxima in parentheses. The following Gyrodactylus specimens were re-examined for the current study: a paratype of Gyrodactylus jarocho from Xiphophorus hellerii (Colección Nacional de Helmintos, Mexico City, accession no. CNHE 7130), a paratype of Gyrodactylus xalapensis from Pseudoxiphophorus bimaculatus (accession no. CNHE 7131), a voucher of Gyrodactylus bullatarudis from Xiphophorus hellerii (accession no. CNHE 7132) and voucher specimens collected from Poecilia mexicana (accession no. CNHE 7133), Gyrodactylus turnbulli from Poecilia reticulata (four voucher specimens from Dr. A. P. Shinn). Morphometric measurements of all previously described species of Gyrodactylus infecting poeciliid fishes reported by Rubio-Godoy et al. (2010) were also used for comparison.
Molecular analysis
Two to ten bodies of excised specimens collected from the four species of poeciliids of the different sampling sites were placed individually in a 1.5-ml Eppendorf tube for genomic DNA extraction. Genomic DNA of each individual was extracted using the DNeasy® Blood and Tissue Kit (QIAGEN, Valencia, California) according to the manufacturer’s protocol. The ribosomal region spanning the 3′ end of the 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2, and the 5′ end of the 28S rRNA gene was amplified by PCR using the primer pairs: ITS1A (5′-GTAACAAGGTTTCCGTAGGTG- 3′) and ITS2 (5′-TCCTCCGCTTAGTGATA-3′) (Matějusova et al. 2001) and ITS1-fm (5′- TAGAGGAAGTACAAGTCG-3′) and ITS2-rm (5′-CGCTYGAATCGAGGTCAGGAC-3′) (Dr. Mark A. Freeman, pers. comm.). All PCR reactions were performed in a final volume of 12.5 μl, including 0.625 μl 10× PCR buffer, 0.25 μl 10 mM dNTPs mixture (200 μM each), 1.0 μl 50 mM MgCl2, 0.15 μl of each primer (10 pmol), 1.5 μl template DNA, 0.0625 μl Taq DNA polymerase (0.312 units), and 8.77 μl of sterile distilled water. PCR were run in a thermocycler (BioRad C1000, Hercules, California) under the following conditions: initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 45 s, annealing at 55 °C, and extension at 72 °C for 90 s; reactions were incubated at 72 °C for 10 min to complete extension and then brought to 8 °C. Unincorporated nucleotides and primers of each PCR amplicon were removed using ExoSap-IT (USB Corporation, Ohio). Sequencing reactions were performed in a final volume of 10 μl, using 3.5 μl of sequencing buffer 2.5×, 0.5 μl of the BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, California), 1 μl of purified amplicons, 1 μl of primer (10 pmol) used in the amplification, and 4 μl of sterile distilled water. Sequencing products were purified by filtration with Sephadex™ G50 (Sigma-Aldrich, St. Louis, MO) and analyzed on an ABI PRISM 3100 automated DNA sequencer (Applied Biosystems). Chromatograms were checked using FinchTV (Geospiza Inc., Seattle, Washington), and proofread contigs were assembled using the computer program BioEdit v. 7.0.9 (Hall 1999). Sequences generated in this study were deposited in GenBank and the accession numbers are cited in the description of each species.
Alignment, phylogenetic analyses, and sequence divergence
New sequences of the ITS1, 5.8S rRNA gene, and ITS2 were compared with the following Gyrodactylus sequences available in GenBank: Gyrodactylus arcuatus Bychowsky, 1933 (AY338443); Gyrodactylus bullatarudis Turnbull, 1956 (AJ011410, AY692024); Gyrodactylus hildae García-Vásquez, Hansen, Christison, Bron and Shinn, 2011 (FJ231869); Gyrodactylus jarocho Rubio-Godoy, Paladini, García-Vásquez and Shinn, 2010 (KJ621984); Gyrodactylus longipes Paladini, Hansen, Fioravanti and Shinn, 2011 (GQ150536); Gyrodactylus ostendicus Huyse and Malberg, 2004 (DQ821767); Gyrodactylus pictae Cable, van Oosterhout, Barson and Harris, 2005 (AY692023); Gyrodactylus poeciliae Harris and Cable, 2000 (AJ001844); Gyrodactylus turnbulli Harris, 1986 (EF445942); Gyrodactylus xalapensis Rubio-Godoy, Paladini, García-Vásquez and Shinn, 2010 (KJ621985); and Gyrodactylus zimbae Vanhove, Snoeks, Volckaert and Huyse, 2011 (HQ214482). All sequences were aligned using MUSCLE v. 3.5 (Edgar 2004), implemented in the software SEAVIEW v. 4.2 (Galtier et al. 1996). Phylogenetic analyses of ITS sequences were performed under maximum likelihood (ML) and Bayesian inference (BI). Prior to ML and BI analyses, Bayesian Information Criterion (BIC) (Schwarz 1978), implemented in jModelTest 2 (Guindon and Gascuel 2003; Darriba et al. 2012), was used as model selection strategy to inferring the optimal model of nucleotide substitution and parameter settings for ITS dataset. The selected likelihood model was TPM2uf+G. ML analyses were performed using the Genetic Algorithm for Rapid Likelihood Inference (GARLI) v. 0.951-1 (Zwickl 2006) under a GTR model, allowing the program to estimate the parameters. Analyses were terminated after 10,000 generations without additional improvement in the likelihood scores of trees. Two likelihood analyses were performed for each data set to ensure convergence. Nodal support was evaluated using 1000 bootstrap replicates, with each replicate terminated after 10,000 generations without an improvement in topology. MrBayes v. 3.2.1 (Ronquist et al. 2012) was used to perform BI analysis. Posterior probabilities (pp) were calculated over 1 × 106 generations, sampling the Markov chain every 100 generations. Parameter settings used were nst = 6 and rates = gamma. Fifteen percent of the sampled trees were discarded as “burn-in” and a 50 % majority rule consensus tree representing the posterior probability (pp) distribution of clades was produced from the 17,002 remaining trees. Finally, uncorrected pairwise distances “p” among species of Gyrodactylus parasitizing poeciliid fishes were obtained with PAUP* 4.0b10 (Swofford 2003) combining ITS1 and ITS2 sequences.
Results
Eight new species of Gyrodactylus were found on four poeciliid fish hosts. Poecilia mexicana was recorded to harbor six new species: Gyrodactylus actzu n. sp., Gyrodactylus apazapanensis n. sp., Gyrodactylus lhkahuili n. sp., Gyrodactylus microdactylus n. sp., Gyrodactylus pseudobullatarudis n. sp., and Gyrodactylus xtachuna n. sp. Poeciliopsis gracilis was infected by four new species: Gyrodactylus pseudobullatarudis n. sp., Gyrodactylus takoke n. sp., Gyrodactylus unami n. sp., and Gyrodactylus xtachuna n. sp. Pseudoxiphophorus bimaculatus was infected by Gyrodactylus takoke n. sp. and Gyrodactylus xtachuna n. sp. Xiphophorus hellerii harbored Gyrodactylus apazapanensis n. sp. and Gyrodactylus pseudobullatarudis n. sp. Details of the localities and hosts where the new species of Gyrodactylus were found are presented in Tables 1 and 2. Morphological descriptions of the new parasite species are presented in alphabetical order, highlighting the most informative characteristics; complete morphological measurements are presented in Table 3. Rather than discussing the systematic position and relationships of each new species separately, morphological descriptions are followed by an analysis of the phylogenetic relationships of the gyrodactylids infecting poeciliid hosts, including the new species presented here as well as those available in GenBank.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:68BB39C4-A867-4FD4-B6FD-C6EE8BA66F87. In addition, species profiles including taxonomic traits, host details, and other metadata are provided on www.gyrodb.net (Harris et al. 2008; Shinn et al. 2011).
Gyrodactylus actzu n. sp. (Fig. 1, Table 3)
urn:lsid:zoobank.org:act:71912FA6-930C-44CE-ABE8-AB16FA1EFEDE
Type host: Poecilia mexicana Steindachner, 1863 (“shortfin molly,” topote del Atlántico) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Apazapan, La Antigua River Basin, Veracruz, Mexico (19° 19′ 31.49″ N; 96° 43′ 31.57″ W).
Type material: Holotype (accession no. CNHE 9385) and one paratype (accession no. CNHE 9386) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City.
DNA reference sequences: Sequences obtained from two individuals deposited in GenBank (Accession nos. KM514475 and KM514476).
