Abstract
In this study, we followed an integrative taxonomy approach to describe two new species of Gyrodactylus von Nordmann, 1832, and to identify specimens of G. breviradix Vega, Razzolini, Arbetman, and Viozzi, 2019, all three collected from ten spotted live-bearer Cnesterodon decemmaculatus (Jenyns, 1842), an endemic and widespread poeciliid from the Pampean region, which is the southernmost occurring species of the Poeciliidae in the Americas. Gyrodactylids were first characterized morphologically and mophometrically, and when possible, sequences of the Internal Transcribed Spacers (ITS1-5.8S-ITS2) and the cytochrome oxidase II (COII) were used to delimit species. Gyrodactylus breviradix, Gyrodactylus marplatensis n. sp., and Gyrodactylus pampeanus n. sp. were found on the fins and body surface of C. decemmaculatus in La Tapera Creek, Mar del Plata, Buenos Aires province, Argentina. A phylogenetic analysis combining newly generated sequences of one of the new species, G. marplatensis n. sp., and of G. breviradix, along with those available in GenBank for a further 36 species of Gyrodactylus, revealed that G. marplatensis n. sp. is a sister taxon of Gyrodactylus decemmaculati Vega, Razzolini, Arbetman, and Viozzi, 2019. Genetic distances for the ITS and COII gene were estimated among Gyrodactylus spp. and further supported the validity of the new species. Overall, morphometric and molecular data coincided in delimiting the new taxa, thus demonstrating the value of integrative taxonomy for the erection of new species of Gyrodactylus and species identification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of Poeciliinae sensu Parenti (1981) (Cyprinodontiformes: Poeciliidae) comprise approximately 275 species in 27 genera, which are widely distributed in the Americas, from the southern USA to Argentina, as well as on many islands throughout the Caribbean (Lucinda 2003; Reznick et al. 2017). The highest diversity occurs in Central America (Hrbek et al. 2007), especially in Mexico, where 81 species are known (Miller 2005), whereas only 5 species have been reported in Argentina (Mirande and Koerber 2015). Due to their diversity and broad distribution, poeciliids have played a prominent role in studies of biogeography (Hrbek et al. 2007), which have recently postulated that the family originated in South America, although its major diversification dates to a later colonization of Central America (Reznick et al. 2017). In addition to their remarkable biological diversity, variation in reproductive traits displayed by this group of fishes, ranging from egg laying to live-bearing and including morphological and behavioral sexual dimorphism, makes them excellent subjects for research on their evolutionary history (Endler 2011).
Recently, 13 species of monogeneans of the genus Gyrodactylus von Nordmann, 1832 parasitizing poeciliid fishes from Mexico (Rubio-Godoy et al. 2010; García-Vásquez et al. 2015, 2019), and two from Argentina (Vega et al. 2019) have been characterized. Previous phylogenetic studies on species of Gyrodactylus infecting poecillids evidenced their polyphyletic origin (García-Vásquez et al. 2015, 2019), revealing that the diversity of Gyrodactylus in poeciliids is largely unknown and highlighting the potential of host-parasite systems for studies on coevolution and cophylogeny. Moreover, gyrodactylids infecting goodeid and profundulid fishes in Mexico, two other families of native cyprinodontiform hosts that are morphologically and ecologically similar to poeciliids, have been recently characterized (Rubio-Godoy et al. 2016; García-Vásquez et al. 2018a, b, 2019). Data suggest that these three fish families are infected by different lineages of morphologically similar and phylogenetically close parasite species, some of which are shared by these hosts. Species of Gyrodactylus possess several biological traits such as alternation of asexual, parthenogenetic, and sexual reproduction, explosive population growth, direct life cycle, high host specificity, and proved colonizing capability through host switching, which makes them interesting models for research on fish-parasite coevolutionary relationships (Huyse and Volckaert 2005).
The ten spotted live-bearer, Cnesterodon decemmaculatus (Jenyns 1842), is an endemic and widespread poeciliid from the Pampean region (Lucinda 2005),that represents the southernmost occurring species of the family in the Americas. This poeciliid has been recently used as a model to examine historical processes shaping the genetic structure of freshwater fishes in the region (Bruno et al. 2016; Ramos-Fregonezi et al. 2017) and has been reported to harbor three undescribed species of Gyrodactylus in Patagonian rivers (Argentina) where it has been recently introduced (Rauque et al. 2018); two of which were recently described (Vega et al. 2019). Consequently, on the basis of the current knowledge on the host biogeography, characterization of its gyrodactylid fauna is a first step towards the understanding of factors driving the diversity, phylogeny, and phylogeography of gyrodactylid-poeciliid systems, not only at local scales but across the entire Neotropical region when new information becomes available. Therefore, the aim of this paper is to describe two new species of Gyrodactylus collected from C. decemmaculatus in the Pampean region, Argentina.
Materials and methods
Sample collection and preparation
Specimens of Cnesterodon decemmaculatus (n = 120) were collected with hand nets (net mesh 0.28 mm) at La Tapera creek, Mar del Plata, Buenos Aires province, Argentina (37° 56′ 40″ S – 57° 32′ 22″ W) on August 10, 2016, austral winter. Fish were sacrificed by spinal cord severing with the aid of dissecting needles under a stereo microscope. Gyrodactylids were removed using surgical needles, and specimens were then fixed in either 95% ethanol or 5% formaldehyde and processed individually. Haptors of specimens fixed in ethanol were excised using a scalpel and subjected to partial proteolytic digestion to remove tissue enclosing the haptoral armature following Rubio-Godoy et al. (2012). Digestion was arrested by the addition of a 50:50 formaldehyde/glycerine solution. Bodies were fixed individually in 95% ethanol and stored at − 20 °C and labeled for subsequent molecular analyses (Harris and Cable 2000). Type material was mounted in Canada Balsam and also in Hoyer’s medium. Morphological analyses were performed on both ethanol-(digestion method as described in García-Vásquez et al. 2015) and formaldehyde-preserved specimens (which were rinsed in tap water for several hours), which were wet-mounted and measured (Fannes et al. 2015; Shinn et al. 2004).
