Abstract
Polyacrylonitrile (PAN)-based activated carbon fiber (ACF) was prepared by CO2 and KOH activation method, respectively, and used for the adsorption of CS2 at room temperature. The adsorbents were characterized by scanning electron microscope, CO2-temperature-programmed desorption and Fourier transform infrared spectroscopy. The adsorption activity of ACF prepared by different conditions (preoxidation process, carbonization temperature, CO2 activation temperature and time, carbon/KOH ratio, KOH activation temperature and time) was studied. The best breakthrough adsorption capacity of CS2 was 55.63 mgS/g when the ACF was activated by CO2 [ACF(I700)-CO2(900 + 90)]. The best breakthrough adsorption capacity of CS2 was 49.52 mgS/g when the activator was KOH [ACF(I700)-KOH(1:1 + 900 + 60)].
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon disulfide (CS2) exists in a variety of industrial waste gases, such as closed calcium carbide furnace exhaust, blast furnace exhaust and yellow phosphorus tail gas [1, 2] which contain a large number of CO (85–95%); CO is an important chemical raw material for one carbon industry. CS2 can pollute the environment and poison the catalyst [3, 4]. Thus, increasingly stringent environmental protection requirements make the removal of CS2 necessary. The common removal methods include adsorption and catalytic hydrolysis [5]. However, the final hydrolysis products CO2 and H2S are air pollutants which need further processing, so the adsorption is regarded as a promising method for the removal of CS2.
Efforts have been made for the removal of CS2. Chen et al. [6] investigated the CS2 on ion-exchanged zeolites Y by dynamic adsorption tests. The results showed that Ag–Y zeolite holds the highest CS2 breakthrough adsorption capacity up to 44.8 mg/g due to the formation of the S−M (σ) bond involving CS2 and Ag+. The introduction of precious metal and the breakthrough adsorption capacity of only 44.8 mg/g shows the poor economy of the adsorbent. Li et al. [7] synthesized a series of Cu/Ni/Fe ternary hydrotalcite-like compounds. Although introduction of alkali could produce more basic sites which were the active centers for the hydrolysis of CS2, the best efficiency of catalytic hydrolysis of CS2 is less than 100%. Wang et al. [8] reported that activated carbon modified by Cu and cobalt sulfonated phthalocyanine (CoSPc) denoted as ACCu–CoSPc showed significantly enhanced CS2 adsorption ability. The adsorption capacity of 13.35 mg CS2/g AC was obtained. Based on the complex adsorbent preparation process, the adsorption capacity appears to be low.
Meanwhile, activated carbon was widely used in the removal of CS2 by catalytic hydrolysis and oxidation methods [9,10,11], but these methods are sensitive to oxygen and water content in the atmosphere which seriously affects the activity of the catalyst. Adsorption of CS2 at room temperature has better environmental and economic benefits. Activated carbon fiber or fabric (ACF) is considered as an advanced porous material as it has many advantages over activated carbon in granular, powder or other forms. ACF is generated via a series of processes, including stabilization, carbonation and activation. The selection of raw material is essential in preparation high-quality ACF. Some of the precursors which have been used for the preparation of ACF include fiber spun from phenolic resins [12], polyimides [13], polyacrylamides [14], pitch [15], as well as from cellulose-based fibers [16] and polyacrylonitrile-based fibers [17]. Most of (90%) the carbon fibers produced are obtained from polyacrylonitrile (PAN). Although PAN fiber is more expensive than rayon fiber, they are used extensively as a source of carbon fiber because their carbon yield is almost twice that of rayon [18]. PAN-based ACF is amorphous graphitic carbon, consisting of sp2 hexagonal carbon layers with different pore sizes ranging from micropores to macropores. Besides, PAN-based ACF contains nitrogen, which is responsible for adsorbent and catalytic functions [19]. The excellent feature of ACF is that the micropores are directly exposed to the surface and extremely high surface area (surface-to-volume ratio) between 2000 and 2500 m2/g, which reduces the mass transfer resistance and encourages the adsorption of wide range of organic as well as inorganic compounds [20]. The adsorption capability of ACF has already been explored for the removal of pesticides, dyes, pharmaceuticals, heavy metals, oxides of nitrogen, oxides of sulfur and volatile organic compounds [21,22,23,24,25,26]. The adsorption of CS2 by ACF was only investigated in water environment [27].