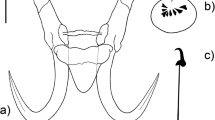
Description: (average (range), in micrometers): Morphological description based on two specimens whose haptors were proteolytically digested. Hamuli 52.9 (52.8–53.0) long, roughly same thickness through all length and widening slightly at dorsal bar attachment point, 4.2 (4.0–4.4) wide; shaft 35.3 (35.2–35.4) long; point 23.0 (22.9–23.3) long, constituting over half the shaft length; hamulus aperture distance 19.0 (18.3–19.7) long; tight hamulus aperture angle 34.8 ° (34.4–35.2 °) long; hamulus root 17.9 (17.7–18.1) long, same width in all its length, rounded and dense ends (Fig. 1a). Dorsal bar 23.0 (21.7–23.6) wide, 0.9 (0.9–0.9) long, oval and elongated attachment points 7.4 (7.0–7.7) long, narrow at union with hamulus; dorsal bar proper formed by two triangular sections both tapering towards the middle, forming angled protuberance on dorsal edge of bar (Fig. 1b). Ventral bar 26.2 (24.8–27.6) wide, 32.7 (30.4–35.1) long; protuberant ventral bar processes 8.8 (7.9–9.6) long, curved and wide ends; ventral bar median portion 7.6 (6.3–8.9) long, trapezoid shape, slightly curved basal section; ventral bar membrane 13.8 (12.0–15.7) long, “V” shaped and stout (Fig. 1c). Marginal hook 22.5 (21.9–23) long; shaft slender, 17.2 (16.5–17.9) long. Marginal hook instep straight. Marginal hook sickle 5.2 (5.1–5.4) long, sickle shaft angled forward with point ending just after the toe. Marginal hook distal width 1.3 (0.9–1.7) long; very short point, ending beyond distal end of the toe. Toe 1.6 (1.4–1.7) long; short, round bridge (Fig. 1d, e). Sickle heel semi-rounded, extending straight into sickle shaft. Marginal hook aperture 5.2 (4.9–5.5) long. Filament loop 10.7 (10.6–10.9) long, half the total shaft length (Fig. 1d, e).
Etymology: This species is named after the small size of the point of the marginal hooks. The word actzú is from the Totonaca Mexican language, which means “small”. The Totonaca people resided in the eastern coastal and mountainous regions of Mexico at the time of the Spanish arrival in 1519, in what today are the states of Veracruz, Puebla, and Hidalgo: roughly the same geographical region where this study was undertaken.
Comments: Gyrodactylus actzu n. sp. is the second gyrodactylid species found in Poecilia mexicana. Previously, Rubio-Godoy et al. (2010) reported that this host harbored Gyrodactylus bullatarudis. However, morphological re-examination of specimens from that study (CNHE nos. 7132 and 7133) indicated that they correspond to previously undescribed species of Gyrodactylus. Here, we correct the erroneous record: Poecilia mexicana collected in Río Moctezuma, Hidalgo, Mexico, were infected by Gyrodactylus pseudobullatarudis n. sp. and by Gyrodactylus xtachuna n. sp. The marginal hook morphology of Gyrodactylus actzu n. sp. is similar to that of Gyrodactylus poeciliae. However, these two species can be easily separated from one another based on the angle of the sickle shaft and the shape of the marginal sickle base. The sickle shaft in Gyrodactylus poeciliae is barely angled forward, and the marginal sickle base is triangular. In Gyrodactylus actzu n. sp., the sickle shaft is tilted forward at an angle of almost 45 ° with respect to the marginal toe, and the sickle base is trapezoid in shape.
ᅟ
Gyrodactylus apazapanensis n. sp. (Fig. 2, Table 3)
urn:lsid:zoobank.org:act:950A0764-F365-4B38-96B7-9999D4B33D88
Type host: Poecilia mexicana Steindachner, 1863 (shortfin molly, topote del Atlántico) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Apazapan, La Antigua River Basin, Veracruz, Mexico (19° 19′ 31.49″ N; 96° 43′ 31.57″ W).
Other host: Xiphophorus hellerii from Apazapan, La Antigua River Basin, Veracruz.
Type material: Holotype (accession no. CNHE 9387) and four paratypes (accession no. CNHE 9388) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City. In addition, three paratypes (accession nos. USNM 1267910 through 1267912) deposited in the Smithsonian US National Parasite Collection (USNM), Washington D.C., USA.
DNA reference sequences: Sequences obtained from six individuals deposited in GenBank (Accession nos. KM514463-KM514468).
Description (average (range), in micrometers): Morphological description based on eight specimens whose haptors were proteolytically digested. Hamuli 57.2 (55.9–59.5) total length; robust with broad distal shaft 4 (3.7–4.4) wide; shaft 37.8 (36.1–40.2) long, curved, becoming slimmer towards distal end; point 22.9 (21.2–25) long, constituting half the shaft length; hamulus aperture distance 24.3 (23.1–26.8) long; hamulus aperture angle 46.2 ° (43.5–50.1 °); root 20.8 (19.1–21.8) long, same width in all its length, with rounded ends (Fig. 2a). Dorsal bar 27.1 (23.8–35.1) wide, 1.9 (1.5–2.4) long, oval and elongated attachment points 9.9 (8.8–11.4) long, thickening at hamulus articulation, formed by two irregular basal triangular sections narrowing towards the middle (Fig. 2b). Ventral bar small, triangular shaped, 25.6 (23.9–27.8) wide, 25 (23.2–28.3) long; ventral bar processes narrow, 4.5 (3.4–5.7) long, pointed laterally to each side of the ventral bar, curved ends; ventral bar median portion 7.1 (5.9–9.3) long, rectangularly shaped with curved edges; ventral bar membrane lingulate, 14.1 (13–18-2) long (Fig. 2c). Marginal hook 41.4 (37.9–45.5) long; shaft svelte, 35.4 (33.1–39.6) long. Marginal hook instep 0.3 (0.2–0.4) deep, slightly curved. Marginal hook sickle 6.1 (5.6–6.6) long, shaft angled forward. Sickle distal width 3.7 (3.1–4.2) long; straight and thin point facing down towards toe, point ends beyond level of toe, point aperture angle 32.5 ° (30.7–35.8 °). Toe 1.7 (1.3–2.9) long, “U” shaped, long bridge curving smoothly, toe point end level with sickle heel base (Fig. 2d–f). Sickle heel rounded, extending straight to form sickle shaft proper. Sickle aperture 5.44 (4.5–6.5) long; filament loop 14.5 (13–15.4) long, half the total shaft length.
Etymology: This species is named after the town of Apazapan (Veracruz, México) from whose vicinity samples were taken.
Comments: Gyrodactylus apazapanensis n. sp. is the third gyrodactylid species found in Poecilia mexicana; and the fourth on Xiphophorus hellerii. Morphologically, the marginal hooks of Gyrodactylus apazapanensis n. sp. are similar to those of Gyrodactylus xalapensis. Nonetheless, these species can be differentiated because the heel is squarish in Gyrodactylus xalapensis and it has a clear indentation where the sickle shaft begins, at a position higher than the toe bridge; while in Gyrodactylus apazapanensis n. sp., the heel is rounded, and it is practically continuous with the sickle shaft; and because the sickle point of Gyrodactylus xalapensis is delicate and slightly curved upwards at its end, and in Gyrodactylus apazapanensis n. sp., it is straight and points down towards the toe.
ᅟ
Gyrodactylus lhkahuili n. sp. (Fig. 3, Table 3)
urn:lsid:zoobank.org:act:45B84120-96C3-4113-9840-74966CDB6143
Type host: Poecilia mexicana Steindachner, 1863 (shortfin molly, topote del Atlántico) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Apazapan, La Antigua River Basin, Veracruz, Mexico (19° 19′ 31.49″ N; 96° 43′ 31.57″ W).
Type material: Holotype (accession no. CNHE 9390) and one paratype (accession no. CNHE 9391) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City.
DNA reference sequences: Sequences obtained from two individuals deposited in GenBank (Accession nos. KM514477 and KM514478).
Description (average (range), in micrometers): Morphological description based on two specimens whose haptors were proteolytically digested. Hamuli 67.6 (67.4–67.7) total length; slender with broad proximal shaft width 8.4 (8.2–8.7) wide; shaft 44.9 long, smoothly curved, slimmer distal shaft width 3.6 (3.3–3.9) wide; point 24.1 (23.4–24.8) long, constituting half the shaft length; hamulus aperture distance 35.2 long; wide open aperture angle 58.5 ° (58.3–58.7 °); hamulus root 25.1 (24.9–25.3) long, narrower in mid length, rounded ends (Fig. 3a). Dorsal bar 27 (23.1–30.9) wide, 2.7 (2.4–3) long, oval, elongated attachment points 7.9 (7.3–8.4) long, formed by two irregular slightly triangular basal sections with rounded protuberances on ventral edge close to attachment points, slender towards mid-section (Fig. 3b). Ventral bar 26.9 (26.7–27.2) wide, 38.2 (37.4–39.1) long; ventral bar processes narrow, 8.7 (8.1–9.3) long, laterally pointed to each side of ventral bar, curved ends; ventral bar median portion 6.8 (6.4–7.2) long, rectangular; ventral bar membrane 21.4 (20.8–22.1) long, lingulate (Fig. 3c). Marginal hook 28.2 (26.9–29.4) long; slim shaft 21.9 (20.6–23.2) long. Marginal hook sickle 6.2 (6.2–6.3) long, shaft straight ending in thin, short curved point. Marginal sickle point tip ends before toe point, point aperture angle 34.2 ° (31.6–36.7 °). Toe pointed, 2.1 (1.7–2.5) long, short and slightly curved bridge (Fig. 3d, e). Sickle heel rounded, ending at same level as bridge. Marginal hook aperture 6.3 (5.9–6.7) long (Fig. 3d, e). Filament loop 12.5 (11.7–13.4) long, half the total shaft length.