Morphological analysis
Images of the haptoral attachment hooks were captured using Leica BFC 295 and Leica ICC50 HD digital cameras interfacing with Leica DM 2500 and DM750 microscopes (magnification of 10 × 100 with oil immersion, 100 objective lens for hamuli, and the marginal hooks with phase contrast). Twenty-eight point-to-point measurements of haptoral structures based on Shinn et al. (2004) and García-Vásquez et al. (2015) were taken on images using the ImageJ (Java 1.6.0_12) software, including for the hamulus (H): total length (HTL), aperture distance (HAD), proximal shaft width (HPSW), point length (HPL), distal shaft width (HDSW), shaft length (HSL), inner curve length (HICL), aperture angle (HAA), point curve angle (HPCA), inner aperture angle (HIA), and root length (HRL); for the ventral bar (VB): total length (VBTL), total width (VBTW), process-to-mid length (VBPML), median length (VBML), process length (VBPL), and membrane length (VBMBL); for the dorsal bar (DB): length (DBL), width (DBW), and attachment point length (DBAPTL); and for the marginal hook (MH): total length (MHTL), shaft length (MHSHL), sickle length (MHSL), sickle proximal width (MHSPW), sickle toe length (MHSTL), sickle distal width (MHSDW), aperture (MHAD), and instep/arch height (MHIH). All measurements are given in micrometers (Table 1). Specimens found in the present study were morphometrically compared with those species recorded in poeciliids: G. actzu García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. apazapanensis García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. breviradix Vega, Razzolini, Arbetman, and Viozzi, 2019; G. bullatarudis Turnbull, 1953; G. chiapaneco García-Vásquez, Pinacho-Pinacho, Guzmán-Valdivieso, Salgado-Maldonado, and Rubio-Godoy, 2019; G. decemmaculati Vega, Razzolini, Arbetman, and Viozzi, 2019; G. costaricensis Kritsky and Fritts, 1970; G. cytophagus Paperna, 1968; G. guatopotei García-Vásquez, Pinacho-Pinacho, Guzmán-Valdivieso, Salgado-Maldonado, and Rubi o-Godoy, 2019; G. jarocho Rubio-Godoy, Paladini, García-Vásquez, and Shinn, 2010; G. lhkahuili García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. microdactylus García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. milleri Harris and Cable, 2000; G. pictae Cable, von Oosterhout, Barson, and Harris, 2005; G. poeciliae Harris and Cable, 2000; G. pseudobullatarudis García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. takoke García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. tlaloci García-Vásquez, Pinacho-Pinacho, Guzmán-Valdivieso, Salgado-Maldonado, and Rubio-Godoy 2019; G. turnbulli Harris, 1986; G. unami García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015; G. xalapensis Rubio-Godoy, Paladini, García-Vásquez, and Shinn, 2010; and G. xtachuna García-Vásquez, Razo-Mendivil, and Rubio-Godoy, 2015. Morphometric measurements of all previously mentioned species of Gyrodactylus were obtained from Rubio-Godoy et al. (2010) and García-Vásquez et al. (2015, 2019), except for measurements of G. breviradix and G.decemmaculati that were obtained from type material (MACN-Pa 680/1 and MACN-Pa 677/1–2, respectively), and images kindly provided by the authors (Vega et al. 2019).
Moreover, the following species parasitizing neotropical goodeids and profundulids were also compared: G. iunuri García-Vásquez, Guzmán-Valdivieso, Razo-Mendivil, and Rubio-Godoy, 2018; G. katamba García-Vásquez, Guzmán-Valdivieso, Razo-Mendivil, and Rubio-Godoy, 2018; G. lamothei Mendoza-Palmero, Sereno-Uribe, and Salgado-Maldonado, 2009; G. montealbani García-Vásquez, Rubio-Godoy, Guzmán-Valdivieso, and Razo-Mendivil, 2018; G. tepari García-Vásquez, Guzmán-Valdivieso, Razo-Mendivil, and Rubio-Godoy, 2018; and G. tomahuac Rubio-Godoy, Razo-Mendivil, García-Vásquez, Freeman, Shinn, and Paladini, 2016. Measurements used in these analyses were taken from García-Vásquez et al. (2018a, b) and Rubio-Godoy et al. (2016).
Statistical analysis
Morphometric similarities between specimens found in the present study and their 30 known congeners parasitizing poeciliid, goodeid, and profundulid fishes from the Neotropical region were simultaneously compared by Non-Metric Multidimensional Scaling (nMDS) analyses. Analyses were based on Euclidean distances of the 25 point-to-point measurements of haptoral structures (H, MH, and VB), when data were available. Only those specimens of this study for which all the measurements were obtained were included in the analyses. In the case of described species, average values were used, depending on the availability of data in the literature (Rubio-Godoy et al. 2010, 2016; García-Vásquez et al. 2015, 2018a, b, 2019). In the case of G. breviradix, the VB could not be measured from type material neither images provided by the authors. Vector overlays were used to identify those point-to-point measurements determining the similarity between species. Hierarchical agglomerative cluster analysis, with group-average linking (Clarke and Warwick 2001), was applied to the same matrices, and those resemblance levels that include all specimens of each new species were overlaid on the nMDS plot.
Phylogenetic analysis
Bodies of excised specimens, whose haptors were morphometrically characterized, were placed individually in 1.5 ml Eppendorf tubes for genomic DNA extraction using the DNeasy® Blood & Tissue Kit (Qiagen, Valencia, California) following the manufacturer’s instructions. Two regions, the ribosomal region spanning the 3′end of the 18S rRNA gene, ITS1, 5.8S rRNA gene, and ITS2, and the 5′end of the 28S rRNA were amplified by PCR adding the forward primer BD1 (5′-GTCGTAACAAGGTTTCCGTA-3′) and the reverse primer BD2 (5′-ATCTAGACCGGACTAGGCTGTG-3′) (Bowles et al. 1995), and the cytochrome oxidase II (COII) gene was amplified using the primer pairs COX2 F1 (5′-TACATAYCGCCCGTCAATYT-3′) and COX2 R1 (5′-TCARTAYCACTGDCGDCCYA-3′) (Xavier et al. 2015). All PCR reactions were performed following the protocols of García-Vásquez et al. (2015) and Xavier et al. (2015). Amplicons were visualized on GelRed (Biotium, San Francisco, California) stained 1% agarose gel and then unincorporated nucleotides, and primers of each PCR amplicon were removed using ExoSap-IT (USB Corporation, Ohio). Sequencing reactions were carried out with the use of BigDye terminator chemistry, incorporating the same primers used in PCR reactions, and cleaned by filtration with Sephadex G50. The sequenced products were read on an ABI PRISM 3100–automated DNA sequencer (Applied Biosystems, Foster City, California).