Therefore, the adsorption of CS2 over PAN-based ACF at room temperature was investigated in this work, and the effects of preparation conditions (preoxidation, carbonization and activation) on adsorption activity were also studied. Besides, the surface characterization methods (SEM, CO2-TPD and FTIR) were used to discuss the surface topography, basic sites and the chemical characteristics of ACF.
Experimental methods
Material
The preparation process of PAN-based ACF is shown in Fig. 1. Polyacrylonitrile (PAN) fiber was purchased from Nantong Sutong Fiber Co., LTD. (Jiangsu, China). The PAN fiber was stirred and washed for 30 min with distilled water to remove any ash or impurities from the surface and then dried at 80 °C for 6 h. Preoxidation was attained at four different process in anhydrous air atmosphere: preoxidation I, II, III and IV (see details in Supporting Materials).
Then, the fiber was carbonized at 600–900 °C for 60 min in nitrogen. The carbonized material was ground and sieved to 60- to 80-mesh size. Next, it was activated with CO2 or KOH (the ratio of alkali to carbon was 2:1–1:3) at 700–1000 °C for 30–120 min. When it was activated with KOH, the fiber was washed with 0.1 mol/L HNO3 1–2 times and then washed with distilled water to a constant pH (pH = 7) to remove the ash and the activating agent after activation. Finally, it was dried at 100 °C in the drying oven for 6 h. These materials are designated as ACF(XT1) − a(Y + T2 + t), respectively. X represents the preoxidation process (I, II, III and IV), T1 is the carbonization temperature in °C, a is the activator (CO2 and KOH), Y is the ratio of alkali to carbon (only in KOH activation), T2 is the activation temperature in °C, and t is the activation time in minute. For example, ACF(I600)-KOH(2:1 + 700 + 30) means that the ACF was prepared via preoxidation process I, carbonized at 600 °C and then activated by KOH (the ratio of alkali to carbon is 2:1) at 700 °C for 30 min.
Characterization
SEM studies were carried out with a Zeiss DSM microscope. Individual fibers were examined, and the typical operation conditions were a voltage and working distance of 15 kV and 5 mm, respectively. The CO2-temperature-programmed desorption (CAT II, MicrotracBEL Corp.) was used to characterize the basic sites of the catalyst. Using CO2 as the acid probe molecule, the catalysts were characterized after CO2 adsorption on catalysts. FTIR spectra were recorded on a Thermo spectrometer in the 4000–400 cm−1 wavenumber range using the KBr pellet technique to analyze the chemical characteristics on the ACF surface.
Evaluation of adsorption performance
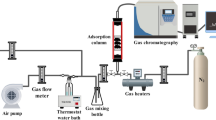
The CS2 removal experiments were performed in a fixed-bed glass reactor under atmospheric pressure. The experimental apparatus of the system is shown in Fig. 2. The overall flow rate was controlled using calibrated mass flow meters. At steady state, N2 gas containing 80–100 ppm CS2 was introduced into the reactor. In all of the tests, 0.1 g of ACF was used and the total flow rate was fixed at 60 mL/min. The adsorption experiment was carried out at room temperature (about 25 °C). The CS2 concentrations of the gaseous feed and the effluent from the reactor were determined by an online gas chromatographic system (FULI-9790 II, Zhejiang, China). The flame photometric detector (FPD) was used in gas chromatographic system, high-purity nitrogen (99.999%) was used as carrier gas, and the temperature of column, injector and detector was 150 °C, 50 °C and 150 °C, respectively. In this investigation, the adsorption efficiency of CS2 was calculated using the following equation:
when the removal efficiencies of CS2 was 80%, the adsorption capacity was defined as the breakthrough adsorption capacity.