Etymology: This species is named after the curved shape of the marginal hook point. The word lhk’ahuili comes from the Totonaca Mexican language, which means “curved”.
Comments: Gyrodactylus lhkahuili n. sp. is the fourth gyrodactylid described from Poecilia mexicana. Morphologically, the marginal hooks of Gyrodactylus lhkahuili n. sp. resemble those of Gyrodactylus jarocho; however, they can be differentiated in the sickle shaft, point, and sickle base. The marginal sickle shaft of Gyrodactylus jarocho is erect and ends in a strong curve, formed by a long sickle point terminating in line with the toe; and the sickle base is “continuous,” with almost no angle formed between the heel and the sickle shaft. In Gyrodactylus lhkahuili n. sp., the sickle shaft is slightly angled forward towards the toe, the shaft point is comparatively shorter and ends just before the toe limit; and there is a clear angle formed by the end of the round heel and the base of the sickle shaft.
ᅟ
Gyrodactylus microdactylus n. sp. (Fig. 4, Table 3)
urn:lsid:zoobank.org:act:346663E0-12B5-4CB6-BB77-2CC644BE03BD
Type host: Poecilia mexicana Steindachner, 1863 (shortfin molly, topote del Atlántico) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Río Bobos in Filipinas, Nautla River Basin, Veracruz, Mexico (20° 01′ 34″ N; 97° 09′ 41″ W).
Type material: Holotype deposited at the Colección Nacional de Helmintos (accession no. CNHE 9392), Mexico City.
DNA reference sequences: Sequence obtained from one individual deposited in GenBank (Accession no. KM514474).
Description (average (range), in micrometers): Morphological description based on one specimen, whose haptor was proteolytically digested. Hamuli 52.7 total length; robust with a broad proximal shaft width 8.3 wide; shaft 34.6 long, curved, progressively slimmer, distal shaft width 4.4 long; point 25.5 long, constituting half of shaft length; hamulus aperture distance 17.4 long; aperture angle 32.2 °; hamulus root 18.2 long, same width throughout whole length, rounded ends (Fig. 4a). Dorsal bar 21.0 wide, 1.7 long, oval and extended attachment points 7.7 long, “sunglass” shape with squarish corner on dorsal edge and round protuberances on ventral edge close to attachment points, gradually tapering towards middle section (Fig. 4b). Ventral bar small, lingulate shape 26.3 wide, 35.0 long; ventral bar processes narrow 11.1 long, laterally pointed to each side of ventral bar, curved ends; ventral bar median portion 12.2 long, rectangular shape; ventral bar membrane 16.3 long, broad anterior part, short cleavage at the end of membrane (Fig. 4c). Marginal hook 23.5 long; shaft svelte, 18.3 long. Marginal hook instep 0.2 deep, slightly curved. Sickle proper 5.2 long, sickle shaft tilted forward towards toe. Distal width 1.2 long; straight and short point; point aperture angle 9.12 °. Toe 1.6 long, end pointing downwards but at same level of sickle base, short bridge. Sickle heel rounded, extending to form sickle shaft proper. Sickle aperture 3.5 long (Fig. 4d, e); filament loop 11.0 long, half the total shaft length.
Etymology: This species is named after the small dimension of the marginal hook sickle point, which is the second smallest of the known gyrodactylids infecting poeciliid fishes—see Comments section.
Comments: Gyrodactylus microdactylus n. sp. is the fifth gyrodactylid species to be described from Poecilia mexicana. Morphologically, the marginal hooks of Gyrodactylus microdactylus n. sp. are similar to those of Gyrodactylus poeciliae, a parasite of Poecilia caucana: both are characterized by having a very short sickle point and a robust sickle base—in fact, the sickle point of Gyrodactylus poeciliae is shorter than that of Gyrodactylus microdactylus n. sp., as the marginal hook sickle distal width of the first is 0.9 μm (Harris and Cable 2000), while that of the former is 1.2 μm. Despite their similarity, these species can be differentiated: the sickle shaft in Gyrodactylus poeciliae is straight while in Gyrodactylus microdactylus n. sp., it is angled forward; and the toe of Gyrodactylus poeciliae is triangular and has a straight base, while in Gyrodactylus microdactylus n. sp., it is pointed and possesses a slightly curved base.
ᅟ
Gyrodactylus pseudobullatarudis n. sp. (Fig. 5, Table 3)
urn:lsid:zoobank.org:act:AD0A1B6F-246A-43F1-8688-D95488A1A460
Type host: Xiphophorus hellerii Heckel, 1848 (“green swordtail”, cola de espada) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins and body surface.
Type locality: Apazapan, La Antigua River Basin, Veracruz, Mexico (19° 19′ 31.49″ N; 96° 43′ 31.57″ W).
Other hosts and localities: Poeciliopsis gracilis (Heckel, 1848) from Rancho El Clarín, Tlapacoyan, Nautla River Basin, Veracruz, Mexico (20° 02′ 07.01″ N; 97° 06′ 22.70″ W); Poecilia mexicana Steindachner, 1863 from Apazapan, Veracruz (this study) and from Río Moctezuma, Pánuco river basin, Hidalgo, Mexico (21° 03′ 31″ N; 99° 28′ 03″ W) (Rubio-Godoy et al. 2010); Xiphophorus hellerii collected in Río Pixquiac, Xalapa, La Antigua River Basin, Veracruz, México (19° 28′ 39″ N; 96° 57′ 00″ W) (Rubio-Godoy et al. 2010).
Type material: Holotype (CNHE reg. no. 9393) and three paratypes (accession nos. CNHE 93994 through 9396) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City. In addition, two paratypes (accession nos. USNM 1267906 and 126707) deposited in the Smithsonian US National Parasite Collection (USNM), Washington D.C., USA.
DNA reference sequences: Sequences obtained from six individuals deposited in GenBank (Accession nos. KM514436-KM514441).
Description (average (range), in micrometers): Morphological description based on seven specimens whose haptors were proteolytically digested. Hamuli 51.3 μm (48.7–56.2) total length; proportionally slim, distal width 4.0 (3.7–4.4); proximal width 7.7 (6.9–8.5); shaft 34.3 (32.8–37.5) long; point 24.1 (22.9–27.6) long and slim, extending to half the shaft length; hamulus aperture distance 5.5 (4.4–7.3); aperture angle 32.8 ° (30.6–35.3 °); hamulus root 16.7 (14.9–18) long, comprising ca. one third of total hamulus length, with rounded ends (Fig. 5a), ventral edge apparently curved just after dorsal bar attachment point. Dorsal bar 24.7 (23.9–26.4) wide, 30.6 (28.1–33) long, attachment point small 6.9 (6.4–7.3), narrow at union with hamulus, formed by two triangular basal sections tapering towards middle section (Fig. 5b). Ventral bar including membrane, approximately triangular in shape, 24.7 (23.9–26.4) wide, 30.6 (28.1–33) long; prominent ventral bar processes 8.3 (6.8–10.2) long, reaching middle of hamulus root; ventral bar median portion with rounded ends, 8.3 (6.8–10.2) long, dense suture connecting ventral edge and base of process, forming semicircular depression in extremes of ventral bar median portion (Fig. 5c); ventral bar membrane 13.8 (11.7–15.5) long, triangular, reaching one third of hamulus shaft length (Fig. 5c). Marginal hooks 26.5 (24.5–28.7) long; shaft 21.5 (19.5–23.3) long; shaft attaches almost in the middle of sickle base. Marginal hook instep curved, 0.6 (0.5–0.7) long. Sickle proper 5.2 (4.9–5.8) long; shaft straight, slender and long (Fig. 5d–g). Distal width 2.2 (1.7–3) wide; point straight, forming almost right angle with sickle shaft; point ends slightly before toe limit, proximally 2.2 (1.7–2.9) wide. Toe 1.7 (1.5–2) long, semi trapezoid in shape, toe pointing downwards and ending just below line of sickle heel base; flat short bridge (Fig. 5e–g). Sickle heel short, almost squared, curving smoothly to shaft articulation; sickle base deeply curved. Sickle aperture 5.2 (4.8–5.6) long; filament loop 11.3 (9.5–12.4) long, one third of the total shaft length (Fig. 5d).
Etymology: This species is named after the morphological similarity of its marginal hooks to those of Gyrodactylus bullatarudis Turnbull, 1956, which it closely resembles. Phylogenetically, Gyrodactylus pseudobullatarudis n. sp. is the sister taxon to Gyrodactylus bullatarudis.