Electropherograms were visually inspected with FinchTV (Geospiza Inc., Seattle, Washington), and overlapping fragments of forward and reverse sequences were assembled with BioEdit v. 7.0.9 (Hall 1999). Sequences were deposited in GenBank and their accession numbers are cited in the description of each species. Partial sequences obtained from the ITS and COII were aligned separately with sequences of other species of Gyrodactylus (see Table 2) available in GenBank using ClustalW with default parameters implemented in MEGA 7.0 (Kumar et al. 2016). The best-fitting nucleotide substitution model (GTR + I + G for both ITS and COII) was estimated with the Akaike Information Criterion (AIC) implemented in MEGA 7.0 (Kumar et al. 2016). Phylogenetic trees were reconstructed with unique sequences, inferred by Maximum Likelihood (ML) and Bayesian Inference (BI) analyses. For ML analyses, the program RAxML v7.0.4 (Stamatakis 2006) was used. A GTRGAMMAI substitution model was used for ML analyses, and 1000 bootstrap replicates were run to assess nodal support. BI trees were generated using MrBayes v3.2 (Ronquist et al. 2012), running two independent MC3 runs of four chains for 10 million generations and sampling tree topologies every 1000 generations. “Burn-in” periods were set to 2.5 million generations according to the standard deviation of split frequencies values (p ˂ 0.01). Posterior probabilities of clades were obtained from 50% majority rule consensus of sample trees after excluding the initial 25% as “burn-in”. The genetic divergence among species of Gyrodactylus was estimated using uncorrected “p” distances with MEGA 7.0 (Kumar et al. 2016). Finally, trees were drawn using FigTree version 1.3.1 (Rambaut 2006).
Results
Three species of Gyrodactylus, two new taxa (Gyrodactylus marplatensis n. sp. and Gyrodactylus pampeanus n. sp.) and G. breviradix were found parasitizing the tegument of 46 of the 120 (38%) specimens of C. decemmaculatus examined. Gyrodactylus breviradix was the most prevalent (14%) species infecting C. decemmaculatus from La Tapera Creek. Measurements of haptoral structures of the two new species and G. breviradix are given in Table 1. Molecular and multivariate analyses (nMDS) of morphometric data corroborated the presence of G. breviradix and of G. marplatensis n. sp. on C. decemmaculatus from the Pampean region. Gyrodactylus pampeanus n. sp. was characterized by its morphometry (nMDS) and morphology only, as no molecular data were obtained. Finally, the phylogenetic position of both G. breviradix and G. marplatensis n. sp. is presented.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank Life Science Identifiers (LSIDs) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http:/zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank:pub: 67B0676C-51EC-4E0D-89B8-FB8428F3C64E. In addition, species profiles including taxonomic traits, host details, and other metadata are provided on www.gyrodb.net (Harris et al. 2008, Shinn et al. 2011).
Descriptions
Gyrodactylus marplatensis n. sp. (Fig.1; Table1).
urn:lsid:zoobank.org:act:2513F514-76FC-4E3E-9DAC-39BDB01E6420.
Type host
Cnesterodon decemmaculatus (Jenyns, 1842) (Cyprinodontiformes: Poeciliidae).
Site of infection
Fins and body surface.
Type locality
La Tapera Creek, Mar del Plata, Buenos Aires province, Argentina (37° 56′ 40″ S – 57° 32′ 22″ W).
Type material
Holotype (accession no. MLP-HE XXX) and seven paratypes (accession no. MLP-HE XXX) were deposited in the Helminthological Collection of the Museo de La Plata (HCMLP), La Plata, Argentina. Two additional paratypes (acc. no. CNHE 11065) are deposited in Colección Nacional de Helmintos (CNHE), Mexico City, Mexico.
DNA reference sequences
Sequences obtained from 2 individuals are deposited in GenBank (accession nos.: MK965393-965394 for ITS, and accession nos.: MN927194-MN927195 for COII).
Etymology
The specific name refers to the type locality of the new species: Mar del Plata, Argentina.
Prevalence
8.4%
Number of specimens collected
9
Description (based on 8 specimens)
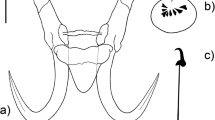
Body (based on 2 specimens with fully extended body) 341.3–427.2 long and 69.7–114.8 wide. Haptor circular, not clearly delineated from the body, 77.0–85.6 long, and 82.3–82.7 wide. Pharynx (n = 1) almost spherical 37.9 long, 36.8 wide, anterior, and posterior bulb not clearly delimited. Male copulatory organ (MCO, n = 1), spherical, 18.8 long × 18.9 wide, armed with one big principal hook (4.2 long), and a single ring of 5 small thin spines (all similar in size) (2.3, n = 2), positioned off centre, arranged 1 on the right, 1 central (the point of the principal hook point ends in the top of the point of the spine) and 3 on the left side, and pointing to the direction of the principal hook point, adjacent to the posterior end of pharynx (Fig. 1a, f). Hamulus (n = 8), total length 67.5 (63.4–70.7) long, roughly same thickness through all length and widening slightly at dorsal bar attachment point, shaft 38.0 (33.9–41.1) long; point 27.1 (24.6–28.3) long, constituting approximately half of the shaft length; proximal shaft width 9.2 (7.6–10.3), distal shaft width 4.9 (4.0–5.3); aperture distance 21.8 (20.6–25.1); aperture angle 38.8 (36.7–41.6); root 27.4 (24.6–31.1) long (Fig. 1b, g), with rounded end. Dorsal bar (n = 2), 1.8–2.5 long, 24.2–28.9 wide, becoming thinner at the middle and with postero-lateral protuberances, immediately before attachment points, becoming narrower when getting close to the attachment points; oval attachment points 24.2–28.9 long (Fig. 1c, h). Ventral bar (n = 6), 38.3 (35.1–42.7) long, 44.5 (38.7–49.9) wide; processes prominent 9.2 (7.1–10.7) long with proximal rounded ends leaning to the hamuli; median portion 8.2 (6.3–12.1) long, trapezoidal, and with semi-triangular ends extended before the start of the ventral bar processes, central region of the median ventral bar proper angled slightly towards its centre; membrane lingulate 23.8 (20.9–25.8) long, with rounded posterior edge (Figs. 1d, i). Marginal hook (n = 6), 29.8 (29.0–30.8) long; slim shaft 24.1 (23.2–24.8) long and slightly curved at its end, sickle base together with the bridge are angled towards the toe, semi-curved, and short bridge; sickle 6.3 (6.0–6.5) long, with erected sickle shaft ending in a deep opened curvature, sickle point extends before the limit of the toe; triangular toe 2.1 (2.0–2.3) long, slightly curved at the end, facing downwards; small squared heel; distal width 3.1 (2.7–3.5); aperture 5.7 (5.3–6.5) 1ong; instep height 0.6 (0.4–0.7) long, curved in shaft attachment point (Figs. 1e, j).