Comments: This is the fourth Gyrodactylus species described from Xiphophorus hellerii; the fifth from Poecilia mexicana; and the first record for Poecilia gracilis. Previously, Gyrodactylus bullatarudis and Gyrodactylus rasini had been described from Xiphophorus hellerii kept in aquaria in the UK and the Czech Republic, respectively; Gyrodactylus bullatarudis was recorded from feral invasive fish in Australia; and Gyrodactylus jarocho was described from wild hosts within their natural distribution range in Mexico. Rubio-Godoy et al. (2010) reported that wild Xiphophorus hellerii in Veracruz, Mexico, harbored Gyrodactylus bullatarudis. However, re-examination of specimens from that study (CNHE 7132 and CNHE 7133 (one specimen)) indicated that they correspond to a previously undescribed species of Gyrodactylus. Here, we correct the erroneous previous records: Xiphophorus hellerii collected in Río Pixquiac, Xalapa, Veracruz, México (19° 28′ 39″ N; 96° 57′ 00″ W) and Poecilia mexicana from Río Moctezuma, Vega de Ramírez, Hidalgo, Mexico (21° 03′ 31″ N; 99° 28′ 03″ W) were infected by Gyrodactylus pseudobullatarudis n. sp. From the Gyrodactylus spp. described in this paper, Gyrodactylus pseudobullatarudis n. sp. has the widest host and geographical range: it was recorded on three fish species from two host genera (Poecilia and Xiphophorus) from three different river basins. Of the known Gyrodactylus species described from poeciliids, the marginal hook sickles of Gyrodactylus pseudobullatarudis n. sp. closely resemble those of Gyrodactylus bullatarudis. Both possess sickles with triangular shaped bases and large heels. Differences in the toe and the sickle point, however, permit their discrimination from each other. The toe of Gyrodactylus bullatarudis is longer 2.3 μm (cf. 1.7 μm in Gyrodactylus pseudobullatarudis n. sp.), pointed and facing downwards beyond the sickle base; while in Gyrodactylus pseudobullatarudis n. sp., the toe point ends at the same level as the sickle base, which has a deeper instep/arch height (0.4 μm in Gyrodactylus bullatarudis cf. 0.6 μm in Gyrodactylus pseudobullatarudis n. sp.). The marginal hook sickle point reaches the limit of the toe in Gyrodactylus bullatarudis, while in Gyrodactylus pseudobullatarudis n. sp., it ends just after the bridge.
ᅟ
Gyrodactylus takoke n. sp. (Fig. 6, Table 3)
urn:lsid:zoobank.org:act:C90A307A-E607-485E-B386-ED68F40C4294
Type host: Pseudoxiphophorus bimaculatus [syn. = Heterandria bimaculata] Heckel, 1848 (“two-spot livebearer”, guatopote manchado) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Apazapan, La Antigua River Basin, Veracruz, Mexico (19° 19′ 31.49″ N; 96° 43′ 31.57″ W).
Other host and localities: Poeciliopsis gracilis from Rancho El Clarín, Tlapacoyan, Nautla River Basin, Veracruz (20° 02′ 07.01″ N; 97° 06′ 22.70″ W); Pseudoxiphophorus bimaculatus from Río Bobos in Filipinas, Nautla River Basin, Veracruz (20° 01′ 34″ N; 97° 09′ 41″ W) and from Tenampulco, Tecolutla River Basin, Puebla (20° 10′ 13″ N; 97° 24′ 20″ W), Mexico.
Type material: Holotype (accession no. CNHE 9397), five paratypes (accession nos. CNHE 9398, 9399, 9404 (2), 9405) and 10 voucher specimens (accession nos. CNHE 9400 (2), 9401 (2), 9402 (4), 9403 (2)) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City. In addition, four voucher specimens (accession nos. USNM 1270617–1270620) deposited in the Smithsonian US National Parasite Collection (USNM), Washington D.C., USA.
DNA reference sequences: Sequences obtained from 16 individuals deposited in GenBank (Accession nos. KM554447-KM554462).
Description (average (range), in micrometers): Morphological description based on 23 specimens whose haptors were proteolytically digested. Hamuli 47.6 (43.6–50) total length; distal shaft width 4 (3.4–4.7) wide; shaft 34.1 (30.1–38.3) long; point 23.6 (21.9–26.3) long, forming narrow angle; hamulus aperture distance 17.1 (14.7–18.5) long; aperture angle 32.6 ° (27.9–39.6 °); hamulus root 13.7 (11.6–15.7) long, depressed after dorsal bar attachment point, rounded ends (Fig. 6a). Dorsal bar 22.7 (18.1–26.9) wide, 1.8 (1.1–2.6) long, oval and irregular attachment points, slender at union with hamulus, formed by two irregular basal triangular sections tapering towards the middle section in specimens found in Pseudoxiphophorus bimaculatus (Fig. 6b), and crescent shaped with uniform thickness in worms collected from Poeciliopsis gracilis. Ventral bar triangular shaped, 24.3 (20.9–28.3) wide, 28.9 (25.1–35.2) long; prominent ventral bar processes 9.1 (7.1–11.8) long, internally straight and curved externally, forming a wide aperture between both processes, almost same length as hamulus root, pointed curved ends; ventral bar median portion 5.8 (4.5–7.6) long, trapezoid shaped with bulbous ends, fine sutures connecting ventral edge and base of processes; ventral bar membrane 13 (7.8–18-1) long, triangular shaped, one third of the hamulus shaft length (Fig. 6c). Marginal hook 26.8 (21.6–29-8) long; slender shaft 23 (18.7–28.8) long. Marginal hook instep 0.5 (0.3–0.6) high. Sickle proper 4.9 (4–5.5) long, sickle shaft slightly angled forwards. Distal width 3.2 (2.4–3.8); straight point facing downwards to toe, sickle point ends almost at same level as toe, point aperture angle 35.6 ° (28.2–39.1 °) (Fig. 6d, e). Toe slightly round at its end, 1.6 (1.1–2.6) long, contracted between bridge and sickle base, toe point above the level of the line of the sickle heel base; no well-developed bridge (Fig. 6d, e). Sickle heel protuberant and semi-squared, forming a deep depression at sickle shaft attachment point, which is almost level with bridge-shaft attachment point. Sickle aperture 4.2 (3.9–4.9) long (Fig. 6d, e); filament loop 13.3 (11.7–15.5) long, half of the total shaft length.
Etymology: This species is named after the similarity of its marginal hooks to those of Gyrodactylus xalapensis, which it resembles. The word takoke (tak’ok’é) is from the Totonaca Mexican language, which means “to imitate”.
Comments: Gyrodactylus takoke n. sp. is the second gyrodactylid species described from Pseudoxiphophorus bimaculatus, the first one being Gyrodactylus xalapensis, also collected from the La Antigua River Basin, in Veracruz, Mexico; it is also the second gyrodactylid species recorded for Poeciliopsis gracilis. Gyrodactylus takoke n. sp. has a wide host and geographical range: it was recorded on two host genera (Pseudoxiphophorus and Poecilia) in three different river basins. The marginal hooks of Gyrodactylus takoke n. sp. and Gyrodactylus xalapensis are morphologically similar, but these species can be easily separated by the shape of their sickles. The sickle base in Gyrodactylus xalapensis is trapezoidal, while in Gyrodactylus takoke n. sp., it is ovoid. The toe is broadly triangular and rounded at its end in Gyrodactylus xalapensis, but pointed and narrow in Gyrodactylus takoke n. sp. The sickle heel is “circular to square-ish” in Gyrodactylus xalapensis, and joins the sickle shaft at a level higher than the union of the bridge and the sickle shaft; and in Gyrodactylus takoke n. sp., the heel is clearly rounded, and both the heel and the very narrow bridge join the sickle shaft at approximately the same level. Finally, the sickle point terminates beyond the distal point of the toe in Gyrodactylus xalapensis, whereas the sickle point of Gyrodactylus takoke n. sp. ends at the same level as the toe limit.
ᅟ
Gyrodactylus unami n. sp. (Figs. 7, Table 3)
urn:lsid:zoobank.org:act:81F1DA05-63DC-4FBF-A893-44B539FAEDB1
Type host: Poeciliopsis gracilis (Heckel, 1848) (“porthole livebearer”, guatopote jarocho) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Rancho El Clarín, Tlapacoyan, Nautla River Basin, Veracruz, Mexico (20° 02′ 07.01″ N; 97° 06′ 22.70″ W).
Type material: Holotype (accession no. CNHE 9406) and five paratypes (accession no. CNHE 9407) deposited in the Colección Nacional de Helmintos (CNHE) from Mexico. In addition, three paratypes (accession nos. USNM 126713 through 126715) deposited in the Smithsonian US National Parasite Collection (USNM), Washington D.C., USA.
DNA reference sequences: Sequences obtained from five individuals deposited in GenBank (Accession nos. KM514469-KM514473).
Description (average (range), in micrometers): Morphological description based on eight specimens whose haptors were proteolytically digested. Hamuli 17.9 (17.2–18.9) total length; proportionally slim, distal width 2.7 (2.3–3.1); proximal width 6.5 (5.9–6.9); shaft 31.9 (30.3–32.7) long; point 20.3 (18.9–21.6) long and slim, reaching half the shaft length; hamulus aperture distance 17.9 (17.2–18.9); aperture angle 38.7 ° (36.3–44.5 °); hamulus root 14.2 (13.1–15.6) long, comprising approximately one third of the total hamulus length, wider at its end (Fig. 7a), ventral edge apparently curved just after dorsal bar attachment point. Dorsal bar 19.5 (15–22.1) wide, 1 (0.7–1.7) long, attachment point small, narrow at its union with hamulus, with bridge-like shape formed by two robust triangular basal sections diminishing towards the middle (Fig. 7b). Ventral bar including the membrane, approximately triangular in shape, 26.9 (22.5–32.4) wide, 26.9 (22.8–28.6) long; prominent ventral bar processes 8.1 (7.3–8.6) long, curved external edge, approaching the middle of hamulus root; ventral bar median portion, rectangular shaped, base slightly wider than dorsal edge; dense suture extending on the lateral extreme of median ventral bar, connecting ventral edge with base of process (Fig. 7c). Ventral bar membrane 13.2 (11.5–14.7) long; triangular, one third of the hamulus shaft length (Fig. 7c). Dorsal edge of ventral bar possesses a shallow “M” shaped median notch (Fig. 7a, c). Marginal hooks 25.1 (22.7–26.9) long; shaft attaches almost in the middle of sickle base; shaft 19.1 (16.6–21) long. Marginal hook instep rounded, 0.6 (0.5–1) high. Sickle proper 6.4 (5.9–6.8) long; shaft straight, slender and long; distal width 3.5 (3.1–4.1); point almost straight, forms right angle with sickle shaft, point ends slightly after toe limit, point aperture angle 26.8 ° (24.4–29.4 °). Toe 1.8 (1.7–1.9) long, semi triangular to trapezoid in shape (Fig. 5d, e), toe point ending just below line of sickle heel base; flat short bridge (Fig. 5d, e). Sickle heel short, almost squared which then curves smoothly to shaft articulation with sickle, rounded base. Sickle aperture 5.9 (5.7–6.1) long; filament loop 14.7 (12.9–16.5) long, half of the total shaft length.
Etymology: This species is named after the institution in which premises it was collected, at Rancho El Clarín, one of the research stations of the Universidad Nacional Autónoma de México (UNAM), in Tlapacoyan, Veracruz.
Comments: Gyrodactylus unami n. sp. is the first gyrodactylid species described from Poeciliopsis gracilis (Heckel); additionally, this host harbors Gyrodactylus pseudobullatarudis n. sp. and Gyrodactylus takoke n. sp. Previously, Salgado-Maldonado et al. (2001, 2005) found Gyrodactylus sp. on this host species collected in the states of Morelos and Oaxaca, Mexico, but these authors did not describe the parasites morphologically, nor deposited specimens in any collection; therefore, Gyrodactylus unami n. sp. could not be contrasted to those previous records. The marginal hook morphology of Gyrodactylus unami n. sp. is similar to that of Gyrodactylus pictae, as both species possess elongated, upright sickle shafts supported by trapezoidal sickle bases. Nonetheless, it is possible to discriminate between these species. The sickle shaft in Gyrodactylus pictae appears to be comparatively shorter than in Gyrodactylus unami n. sp., but it is actually comparably long (marginal hook sickle length 6.5 μm in Gyrodactylus pictae (Cable et al., 2005) cf. 6.4 μm in Gyrodactylus unami n. sp.). The apparent difference in length derives from the shape of the sickle shaft, which is robust and starts gradually curving forwards halfway up the shaft length until reaching the sickle point in Gyrodactylus pictae, while in Gyrodactylus unami n. sp., the sickle shaft is slender, long and straight, ending in a small curve when reaching the point, which stands at almost a right angle to the shaft. The sickle base is trapezoidal in both species, but the toe drops considerably in Gyrodactylus pictae, while in Gyrodactylus unami n. sp., the distal end of the toe and the base of the heel are approximately level.
ᅟ
Gyrodactylus xtachuna n. sp. (Fig. 8, Table 3)
urn:lsid:zoobank.org:act:BE5B7178-B6C9-432D-9334-0F0E7688D063
Type host: Poeciliopsis gracilis (Heckel, 1848) (porthole livebearer, guatopote jarocho) (Cyprinodontiformes: Poeciliidae).
Site of infection: Fins.
Type locality: Río Bobos in Filipinas, Nautla River Basin, Veracruz, Mexico (20° 01′ 34″ N; 97° 09′ 41″ W).
Other hosts and localities: Poecilia mexicana and Pseudoxiphophorus bimaculatus both from Nautla River Basin, Filipinas, Veracruz; and Poeciliopsis gracilis from Tenampulco, Tecolutla River Basin, Puebla, Mexico (20° 10′ 13″ N; 97° 24′ 20″ W).
Type material: Holotype (accession no. CNHE 9408) and four paratypes (accession nos. CNHE 9409, 9410 (2), 9411) deposited in the Colección Nacional de Helmintos (CNHE), Mexico City. In addition, two voucher specimens (USNM 1267908 and 1267909) deposited in the Smithsonian US National Parasite Collection (USNM), Washington D. C., USA.
DNA reference sequences: Sequences obtained from five individuals deposited in GenBank (Accession nos. KM514442-KM514446).
Description (average (range), in micrometers): Morphological description based on seven specimens whose haptors were proteolytically digested. Hamuli 53.8 μm (52.3–56.7) total length; proportionally slim with a broad distal shaft 4.3 (4.1–4.5) wide; shaft 35.7 (34.8–36.5) long, solid and uniform in all its length; point 25.0 (24.2–25.9) long and slim, extending over half the shaft length; hamulus aperture distance 18.4 (17.4–19.5) long; narrow aperture angle 32.6 ° (30.6–34.4 °); hamulus root straight, 17.6 (17.9–19.1) long, rounded ends, representing approximately one third of total hamulus length (Fig. 8a). Dorsal bar 25.8 (23.3–29.1) wide, 1.3 (1.0–1.7) long, oval, irregular and broad attachment points 7.2 (6.3–7.7) long, narrow at union with hamulus; basal edge of dorsal bar broadens into a “W” shaped protuberance on either side and then tapers to form an arch that is narrowest towards the middle (Fig. 8b). Ventral bar trapezoid shaped, 25.3 (24.3–27.4) wide, 32.4 (30.6–36.7) long; lingulate prominent ventral bar processes, 10.4 (9.4–11.4) long, reaching middle of hamulus root, processes wider in the middle and narrowing at base; ventral bar median portion trapezoid shaped with rounded ends; dense suture connecting basal edge of median portion to process base; ventral bar membrane 15.8 (14.3–19.8) long, lingulate, ending in narrow square point, comprising one third of hamulus shaft length (Fig. 2c). Marginal hook 25.0 (24.5–29.0) long; slim shaft, 19.9 (19.1–20.7) long. Marginal hook instep rounded, 0.5 (0.4–0.7) high. Sickle proper 5.3 (4.6–5.6) long. Distal width 2.2 (1.9–2.8); point almost straight and short, point ends just after the bridge, point aperture angle 24.8 ° (21.0–28.8 °). Toe long, semi trapezoid in shape with a rounded point, 1.7 (1.5–2) long, toe point ending at same level as sickle heel base; flat, short bridge (Fig. 8d–f). Sickle heel short and rounded, starting below anterior end of bridge. Sickle aperture 3.9 (3.5–4.2) long; filament loop 12.3 (10.9–13.5) long, half of the total shaft length (Fig. 8d).
Etymology: This species is named after the similarity of its marginal hooks to those of Gyrodactyl bullatarudis, which it resembles. The word xtachuna (xt’achuná) is from the Totonaca Mexican language, which means “similar to.”
Comments: Gyrodactylus xtachuna n. sp. is the second gyrodactylid species to be described from Poeciliopsis gracilis; the sixth gyrodactylid recorded for Poecilia mexicana; and the fourth for Pseudoxiphophorus bimaculatus. Gyrodactylus xtachuna n. sp. has a wide host and geographical range: it was recorded on three host species from three host genera (Poecilia, Poeciliopsis, and Pseudoxiphophorus) in two different river basins. From the Gyrodactylus species described from poeciliids, the marginal hook sickles of Gyrodactylus xtachuna n. sp. are most similar to those of Gyrodactylus bullatarudis. Both species possess slightly angled sickle shafts, approximately triangular shaped bases and large heels. However, differences in the toe, heel, and bridge permit their discrimination from one another. The toe of Gyrodactylus bullatarudis is round and points downwards past the sickle base; the heel is rhomboid and the toe region has a narrow, flat and prominent bridge. The toe of Gyrodactylus xtachuna n. sp., however, is pointed and reaches the level of the sickle base; the heel is rounded, and the toe region has a short and slightly curved bridge. The marginal hooks of Gyrodactylus pseudobullatarudis n. sp. are also similar to those of Gyrodactylus xtachuna n. sp. Nevertheless, these species can be readily discriminated from each other in the heel, point, and toe regions. In Gyrodactylus pseudobullatarudis n. sp., the sickle base is deep, while in Gyrodactylus xtachuna n. sp., it is curved. The heel of Gyrodactylus xtachuna n. sp. is rounded, while in Gyrodactylus pseudobullatarudis n. sp., it is more angular. The sickle point in Gyrodactylus xtachuna n. sp. is short and reaches the level of the bridge, while in Gyrodactylus pseudobullatarudis n. sp., the point is longer, reaching the mid-line of the toe.