Remarks
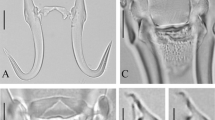
Gyrodactylus marplatensis n. sp. exhibited a unique combination of morphometric features of haptoral attachment structures that allows differentiating it from its congeners parasitizing poeciliids, goodeids, and profundulids (Fig. 4). Only G. jarocho, a parasite of Xiphophorus hellerii from Mexico (Rubio-Godoy et al. 2010), showed MH morphometry similar to the new species (Fig.4b). Nonetheless, these species are readily differentiated by the shape of the marginal hook, as well as that of their ventral bars and hamuli (Fig. 4a, c). Indeed, the ventral bar of G. jarocho has long, narrow, and pointed processes, whereas in G. marplatensis n. sp., these are rounded and short. Moreover, the lateral margins of the ventral bar membrane in G. jarocho are not attached to the external edges of the ventral bar median portion, while in G. marplatensis n. sp., they are (Fig. 4c). Moreover, although measurements of the MH, H, and VB of G. decemmaculati, parasite of C. decemmaculatus from Patagonia, were never included within the region that delimits intraspecific variation of G. marplatensis (Fig. 4), the three structures from G. decemmaculati were located close to those of G. marplatensis in each nMDS plot (Fig. 4a, b, c).
Gyrodactylus pampeanus n. sp. (Fig. 2, Table 1).
urn:lsid:zoobank.org:act:E0DA95B6-0E10-47C3-A9F5-F7D7BDE6615D.
Type host
Cnesterodon decemmaculatus (Jenyns, 1842) (Cyprinodontiformes: Poeciliidae).
Site of infection
Fins and body surface.
Type locality
La Tapera Creek, Mar del Plata, Buenos Aires province, Argentina (37° 56′ 40″ S – 57° 32′ 22″ W).
Type material
Holotype (accession no. MLP-HE XXX) and five paratypes (accession no. MLP-HE XXX) were deposited in the Helminthological Collection of the Museo de La Plata (HCMLP), La Plata, Argentina. One additional paratype (acc. no. CNHE 11066) deposited in Colección Nacional de Helmintos (CNHE), Mexico City, Mexico.
DNA reference sequences
No sequences were obtained from the specimens processed.
Etymology
The species names refers to the region where the species was found, the Pampa, Argentina.
Prevalence
5.6%
Number of specimens collected
9
Description (based on 9 specimens)
Body (based on 3 specimens with fully extended body) 395.7 (349.0–424.6) long, 99.5 (76.4–131.5) wide. Haptor circular 95.3 (93.1–97.5) long, 73.9 (72.6–75.2) wide. Pharynx (n = 2) spherical 52.9–61.5 long, 46.3–57.2 wide. MCO was visible in one specimen, but the arrangement and number of spines were difficult to recognize, only the principal hook was visible. Hamulus (n = 9), 53.7 (52.0–55.7) long, with curved shaft 36.2 (34.0–37.7) long; point 21.8 (20.5–23.6) long and slender, constituting less than half of the shaft length; proximal shaft width 7.5 (6.5–8.4) narrow; aperture distance 25.5 (18.0–28.9) long; wide aperture angle 49.1° (37.2°–53.5°); straight root 20.5 (17.9–22.6) long, proximal end rectangular, and almost the same with than the hamulus shaft (Fig. 2a, e). Dorsal bar (n = 2), 1.3–1.6 long, and straight, with uniform length along its width, becoming narrower in the hamulus attachment points, 20.1–21.7 width, oval attachment points 7.8–8.1 long (Fig. 2b, f). Ventral bar (n = 7), 22.4 (18.6–26.3) long “V”-shaped, 20.0 (18.0–22.3) wide; small and short processes 2.0 (0.8–3.3) long, pointed laterally with curved ends; median portion 5.2 (4.2–6.7) long, rectangular with rounded postero-lateral ends; membrane “V”-shaped, with truncate distal end, 14.5 (11.2–18.8) long (Fig. 2c, g). Marginal hook (n = 8), 25.7 (21.4–27.8) long; shaft slim 20.3 (16.1–21.8) long; sickle 6.1 (5.7–6.5) long, with erected shaft developing a small curve ending in a short point facing slightly upwards, ending just at the level of the bridge, sickle point 2.1 (1.6–2.9) long, short, and angled bridge, toe 1.9 (1.6–2.1) long, trapezoidal, and straight at the level of the sickle base, with a sort of light curvature where the shaft attaches to the sickle, heel rounded, and short; distal width 2.1 (1.6–2.8); aperture 5.3 (4.8–5.6); instep height 0.5 (0.2–1.1) (Fig. 2d, h).
Remarks
Gyrodactylus pampeanus n. sp. is readily distinguishable from its congeners parasitizing poeciliids, goodeids, and profundulids, by showing a particular combination of morphometric characteristics of haptoral attachment structures (Fig. 4). Despite this, its MH shows morphometric similarities with several congeners (Fig. 4b), among which it is morphologically most similar to G. costaricensis described from Poecilia sphenops from Costa Rica (Kritsky and Fritts 1970). However, the MH point is slim in G. costaricensis, whereas it is thicker in G. pampeanus n. sp.; also, the heel is straight in its union with the marginal hook shaft in G. costaricensis while in G. pampeanus n. sp., it forms a deep curve with the marginal hook shaft. Moreover, the new species differs from its congeners in the morphology and morphometry of both the hamuli (Fig. 4a) and ventral bar (Fig. 4c).
Gyrodactylus breviradix Vega, Razzolini, Arbetman, and Viozzi, 2019 (Fig. 3; Table 1).
Site of infection
Fins and body surface.
Locality
La Tapera Creek, Mar del Plata, Buenos Aires province, Argentina (37° 56′ 40″ S – 57° 32′ 22″ W).
Voucher specimens
Six specimens (accession no. MLP-HE XXX) deposited in the Helminthological Collection of the Museo de La Plata (HCMLP), La Plata, Argentina; two specimens (acc. no. CNHE 11064) deposited in Colección Nacional de Helmintos (CNHE), Mexico City.
DNA reference sequences
Sequences obtained from 3 individuals are deposited in GenBank (accession nos.: MK965395-965397 for ITS and MN927192 for COII).
Prevalence
14%
Number of specimens collected
15
General measurements
Body (based on 4 specimens with fully extended body) 550.7 (475.3–623.8) long, 116.3 (97.9–140.3) wide. Pharynx (n = 3) ovoid 45.8 (42.4–47.5) long, 34.2 (30.9–36.9) wide. Male copulatory organ (MCO, n = 3), 18.8 (16.1–21.1) long, 15.05 (13.9–15.9) wide, principal hook (6.18 long, n = 1), spines (3.08 long, n = 4) (Fig. 3a, f). Haptor (based on 4 specimens with fully extended haptor) 83.0 (80.0–86.3) long, 73.4 (66.6–81.0) wide. Measurements of H, MH, and VB given in Table 1 (Fig. 3j).