Phylogenetic analyses
The length of the new ITS sequences of 44 individuals of Gyrodactylus spp. varied from 928 base pairs (bp) to 1054 bp (Table 4). Combination of the new sequences with those from GenBank produced an alignment constituted by 56 sequences with a length of 1190 bp. The topology of the best scoring tree recovered with ML was identical to the consensus BI tree (Fig. 9), with several well-supported nodes. Phylogenetic trees showed that not all the new and previously described species of Gyrodactylus from poeciliid fishes constitute a monophyletic assemblage; and that the new sequences obtained in this study constituted eight monophyletic groups, nested in two of the three recovered clades. Monophyletic groups recovered from the new sequences allied to the same number of new species delimited with morphological characters.
Bayesian 50 % majority rule consensus phylogram inferred for 56 sequences from ITS1, 5.8S rRNA and ITS2 from several species of Gyrodactylus. The different colors indicate the eight monophyletic reciprocal groups obtained in this study; these correspond to the proposed eight new species of Gyrodactylus. Posterior probabilities are given above the internodes. Marginal hook sickles of Gyrodactylus spp. found in Puebla and Veracruz, Mexico, compared with marginal hooks of gyrodactylids known to infect poeciliid fishes. The sickles of the new species found in this study are highlighted with the color of their respective position in the phylogenetic tree. Drawings of marginal hooks of known gyrodactylids taken from Rubio-Godoy et al. 2010. Scale bar 5 μm
Gyrodactylus longipes and Gyrodactylus hildae formed a basal clade with high branch support (0.99 of pp and 80 % of bootstrap value). Sixteen sequences of Gyrodactylus takoke n. sp. along with Gyrodactylus xalapensis, Gyrodactylus arcuatus, Gyrodactylus ostendicus, and Gyrodactylus zimbae constituted a second strongly supported clade (0.99 of pp and 80 % of bootstrap value). Within this clade, Gyrodactylus arcuatus (a parasite of sticklebacks and salmonid fishes) appears as sister group of Gyrodactylus xalapensis and Gyrodactylus takoke n. sp. (both species parasites of poeciliid fishes), and values of 1 of pp and 76 % of bootstrap support these relationships. The 16 specimens of Gyrodactylus takoke n. sp. form three well-supported groups, which correspond to the three river basins from which samples were collected (Table 2). Twelve species of Gyrodactylus (all parasites of poeciliid fishes) constituted a third clade. This clade included five sequences of Gyrodactylus unami n. sp. occupying a basal position, and three derived groups. One of the latter comprises Gyrodactylus turnbulli plus Gyrodactylus pictae. The second derived group is formed by two clusters: the first included Gyrodactylus poeciliae and six sequences of Gyrodactylus apazapanensis n. sp.; whereas the second included two subgroups, the first containing Gyrodactylus jarocho and five sequences of Gyrodactylus lhkahuili n. sp., and the second constituted by one sequence of Gyrodactylus microdactylus n. sp. and two sequences of Gyrodactylus actzu n. sp.. Finally, in the third derived group, Gyrodactylus bullatarudis appears closely related to seven sequences of Gyrodactylus pseudobullatarudis n. sp., with Gyrodactylus actzu n. sp. (constituted by five sequences) as the sister taxon of both species. Interrelationships between most of the species were well supported, with values ranging from 0.85 to 1 of pp and from 74 to 100 % of bootstrap, with the exception of the 0.53 pp value at the base of the clade.
Molecular characterization, and intra- and interspecific genetic variation
Size of the ITS1 and ITS2 sequences varied among the eight new species of Gyrodactylus described herein. Gyrodactylus takoke n. sp. showed an ITS1 of 374 bp, whereas in Gyrodactylus actzu n. sp. it was of 509 bp. With respect to ITS2, its size was shorter than ITS1, and varied from 352 bp in Gyrodactylus lhkahuili n. sp. to 414 bp in Gyrodactylus unami n. sp. (Table 4). The length of the 5.8S rRNA gene in all the new species was of 158 bp (Table 4).
Intraspecific sequence variation within the ITS region (ITS2 and ITS2 without the 5.8S rRNA gene) was only detected between some isolates of Gyrodactylus pseudobullatarudis n. sp., Gyrodactylus xtachuna n. sp. and Gyrodactylus takoke n. sp. For the first species, we obtained sequences from seven individuals, four of which showed no differences, and the remaining three varied between 0.13 and 0.53 %. For Gyrodactylus xtachuna n. sp., we processed five specimens, of which three exhibited variation ranging from 0.13 to 0.4 %. In Gyrodactylus takoke n. sp., we sequenced 16 individuals and just five showed differences ranging from 0.14 to 1.84 % (Table S1).
Comparison of ITS1 and ITS2 among the eight new species showed a great amount of nucleotide differences, ranging from 1.8 to 36.6 %. The two pairs of species Gyrodactylus pseudobullatarudis n. sp.—Gyrodactylus xtachuna n. sp. and Gyrodactylus microdactylus n. sp.—Gyrodactylus actzu n. sp. showed the lowest values of variation, with 1.85 to 2.3, and 5.7 %, respectively. In contrast, the level of sequence variation between Gyrodactylus pseudobullatarudis n. sp. and Gyrodactylus takoke n. sp. ranged from 35.5 to 36.3 %. In addition, differences of 35.5 to 36.3 % were observed between Gyrodactylus takoke n. sp. and five of the new species (Gyrodactylus apazapanensis n. sp., Gyrodactylus unami n. sp., Gyrodactylus microdactylus n. sp., Gyrodactylus actzu n. sp. and Gyrodactylus lhkahuili n. sp.) (Table S1).
Sequence variation between the new species of gyrodactylids and those described previously from poeciliid fishes reached values from 1.1 to 36.6 %. The lowest nucleotide variation was exhibited between Gyrodactylus takoke n. sp. and Gyrodactylus xalapensis and between Gyrodactylus pseudobullatarudis n. sp. and Gyrodactylus bullatarudis with values ranging from 1.1 to 2.1 % and from 1.6 to 2.4 %, respectively. On the other hand, the highest sequence variation was exhibited between Gyrodactylus takoke n. sp. and Gyrodactylus bullatarudis, with values ranging from 36.1 to 36.6 % (Table S1).
Discussion
Poeciliids are tropical fishes naturally distributed in the Atlantic coast of the Americas. There are 180 species of poeciliids, and from these, only 11 Gyrodactylus species have been described—with roughly half the parasite species originally obtained from translocated aquarium fish. Six gyrodactylid species were described from wild poeciliids collected within their native distribution ranges: Gyrodactylus costaricensis from Costa Rica (Kritsky and Fritts 1970), Gyrodactylus milleri and Gyrodactylus poeciliae from Venezuela (Harris and Cable 2000), Gyrodactylus pictae from Trinidad (Cable et al. 2005), Gyrodactylus jarocho and Gyrodactylus xalapensis from Mexico (Rubio-Godoy et al. 2010). The eight new Gyrodactylus species described here were all collected from wild poeciliid hosts within their native distribution ranges; hence, the host-parasite associations recorded and their geographic distributions are probably “natural”—albeit the complete host and geographical ranges of these and other undescribed gyrodactylids infecting poeciliids will only be known following further parasitological surveys. Given the position of Mexico, between the Nearctic and Neotropical biogeographical provinces, as well as the country’s topography, especially on the Atlantic Slope with its large latitudinal shift and great differences in altitude and vegetation zones between the low-lying Coastal Plain and an elevated Sierra Madre Oriental, as well as its rich freshwater fish fauna (Miller 2005), there is ample scope to find several new species of Gyrodactylus infecting poeciliid—and other—fishes. Indeed, the finding of eight undescribed gyrodactylids in four species of poeciliids in three river basins, suggests that these common and diverse fishes harbor a hidden parasite diversity, which might be discovered employing an integrative taxonomy approach, combining morphological, molecular, ecological, and other data to delimit new parasite taxa. The relevance of an integrative taxonomy approach has recently been highlighted with the discovery of several cryptic species of helminths (Pérez-Ponce de León and Nadler 2010; Razo-Mendivil et al. 2010, 2015), including gyrodactylids (Huyse and Volckaert 2002). In this respect, it is interesting that recent molecular analyses suggest that wild guppies in Trinidad harbor cryptic species of Gyrodactylus bullatarudis, Gyrodactylus poeciliae, and Gyrodactylus turnbulli (Xavier et al. 2015).