Remarks
nMDS biplots showed that the H and MH of the specimens found in C. decemmaculatus from La Tapera creek were similar both morphologically and morphometrically to those of G. breviradix described from the same host species in Patagonia, indicating that they are conspecifics. Unfortunately, the morphometry of the VB could not be included in the analyses, because the low quality of available images from type specimens provided by Vega et al. 2019 did not allow obtaining reliable measurements. Multivariate morphometric analyses indicated that G. breviradix is similar to G. takoke for all the three structures measured, and to G. xalapensis for H and MH (Fig. 4a, b). However, G. breviradix can be differentiated from G. takoke by having a straight and shorter marginal hook sickle, whereas it is longer and angled downwards in G. takoke and G. xalapensis; furthermore, the marginal hook sickle base is at the level of the sickle base line in G. takoke and G. xalapensis, whereas it is angled upwards in G. breviradix; finally, the toe is trapezoid in shape in both G. takoke and G. xalapensis while it is square-shaped in G. breviradix. (Figs. 3e, j, 4b).
Metric Multidimensional Scaling (MDS) ordination plot considering hamulus (a), marginal hook (b), and ventral bar (c) point-to-point measurements of known Gyrodactylus species infecting poeciliid, goodeid, and profundulid fishes. Symbols represent individual worms of the three species found in the present study: Gyrodactylus breviradix (triangle), Gyrodactylus marplatensis n. sp. (square), and Gyrodactylus pampeanus n. sp. (circle). Numbers represent averaged values of the following Gyrodactylus species: (1) G. actzu; (2) G. apazapanensis; (3) G. breviradix; (4) G. bullatarudis; (5) G. chiapaneco; (6) G. decemmaculati; (7) G. costaricensis; (8) G. cytophagus; (9) G guatopotei; (10) G. iunuri; (11) G. jarocho; (12) G. katamba; (13) G. lamothei; (14) G. lhkahuili; (15) G. microdactylus; (16) G. milleri; (17) G. montealbani; (18) G. pictae; (19) G. poeciliae; (20) G. pseudobullatarudis; (21) G. rasini; (22) G. takoke; (23) G. tlaloci; (24) G. tepari; (25) G. tomahuac; (26) G. turnbulli; (27) G. unami; (28) G. xalapensis; (29) G. xtachuna; and (30) G. zapoteco. Results of a hierarchical agglomerative overlaid on the nMDS biplot with distances (2.95 for hamuli, 1.5 for ventral bar, and 2.7 for marginal hook) represented by shaded areas. Vector overlays are Pearson correlations of measurements with the canonical analysis of principal coordinate axes. Vector names as in Table 1
Phylogenetic analyses
Sequences of ITS were obtained from three specimens of G. breviradix and from two specimens of G. marplatensis n. sp.. Unfortunately, sequences from G. pampeanus n. sp. were impossible to obtain, probably due to inadequate specimen fixation. The phylogenetic tree of the ITS dataset included three unique sequences of G. breviradix, two unique sequence of G. marplatensis n. sp., and 57 sequences of 27 species of Gyrodactylus retrieved from GenBank (see Table 2). Gyrodactylus mojarrae and Gyrodactylus sp. B, parasites of non-cyprinodontiform Neotropical fishes were used as outgroup. Phylogenetic hypotheses produced by BI and ML analyses are shown in Fig. 5a. Strong nodal support of bootstrap and posterior probability values were obtained for specimens of G. marplatensis n. sp. and G. breviradix, respectively, in all analyses. Gyrodactylus breviradix formed a clade with high branch support (95/0.99) with the poeciliid fish-infecting G. xalapensis and G. takoke, as a sister species. Gyrodactylus marplatensis n. sp. appears in a further clade, grouped with G. decemmaculati and G. guatopotei, both parasites of poeciliids. Genetic divergence for ITS1-5.8S-ITS2 among G. marplatensis n. sp., G. breviradix and the other 27 Gyrodactylus species showed a great amount of nucleotide variation, ranging from 0.1 to 54.49%. Values of nucleotide inter-specific variation between G. breviradix, G. xalapensis, and G. takoke ranged from 11.9 to 13.3% and 10.8 to 13.5%, respectively. Nucleotide variation between G. marplatensis n. sp., G. decemmaculati and G. guatopotei was 1.7% and 7.9%, respectively. The intra-specific variation of G. breviradix was of 0–5% and in G. marplatensis n. sp. was null.
Phylogenetic hypothesis for Gyrodactylus spp. of Cnesterodon decemmaculatus using ITS data (a), and COII data (b). Phylogenetic trees inferred through Maximum Likelihood (ML) and Bayesian Inference (BI). Numbers near internal nodes show bootstrap and the posterior probability of clade frequencies. Scale bars indicate the number of substitutions per site. The species characterized in the present study are shown in color: Gyrodactylus breviradix (blue), Gyrodactylus marplatensis n. sp. (green). Single asterisk indictaes both G. chiapaneco and G. tlaloci naturally infect fishes from the poeciliid and profundulid families
The COII data set included 47 sequences with 262 nucleotides. Sequences of COII were obtained from one specimen of G. breviradix and two specimens of G. marplatensis n. sp. Phylogenetic hypotheses produced by BI and ML analyses are shown in Fig. 5b. Gyrodactylus breviradix and G. marplatensis n. sp. were found to be reciprocally monophyletic in all analyses, with strong nodal support of bootstrap and posterior probability values. Gyrodactylus breviradix formed a clade with high branch support (98/0.99). Gyrodactylus marplatensis n. sp. appears in a further clade, grouped with G. decemmaculati and G. guatopotei. Genetic divergence for COII among 18 Gyrodacylus spp. ranged from 0.1 to 41.26%. Nucleotide variation between G. marplatensis n. sp., G. decemmaculati and G. guatopotei was of 11.1% and 24.8%, respectively. The intra-specific variation of G. breviradix was of 0.4 to 2.1% and in G. marplatensis n. sp. was null.
Discussion
In the present study, the combination of traditional morphological comparisons and different point to point morphometric measurements under multivariate statistical procedures allowed an easy discrimination and identification of species of Gyrodactylus. Although a broad set of morphometric characters are often provided in the descriptions of Gyrodactylus species (e.g., Shinn et al. 2004; Rubio-Godoy et al. 2010), many papers including recent ones restrict morphometric and morphological comparisons to marginal hook characteristics, since these structures have frequently proven to be enough for differentiating Gyrodactylus congeners (Malmberg 1970; Shinn et al. 1996; Kay et al. 1999; Cunningham et al. 2001; Rubio-Godoy et al. 2010; García-Vásquez et al. 2015). However, significant variability can occur, clouding the capabilities of such structures for species discrimination. Indeed, intra-specimen differences between the second and eighth marginal hooks have been recorded for some species (Huyse and Volckaert 2002; Rubio-Godoy et al. 2010). Furthermore, the shape and size of haptoral hard structures can be subjected to phenotypic plasticity (Olstad et al. 2009) associated with host and environmental parameters whose effect can vary between structures (ventral bar, hamuli or marginal hook), and species (Harris 1998; Geets et al. 1999; Huyse and Volckaert 2002; Dávidová et al. 2005). Therefore, researchers should be cautious when restricting the identification and differentiation of Gyrodactylus species to evidence retrieved from marginal hooks only. In the present study, the diagnostic structures that better differentiated the species from their congeners differed among the two new species, highlighting the need of simultaneously analyzing all possible morphometric data of haptoral structures, as demonstrated in several previous studies (Kay et al. 1999; McHugh et al. 2000; Shinn et al. 2000, 2004; Huyse and Volckaert 2002). This is especially applicable to gyrodactylids infecting poeciliids, which have a broad distribution that includes different biogeographic regions and are, therefore, exposed to highly contrasting environmental variables.
In general, a considerable agreement was observed among morphological, morphometric, and genetic methods in the differentiation and identification of the species reported here, demonstrating the usefulness of combining complementary methodologies for the delimitation of species of Gyrodactylus. However, the genetic distances, recorded for new ITS sequences of G. breviradix, showed a high variability (0–5%) when compared with specimens from Patagonia (Vega et al. 2019), which is also reflected in their division in two clades in the phylogenetic tree. ITS differentiation values higher than 4% have been considered indicative of cryptic species for some morphologically indistinguishable species of Gyrodactylus (Razo-Mendivil et al. 2016). However, there is no consensus on the level of differentiation of ITS that reflects the presence of different taxa for this genus. Distances higher than 1% could be indicative of inter-specific differentiation, when these differences are accompanied with some kind of meaningful ecological differentiation (Ziętara and Lumme 2003). Nevertheless, levels of variation up to 1.84% have been reported for specimens of a single species infecting two poeciliid hosts in three different river basins (García-Vásquez et al. 2015). On the other hand, for mtDNA, a variability smaller than 10% has been suggested to be intra-specific (Kuusela et al. 2008). The variation observed inCOII sequences of G. breviradix (0.4 to 2.1%) are within that range, being similar to those of some congeners infecting poeciliids, such as G. turnbulli and G. poeciliae, whose CCOII sequences vary between 0.4–3.4% and 0.4–2.3%, respectively (Xavier et al. 2015). Because of the contrasting results from mitochondrial and nuclear sequences, and considering that no morphological/morphometrical differences were observed among specimens from both regions, the new material is provisionally regarded as G. breviradix, until further research, based on larger samples, wider geographic areas, and additional genetic markers, allows a definitive identification of this species.
For G. marplatensis n. sp., different haptoral features or their combinations were responsible for morphological similarities among genetically closely related species, confirming that sclerites other than the marginal hooks are also important for species delimitation.
The extant diversity of the genus Gyrodactylus is the result of a combination of two kinds of evolutionary events, namely co-evolution, promoted by the direct life-cycle and high host-specificity of their representatives, and speciation by host switching, facilitated by the ability for auto-infection of their members (Brooks and McLennan 1993; Huyse and Volckaert 2002). Phylogenetic hypotheses of Gyrodactylus species infecting poeciliids propose that they constitute a polyphyletic group (García-Vásquez et al. 2015, 2019), a finding which is supported in the present study.
In South America, only 5 species of Gyrodactylus have been reported on native poeciliids: G. turnbulli from Poecilia reticulata from Peru (An et al. 1991), G. milleri and G. poeciliae from Poecilia caucana from Venezuela (Harris and Cable 2000), and recently, G. decemmaculati and G. breviradix from Cnesterodon decemmaculatus, a host recently introduced in Patagonia, Argentina (Vega et al. 2019). The present study adds two new species to the gyrodactylid fauna of poeciliids, representing the southernmost record of the genus in natural and native populations of poeciliids in the Americas. The present findings illustrate the potential for finding several more species in South America, as C. decemmaculatus is now known to harbor four species of Gyrodactylus.
References
An L, Jara CA, Cone DK (1991) Five species of Gyrodactylus Nordmann, 1832 (Monogenea) from fresh-water fishes of Peru. Can J Zool 69:1199–1202
Bowles J, Blair D, McManus DP (1995) A molecular phylogeny of the human schistosomes. Mol Phyl Evol 4:103–109. https://doi.org/10.1006/mpev.1995.1011
Brooks DR, McLennan DA (1993) Parascript: parasites and the language of evolution. Smithsonian Institution Press, Washington, D.C.