Even though only 19 Gyrodactylus species are known to infect poeciliid fishes, great diversity is evident, both in terms of the morphology of the different parasite species, as well as in the richness of the parasite fauna infecting different fish hosts. The morphology of all known gyrodactylids infecting poeciliids is well characterized (see Rubio-Godoy et al. 2010 for a comprehensive review). The phylogenetic associations of these parasites have not been fully assessed, as not all species have been sequenced; up to date, the phylogenetic tree we present here is the most complete hypothesis of the relationships between gyrodactylids infecting poeciliid fishes. Based on this hypothesis, not all species of Gyrodactylus infecting poeciliids constitute a monophyletic group. Future surveys of the biodiversity of gyrodactylids from this particular group of fishes carried out from an integrative taxonomy approach, might shed light on the factors that have promoted parasite speciation—as demonstrated for gyrodactylids infecting gobiid fishes in Europe (Huyse and Volckaert 2005; Vanhove et al. 2014)
With over 450 known species (Harris et al. 2004; Shinn et al. 2011), Gyrodactylus is one of the most specious genera among Metazoa (Cribb et al. 2002). Traditionally, species delimitation has been based on morphometric analyses of parasite sclerotized attachment hooks, but discrimination is difficult as gyrodactylids are morphologically similar; sometimes, almost identical (Ziętara and Lumme 2002). Therefore, molecular data are increasingly being used to differentiate between taxa. ITS sequences have proved quite useful to discriminate species as these molecular markers generally exhibit low intraspecific and high interspecific variation. ITS variability make this a very useful and precise marker for species discrimination, and it has been suggested that 1 % ITS sequence difference in conjunction with morphological and/or ecologically meaningful differences warrant species delimitation (Ziętara and Lumme 2002), although this proposal has not been universally embraced. It is remarkable that all new Gyrodactylus species described here exhibit ITS sequence differences to other species above the proposed 1 % threshold, and reach as much as 36.6 % differentiation.
Interestingly, Gyrodactylus bullatarudis and both Gyrodactylus pseudobullatarudis and Gyrodactylus xtachuna are morphologically similar, and are phylogenetically closely related. Similarly, Gyrodactylus jarocho and Gyrodactylus lhkahuili, as well as Gyrodactylus actzu and Gyrodactylus microdactylus are both morphologically similar and phylogenetically close. The similarity of Gyrodactylus bullatarudis, Gyrodactylus pseudobullatarudis, and Gyrodactylus xtachuna extends to their having a wide host range, as all three gyrodactylid species have been collected from fish species belonging to different genera. Remarkably, Gyrodactylus bullatarudis can also infect the non-poeciliid Hart’s Rivulus or killifish (Anablepsoides hartii [syn. = Rivulus hartii]) (Cable et al. 2013). Regarding the fish hosts, Poecilia mexicana has the richest gyrodactylid fauna, as it is known to harbor six species; followed by Xiphophorus hellerii, harboring five gyrodactylids; Pseudoxiphophorus bimaculatus, infected by four; Poecilia reticulata and Poeciliopsis gracilis, both infected by three; Poecilia caucana by two; and all other poeciliids known to be infected by gyrodactylids, by one species each. A further interesting observation is that in the phylogenetic tree, three well-supported groups of G yrodactylus takoke n. sp. specimens were found, which correspond to the three different river basins from which the samples were collected. This suggests that a phylogeographical structure may be evident when studying gyrodactylids infecting different fish stocks.
Poeciliids are widely recognized as invasive species, and have been demonstrated to exert negative ecological impacts upon invasion. A further danger of invasive fish is that they can act as carriers of invasive parasites; e.g., the Asian tapeworm Bothriocephalus acheilognathi and the digenean Centrocestus formosanus, both of which have achieved almost global distributions and affected countless native fishes following introduction with their fish or snail hosts, respectively (Salgado-Maldonado and Rubio-Godoy 2014). In Mexico, the potential of monogeneans in general, and of gyrodactylids in particular, to become invasive parasites has been highlighted: of 40 introduced helminths recorded in the country, 33 are monogeneans; and the parasites Cichlidogyrus sclerosus and Gyrodactylus cichlidarum, both infecting tilapia are recognized as established invaders (Salgado-Maldonado and Rubio-Godoy 2014). Remarkably, invasive Ponto-Caspian gobiid fishes, which are quickly spreading into eastern and central Europe through river and inland canal systems, have been shown to be vectors of Gyrodactylus proterorhini, originally a parasite of the Black Sea and the Sea of Azov (Kvach et al. 2014; Ondračková et al. 2005). The first gyrodactylid described from a poeciliid, Gyrodactylus bullatarudis, provides a further example of the invasiveness and potential pathogenic effects of these ectoparasites: it was described from captive Poecilia reticulata in Canada (Turnbull 1956) and later recorded from the same host in Trinidad, West Indies (Harris and Lyles 1992), probably within its native distribution range. Still within the native distribution range of poecillid fishes, Gyrodactylus bullatarudis has been recorded from Poecilia sphenops in Costa Rica (Kritsky and Fritts 1970) and from Pseudoxiphophorus bimaculatus [syn. = Heterandria bimaculata] in Mexico (Salgado-Maldonado et al. 2014). The invasiveness of this parasite, however, is demonstrated by the fact that it has also been found on Xiphophorus maculatus × Xiphophorus hellerii hybrids bought from an aquarium in the UK but imported from Singapore (Harris 1986); and on feral poeciliids in Australia, including Poecilia reticulata, Xiphophorus hellerii (Dove and Ernst 1998) and Gambusia holbrooki (Dove 2000). Increasing its invasiveness potential, Gyrodactylus bullatarudis has been proposed to be able to disperse overland while infecting killifish, which are capable of migrating out of water (Cable et al. 2013). Finally, both Gyrodactylus bullatarudis and Gyrodactylus turnbulli (a further gyrodactylid recorded on both aquarium and wild poeciliids) have been shown to exert negative fitness effects on wild Poecilia reticulata in Trinidad (van Oosterhout et al. 2007). Given the global translocation of poeciliids and their acknowledged ecological impacts upon introduction to new ecosystems, it is important to consider/assess the potential impact of their parasites; this work provides taxonomic information to achieve that goal—this is in line with the recent appeal to consider parasitology in a wider context, where biodiversity discovery should be conceptualized within a changing world where, for instance, parasite invasions are altering regional biodiversity and ecological processes, a reality that must be considered and studied (Hoberg et al. 2015).
References
Agorreta A, Domínguez-Domínguez O, Reina RG, Miranda R, Bermingham E, Doadrio I (2013) Phylogenetic relationships and biogeography of Pseudoxiphophorus (Teleostei: Poecilidae) based on mitochondrial and nuclear genes. Mol Phylogenet Evol 66:80–90
An L, Jara CA, Cone DK (1991) Five species of Gyrodactylus Nordmann, 1832 (Monogenea) from fresh-water fishes of Peru. Can J Zool 69:1199–1202
Cable J, van Oosterhout C, Barson N, Harris PD (2005) Gyrodactylus pictae n. sp. (Monogenea: Gyrodactylidae) from the Trinidadian swamp guppy Poecilia picta Regan, with a discussion on species of Gyrodactylus von Nordmann, 1832 and their poeciliid hosts. Syst Parasitol 60:159–164
Cable J, Archard GA, Mohammed RS, McMullan M, Stephenson JF, Hansen H, van Oosterhout C (2013) Can parasites use predators to spread between primary hosts? Parasitology 140:1–6
Cribb TH, Chisholm LA, Bray RA (2002) Diversity in the Monogenea and Digenea: does lifestyle matter? Int J Parasitol 32:321–328
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Dove ADM (2000) Richness patterns in the parasite communities of exotic poeciliid fishes. Parasitology 120:609–623
Dove ADM, Ernst I (1998) Concurrent invaders—four exotic species of Monogenea now established on exotic freshwater fishes in Australia. Int J Parasitol 28:1755–1764
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Galtier N, Gouy M, Gautier C (1996) SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
García-Vásquez A, Hansen H, Christison KW, Bron JE, Shinn AP (2011) Description of three new species of Gyrodactylus Nordmann 1832 (Monogenea) from oreochromids (Oreochromis, Cichlidae). Acta Parasitol 56:20–33
Gilmore SR, Cone DK, Lowe G, King SK, Jones SRM, Abbott CL (2012) Molecular phylogeny of Gyrodactylus (Monogenea) parasitizing fishes in fresh water, estuarine and marine habitats in Canada. Can J Zool 90:776–786