Bruno MC, Mapelli FJ, Casciotta JR, Almirón AE, Lizarralde MS (2016) Phylogeography of Cnesterodon decemmaculatus (Cyprinodontiformes: Poeciilidae) in Southern Pampas, Argentina: ancient versus recent patterns in freshwater fishes. Environ Biol Fish 99:293–307. https://doi.org/10.1007/s10641-016-0474-0
Bueno-Silva M, Boeger WA (2014) Neotropical Monogenoidea. 58. Three new species of Gyrodactylus (Gyrodactylidae) from Scleromystax spp.(Callichthyidae) and the proposal of COII gene as an additional fragment for barcoding gyrodactylids. Folia Parasitol 61:213–222. https://doi.org/10.14411/fp.2014.028
Cable J, Van Oosterhout C, Barson N, Harris PD (2005) Gyrodactylus pictae n. sp.(Monogenea: Gyrodactylidae) from the Trinidadian swamp guppy Poecilia picta Regan, with a discussion on species of Gyrodactylus von Nordmann, 1832 and their poeciliid hosts. Syst Parasitol 60(3):159–164. https://doi.org/10.1007/s11230-004-6348-4
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Cunningham CA, Mo TA, Collins CM, Buchmann K, Thiery R, Blanc G, Lautraite A (2001) Redescription of Gyrodactylus teuchis Lautraite, Blanc, Thiery, Daniel & Vigneulle, 1999 (Monogenea: Gyrodactylidae); a species identified by ribosomal RNA sequence. Syst Parasitol 48:141–150
Dávidová M, Jarkovský J, Matejusová I, Gelnar M (2005) Seasonal occurrence and metrical variability of Gyrodactylus rhodei Žitnan, 1964 (Monogenea, Gyrodactylidae). Parasitol Res 95:398–405. https://doi.org/10.1007/s00436-005-1311-0
Endler JA (2011) Integrative commentary on ecology and evolution of poeciliid fishes. In: Evans JP, Pilastro A, Schlupp I (eds) Ecology and evolution of poeciliid fishes. University of Chicago Press, Chicago, Ill, pp 301–310
Fannes W, Vanhove MPM, Huyse T, Paladini G (2015) A scanning electron microscope technique for studying the sclerites of Cichlidogyrus. Parasitol Res 114:2031–2034. https://doi.org/10.1007/s00436-015-4446-7
García-Vásquez A, Razo-Mendivil U, Rubio-Godoy M (2015) Morphological and molecular description of eight new species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) from poeciliid fishes, collected in their natural distribution range in the Gulf of Mexico slope, Mexico. Parasitol Res 114:3337–3355. https://doi.org/10.1007/s00436-015-4559-z
García-Vásquez A, Pinacho-Pinacho CD, Martínez-Ramírez E, Rubio-Godoy M (2018a) Two new species of Gyrodactylus von Nordmann, 1832 from Profundulus oaxacae (Pisces: Profundulidae) from Oaxaca, Mexico, studied by morphology and molecular analyses. Parasitol Int 67:517–527. https://doi.org/10.1016/j.parint.2018.03.003
García-Vásquez A, Rubio-Godoy M, Guzmán-Valdivieso I, Razo-Mendivil U (2018b) Three new species of Gyrodactylus von Nordmann, 1832 described from Goodea atripinnis (Pisces: Goodeidae), an endemic freshwater fish from the central highlands of Mexico. Parasitol Res 117:139–150. https://doi.org/10.1007/s00436-017-5680-y
García-Vásquez A, Pinacho-Pinacho CD, Guzmán-Valdivieso I, Salgado-Maldonado G, Rubio-Godoy M (2019) New species of Gyrodactylus von Nordmann, 1832 from native fish from Chiapas, Mexico, studied by morphology and molecular analyses. Acta Parasitol 64:551–565. https://doi.org/10.2478/s11686-019-00088-y
Geets A, Appleby C, Ollevier C (1999) Host-dependent and seasonal variation in opisthaptoral hard parts of Gyrodactylus cf. arcuatus from three Pomatoschistus spp. and G. arcuatus from Gasterosteus aculeatus: a multivariate approach. Parasitol 119:27–40
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuc Ac Symp Ser 41:95–98
Harris PD (1986) Species of Gyrodactylus von Nordmann, 1832 (Monogenea Gyrodactylidae) from poeciliid fishes, with a description of G. turnbulli sp. nov. from the guppy, Poecilia reticulate Peters. J Nat Hist 20:183–191. https://doi.org/10.1080/00222938600770151
Harris PD (1998) Extreme morphological variation between related individuals of Gyrodactylus pungitii Malmberg, 1964 (Monogenea). Syst Parasitol 39:137–140
Harris PD, Cable J (2000) Gyrodactylus poeciliae n. sp. and G. milleri n. sp. (Monogenea: Gyrodactylidae) from Poecilia caucana (Steindachner) in Venezuela. Syst Parasitol 47:79–85. https://doi.org/10.1023/A:1006413804061
Harris PD, Shinn AP, Cable J, Bakke TA, Bron J (2008) GyroDb: gyrodactylid monogeneans on the web. Trends Parasitol 24:109–111. https://doi.org/10.1016/j.pt.2007.12.004
Hrbek T, Seckinger J, Meyer A (2007) A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol Phylogenet Evol 43:986–998. https://doi.org/10.1016/j.ympev.2006.06.009
Huyse T, Volckaert FAM (2002) Identification of a host-associated species complex using molecular and morphometric analyses, with the description of Gyrodactylus rugiensoides n. sp. (Gyrodactylidae, Monogenea). Int J Parasitol 32:907–919. https://doi.org/10.1016/S0020-7519(02)00026-7
Huyse T, Volckaert FAM (2005) Comparing host and parasite phylogenies: Gyrodactylus flatworms jumping from goby to goby. Syst Biol 54:710–718. https://doi.org/10.1080/10635150500221036
Kay JW, Shinn AP, Sommerville C (1999) Towards an automated system for the identification of notifiable pathogens using Gyrodactylus salaris as an example. Parasitol Today 15(5):201–206
Kritsky DC, Fritts TH (1970) Monogenetic trematodes from Costa Rica with the proposal of Anacanthocotyle gen. n. (Gyrodactylidae: Isancistrinae). Proc Helmithol Soc Wash 37:63–68
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Kuusela J, Ziêtara MS, Lumme J (2008) Description of three new European cryptic species of Gyrodactylus Nordmann, 1832 supported by nuclear and mitochondrial phylogenetic characterization. Acta Parasitol 53(2):120–126. https://doi.org/10.2478/s11686-008-0015-x
Lucinda PHF (2003) Family Poeciliidae (Livebearers). In: Reis RE, Kullander SO, Ferraris CJJ (eds) CheckList of the freshwater fishes of south and central America. Edipucrs, Porto Alegre, pp 555–581
Lucinda PHF (2005) Systematics of the genus Cnesterodon Garman, 1895 (Cyprinodontiformes: Poeciliidae: Poeciliinae). Neotrop Ichthyol 3:259–270. https://doi.org/10.1590/S1679-62252005000200003
Malmberg G (1970) The exretory systems and the marginal hooks as basis for the systematics of Gyrodactylus (Trematoda, Monogenea). Arkiv för Zoologi 2:1–235
McHugh ES, Shinn AP, Kay JW (2000) Discrimination of the notifiable pathogen Gyrodactylus salaris from G. thymalli (Monogenea) using statistical classifiers applied to morphometric data. Parasitology 121:315–323