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Harris PD (1986) Species of Gyrodactylus von Nordmann, 1832 (Monogenea: Gyrodactylidae) from poeciliid fishes, with a description of G. turnbulli sp. nov. from the guppy, Poecilia reticulata Peters. J Nat Hist 20:183–191
Harris PD, Cable J (2000) Gyrodactylus poeciliae n. sp. and G. milleri n. sp. (Monogenea: Gyrodactylidae) from Poecilia caucana (Steindachner) in Venezuela. Syst Parasitol 47:79–85
Harris PD, Lyles AM (1992) Infections of Gyrodactylus bullatarudis and Gyrodactylus turnbulli on Guppies (Poecilia reticulata) in Trinidad. J Parasitol 78:912–914
Harris PD, Shinn AP, Cable J, Bakke TA (2004) Nominal species of the genus Gyrodactylus von Nordmann 1832 (Monogenea: Gyrodactylidae), with a list of principal host species. Syst Parasitol 59:1–27
Harris PD, Shinn AP, Cable J, Bakke TA, Bron J (2008) GyroDb: gyrodactylid monogeneans on the web. Trends Parasitol 24:109–111
Hoberg EP, Agosta SJ, Boeger WA, Brooks DR (2015) An integrated parasitology: revealing the elephant through tradition and invention. Trends Parasitol 31:128–133
Holitzki TM, Mackenzie RA, Wiegner TN, McDermid KJ (2013) Differences in ecological structure, function, and native species abundance between native and invaded Hawaiian streams. Ecol Appl 23:1367–1383
Huyse T, Volckaert FAM (2002) Identification of a host-associated species complex using molecular and morphometric analyses, with the description of Gyrodactylus rugiensoides n. sp. (Gyrodactylidae, Monogenea). Int J Parasitol 32:907–919
Huyse T, Volckaert FAM (2005) Comparing host and parasite phylogenies: Gyrodactylus flatworms jumping from goby to goby. Syst Biol 54:710–718
Kritsky DC, Fritts TH (1970) Monogenetic trematodes from Costa Rica with the proposal of Anacanthocotyle gen. n. (Gyrodactylidae: Isancistrinae). Proc Helmithol Soc Wash 37:63–68
Kvach Y, Kornyychuk Y, Mierzejewska K, Rubtsova N, Yurakhno V, Grabowska J, Ovcharenko M (2014) Parasitization of invasive gobiids in the eastern part of the Central trans-European corridor of invasion of Ponto-Caspian hydrobionts. Parasitol Res 113:1605–1624
Lucký Z (1973) Gyrodactylus rasini n. sp. (Monogenoidea: Gyrodactylidae) a parasite on the gills of Xiphophorus hellerii bred as an aquarium fish in Czechoslovakia. Vet Med 18:647–652
Matějusova I, Gelnar M, McBeath AJA, Collins CM, Cunningham CO (2001) Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). Int J Parasitol 31:738–745
Miller RR (2005) Freshwater fishes of Mexico. The University of Chicago Press, USA
Morales-Cazan A, Albert JS (2012) Monophyly of Heterandriini (Teleostei: Poeciliidae) revisited: a critical review of the data. Neotrop Ichthyol 10:19–44
Ondračková M, Dávidová M, Pečinková M, Blažek R, Gelnar M, Valová Z, Černý J, Jurajda P (2005) Metazoan parasites of Neogobius fishes in the Slovak section of the River Danube. J Appl Ichthyol 21:345–349
Paperna I (1968) Monogenetic trematodes collected from freshwater fish in Ghana. Second report. Bamidgeh 20:88–90
Pérez-Ponce de León G, Nadler SA (2010) What we don’t recognize can hurt us: a plea for awareness about cryptic species. J Parasitol 96:453–464
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu Rev Ecol Evol Syst 39:171–191
Razo-Mendivil U, Vázquez-Domínguez E, Rosas-Valdez R, Pérez-Ponce de León G, Nadler SA (2010) Phylogenetic analysis of nuclear and mitochondrial DNA reveals a complex of cryptic species in Crassicutis cichlasomae (Digenea: Apocreadiidae), a parasite of Middle-American cichlids. Int J Parasitol 40:471–486
Razo-Mendivil U, Rosas Valdez R, Rubio-Godoy M, Pérez-Ponce de León G (2015) The use of mitochondrial and nuclear sequences in prospecting for cryptic species in Tabascotrema verai (Digenea: Cryptogonomidae), a parasite of Petenia splendida (Cichlidae) in Middle America. Parasitol Int 64:173–181
Rogers WA, Wellborn TL Jr (1965) Studies on Gyrodactylus (Trematoda: Monogenea) with descriptions of five new species from the southeastern U. S. J Parasitol 51:977–982
Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rubio-Godoy M, Paladini G, García-Vásquez A, Shinn AP (2010) Gyrodactylus jarocho n. sp. and Gyrodactylus xalapensis n. sp. (Platyhelminthes: Monogenea) from Mexican poeciliids (Teleostei: Cyprinodontiformes), with comments on the known gyrodactylid fauna infecting poeciliid fish. Zootaxa 2509:1–29
Rubio-Godoy M, Paladini G, Freeman M, García-Vásquez A, Shinn AP (2012) Morphological and molecular characterisation of Gyrodactylus salmonis (Platyhelminthes, Monogenea) isolates collected in Mexico from rainbow trout (Oncorhynchus mykiss Walbaum). Vet Parasitol 186:289–300
Salgado-Maldonado G, Rubio-Godoy M (2014) Helmintos parásitos de peces de agua dulce introducidos. In: Koleff P, Mendoza R (eds) Especies acuáticas invasoras en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City, pp 269–285
Salgado-Maldonado G, Cabañas-Carranza G, Caspeta-Mandujano JM, Soto-Galera E, Mayén-Peña E, Brailovsky D, Báez-Valé R (2001) Helminth parasites of freshwater fishes of the Balsas River drainage basin southwestern Mexico. Comp Parasitol 68:196–203
Salgado-Maldonado G, Aguilar-Aguilar R, Cabañas-Carranza G, Soto-Galera E, Mendoza-Palmero C (2005) Helminth parasites in freshwater fish from the Papaloapan river basin, Mexico. Parasitol Res 96:69–89
Salgado-Maldonado G, Novelo-Turcotte MT, Vázquez G, Caspeta-Mandujano JM, Quiroz-Martínez B, Favila M (2014) The communities of helminth parasites of Heterandria bimaculata (Teleostei: Poeciliidae) from the upper Río La Antigua basin, east-central Mexico show a predictable structure. Parasitology 141:970–980
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Shinn AP, Harris PD, Cable J, Bakke TA, Paladini G, Bron JE (2011) GyroDb. World Wide Web electronic publication. (http://www.gyrodb.net)
Swofford DL (2003) PAUP*. Phylogenetic Analysis using Parsimony (*and other Methods), version 4.0b10. Sinauer Associates, Sunderland
Turnbull ER (1956) Gyrodactylus bullatarudis n. sp. from Lebistes reticulatus Peters with a study of its life-cycle. Can J Zool 34:583–594
Valero A, Macías-García C, Magurran AE (2008) Heterospecific harassment of native endangered fishes by invasive guppies in Mexico. Biol Lett 4:149–152
van Oosterhout C, Mohammed RS, Hansen H, Archard GA, McMullan M, Weese DJ, Cable J (2007) Selection by parasites in spate conditions in wild Trinidadian guppies (Poecilia reticulata). Int J Parasitol 37:805–812
Vanhove MPM, Economou AN, Zogaris S, Giakoumi S, Zanella D, Volckaert FAM, Huyse T (2014) The Gyrodactylus (Monogenea, Gyrodactylidae) parasite fauna of freshwater sand gobies (Teleostei, Gobioidei) in their centre of endemism, with description of seven new species. Parasitol Res 113:653–668
Xavier R, Faria PJ, Paladini G, van Oosterhout C, Johnson M, Cable J (2015) Evidence for cryptic speciation in directly transmitted gyrodactylid parasites of Trinidadian guppies. PLoS ONE 10:e0117096
Ziętara MS, Lumme J (2002) Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea, Gyrodactylidae). Evolution 56:2445–2458
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. thesis: The University of Texas at Austin, USA
Acknowledgments
We are grateful to Ismael Guzmán (INECOL) for help collecting hosts and worms; Martha Salazar and Cutperto Mora for the collection of fish specimens in Puebla; Diego Santiago Alarcón (INECOL) for the loan of the microscope to take microphotographs; and Luis García Prieto from the Colección Nacional de Helmintos (CNHE), Universidad Nacional Autónoma de México (UNAM) for the loan of Gyrodactylus type material. We thank Mark Freeman (University of Malaya, Malaysia) for designing the primers ITS1-fm and ITS2-rm, and for allowing us to publish the ITS sequences of Gyrodactylus jarocho and Gyrodactylus xalapensis, which he obtained. We thank Germán Muñoz, Mario Garduño and Héctor Basurto (all UNAM) for allowing us to work at Rancho El Clarín, Tlapacoyan. Field sampling was performed according to Mexican codes of practice and received approval of the Mexican Ministry of the Environment and Natural Resources (SEMARNAT) under scientific collection permit SGPA/DGVS/02967/14. This research was supported by grants to MRG from Consejo Nacional de Ciencia y Tecnología (CONACyT no. CB 168306) and Fundación Produce Veracruz/Red Nacional de Información e Investigación en Pesca y Acuacultura (FUNPROVER/RNIIPA). AGV thanks CONACyT for her postdoctoral fellowship (Becario 20340). This study was presented at the XIII ICOPA 2014 conference.
Author information
Authors and Affiliations
Corresponding author
Additional information
Adriana García-Vásquez and Ulises Razo-Mendivil are the principal authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
García-Vásquez, A., Razo-Mendivil, U. & Rubio-Godoy, M. Morphological and molecular description of eight new species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from poeciliid fishes, collected in their natural distribution range in the Gulf of Mexico slope, Mexico. Parasitol Res 114, 3337–3355 (2015). https://doi.org/10.1007/s00436-015-4559-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4559-z