Mendoza-Palmero CA, Blasco-Costa I, de León GPP (2019) Morphological and molecular characterisation of a new species of Gyrodactylus von Nordmann, 1832 (Monogenoidea: Gyrodactylidae) of cichlid fishes (Perciformes) from Mexico. Parasitol Int 70:102–111. https://doi.org/10.1016/j.parint.2019.02.009
Miller RR (2005) Freshwater fishes of Mexico. The University of Chicago Press, USA
Mirande JM, Koerber S (2015) Checklist of the freshwater fishes of Argentina (CLOFFAR). Ichthyological Contributions of PecesCriollos 36:1–68 https://media.hotelwebservice.com/media/pecescriollos/docs/icp_36_-_mirande_koer
Olstad K, Bachmann L, Bakke TA (2009) Phenotypic plasticity of taxonomic and diagnostic structures in gyrodactylosis-causing flatworms (Monogenea, Platyhelminthes). Parasitology 136:1305–1315. https://doi.org/10.1017/S0031182009990680
Parenti LR (1981) A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha). Bull Am Mus Nat Hist 168:335–557
Rambaut A (2006) FigTree v1.3.1. Institute of Evolutionary Biology. University of Edinburgh, Edinburgh
Ramos-Fregonezi AMC, Malabarba LR, Fagundes NJR (2017) Population genetic structure of Cnesterodon decemmaculatus (Poeciliidae): a freshwater look at the Pampa Biome in southern South America. Front Genet 8. https://doi.org/10.3389/fgene.2017.00214
Rauque C, Viozzi G, Flores V, Vega R, Waicheim A, Salgado-Maldonado G (2018) Helminth parasites of alien freshwater fishes in Patagonia (Argentina). Int J Parasitol Parasites Wildl 7:369–379. https://doi.org/10.1016/j.ijppaw.2018.09.008
Razo-Mendivil U, García-Vásquez A, Rubio-Godoy M (2016) Spot the difference: two cryptic species of Gyrodactylus von Nordmann, 1832 (Platyhelminthes: Monogenea) infecting Astyanax aeneus (Actinopterygii, Characidae) in Mexico. Parasitol Int 65(5):389–400. https://doi.org/10.1016/j.parint.2016.05.009
Razzolini E, Murari AL, Baldisserotto B, Boeger WA (2019) Gyrodactylus lilianae n. sp.(Polyonchoinea: Gyrodactylidae) from Rhamdia quelen (Quoy & Gaimard)(Siluriformes: Heptapteridae) from southern Brazil: a potential nuisance for aquaculture. Syst Parasitol 96(4–5):407–415. https://doi.org/10.1007/s11230-019-09858-8
Reznick DN, Furness AI, Meredith RW, Springer MS (2017) The origin and biogeographic diversification of fishes in the family Poeciliidae. PLoS One 12(3):e0172546. https://doi.org/10.1371/journal.pone.0172546
Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference andmodel choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Rubio-Godoy M, Paladini G, García-Vásquez A, Shinn AP (2010) Gyrodactylus jarocho sp. nov. and Gyrodactylus xalapensis sp. nov. (Platyhelminthes: Monogenea) from Mexican poeciliids (Teleostei: Cyprinodontiformes), with comments on the known gyrodactylid fauna infecting poeciliid fish. Zootaxa 2509:1–29. https://doi.org/10.11646/zootaxa.2509.1.1
Rubio-Godoy M, Paladini G, Freeman M, García-Vásquez A, Shinn AP (2012) Morphological and molecular characterisation of Gyrodactylus salmonis (Platyhelminthes, Monogenea) isolates collected in Mexico from rainbow trout (Oncorhynchus mykiss Walbaum). Vet Parasitol 186:289–300. https://doi.org/10.1016/j.vetpar.2011.11.005
Rubio-Godoy M, Razo-Mendivil U, García-Vásquez A, Freeman MA, Shinn AP, Paladini G (2016) To each his own: no evidence of gyrodactylid parasite host switches from invasive poeciliid fishes to Goodea atripinnis Jordan (Cyprinodontiformes: Goodeidae), the most dominant endemic freshwater goodeid fish in the Mexican highlands. Parasit Vectors 9(1):604. https://doi.org/10.1186/s13071-016-1861-2
Shinn AP, des Clers S, Gibson DI, Sommerville C (1996) Multivariate analyses of morphometrical features from Gyrodactylus spp. (Monogenea) parasitising British salmonids: light microscope based studies. Syst Parasitol 33(2):15–125
Shinn AP, Kay JW, Sommerville C (2000) The use of statistical classifiers for the discrimination of species of the genus Gyrodactylus (Monogenea) parasitizing salmonids. Parasitology 120:261–269
Shinn AP, Hansen H, Olstad K, Bachmann L, Bakke TA (2004) The use of morphometric characters to discriminate species of laboratory-reared and wild populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol 51:239–252. https://doi.org/10.14411/fp.2004.029
Shinn AP, Harris PD, Cable J, Bakke TA, Paladini G, Bron JE (2011) GyroDb. World Wide Web electronic publication. http://www.gyrodb.net. Accessed 23 Jul 2018
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Vega R, Razzolini E, Arbetman M, Viozzi G (2019) Two new species of Gyrodactylus von Nordmann, 1832 (Monogenoidea: Gyrodactylidae) parasitizing introduced poeciliids in Patagonia. Zootaxa 4664(3):423–433. https://doi.org/10.11646/zootaxa.4664.3.9
Xavier R, Faria PJ, Paladini G, van Oosterhout C, Johnson M, Cable J (2015) Evidence for cryptic speciation in directly transmitted gyrodactylid parasites of trinidadian guppies. PLoS One 10(1):e0117096. https://doi.org/10.1371/journal.pone.0117096
Ziętara MS, Lumme J (2003) The crossroads of molecular, typological and biological species concepts: two new species of Gyrodactylus Nordmann, 1832 (Monogenea: Gyrodactylidae). Syst Parasitol 55:39–52
Acknowledgments
We thank Delfina Canel and Manuel Irigoitia for the assistance with field collections and Dr. Rocio Vega for providing the images of G. breviradix and G. decemmaculati for the measurement. We also want to thank to Gina Galllo (INECOL) for the help with the illustrations and to Dr. Delfina Cantatore for the help with phylogenetic analyses.
Funding
This work was supported by Grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) – Argentina (PIP no. 112-201501-00973), Fondo para la Investigación Ciencia y Tecnología (FonCyT)– Argentina (PICT 2015 no. 2013, PICT-2016-4175) and Universidad Nacional de Mar del Plata (UNMdP) – Argentina (EXA869/16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national, and international guidelines for the care and use of animals were followed. Permit for fishing provided by Ministerio de Asuntos Agrarios de la Provincia de Buenos Aires, Argentina (Disposición 164, August 23, 2012).
Additional information
Section Editor: Shokoofeh Shamsi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
urn:lsid:zoobank.org:pub:67B0676C-51EC-4E0D-89B8-FB8428F3C64E
Rights and permissions
About this article
Cite this article
Taglioretti, V., García-Vásquez, A., Rossin, M.A. et al. Two new species of Gyrodactylus von Nordmann, 1832 parasitizing Cnesterodon decemmaculatus (Poeciliidae) from the southern limit of the family in the Neotropical region. Parasitol Res 119, 1713–1728 (2020). https://doi.org/10.1007/s00436-020-06680-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06680-w