Results and discussion
Adsorption activity test
Effect of the different preoxidation process
As the first stage of the carbon fiber manufacture from polyacrylonitrile, fiber should undergo preoxidation process, which prevents the decay of their fibrous form during pyrolysis [28]. The preoxidation of precursor fiber can proceed in an oxygen or neutral atmosphere, but ACF with higher mechanical strength and higher porosity is obtained if the preoxidation is carried out in an oxygen atmosphere, and in suitable conditions [29]. In this study, four different preoxidation processes (see Supporting Materials for details) were set up to study its effect on adsorption efficiency. In the study of PAN-based ACF preoxidation, temperature of preoxidation is almost the same [30], and the main difference lies in the different piecewise heating programs. These processes were used in most of the reported studies [19, 31,32,33,34]. The most suitable process for the adsorption of CS2 was tried to find out. The test results are shown in Fig. 3, in which the adsorption efficiency was plotted as a function of time. The CS2 adsorption efficiency decreased with time for all sample eventually reached a minimum. Obviously, the ACF(I700)-CO2(800 + 60) has been identified as the most promising adsorbent for the adsorption of CS2. The experimentally determined breakthrough adsorption capacities of the ACF prepared by different preoxidation processes are listed in Table 1. Compared with the other ACF, the ACF(I700)-CO2(800 + 60) has larger breakthrough adsorption capacities, measured as 22.51 mgS/g for fresh ACF. According to the literature [19], if when the preoxidation temperature was set to below 180 °C, the fibers were not sufficiently thermally stabilized to the central part of the fiber. Moreover, the temperature could not be set above the fusing temperature (about 310 °C) of the precursor, and high-temperature preoxidation causes the over-absorption of oxygen. These reactions worsened the mechanical properties and the defects in microstructure. On the one hand, for ACF(II700)-CO2(800 + 60), the preoxidation temperature was higher than that of ACF(I700)-CO2(800 + 60); maybe, it is because 280 °C is closer to the fusing temperature of the precursor and it is bad for the formation of microstructures. On the other hand, for ACF(III700)-CO2(800 + 60) and ACF(IV700)-CO2(800 + 60), the lower preoxidation temperature did not achieve the desired effect; meanwhile, the staged calcining did not make ACF more stable (better adsorption activity). It is speculated that the ACF(I700)-CO2(800 + 60) may produce more adsorption sites for the adsorption of CS2.
Effect of the different carbonization temperatures
The carbonization process could remove most of the organic composition and volatile components in the raw materials, which could increase the fixed carbon contents and reduce the ash content. This is the key step of producing ACF, because it could have the initial porosity with this step [5]. In this study, the influence of different carbonization temperatures (600, 700, 800 and 900 °C) on the adsorption of CS2 was investigated. The adsorption test results are shown in Fig. 4, in which the adsorption efficiency was plotted as a function of time. From Fig. 4, it can be seen that when the carbonization temperature increased from 600 to 700 °C, the adsorption activity of ACF for CS2 increased significantly. The activity of ACF decreased as the carbonization temperature continued to increase. The activity of ACF(I800)-CO2(800 + 60) was lower than that of ACF(I700)-CO2(800 + 60). The activity of ACF was the worst when the carbonization temperature was 900 °C. When the carbonization temperature was 700 °C, after 60 min the adsorption efficiency reached 100% and was maintained at 150 min. The experimentally determined breakthrough adsorption capacities of the ACF prepared by different carbonization temperatures are listed in Table 2. With the increase in carbonization temperature, the breakthrough adsorption capacity first increased and then decreased. When the carbonization temperature was 700 °C, the ACF(I700)-CO2(800 + 60) had the highest breakthrough adsorption capacity (21.75 mgS/g). The carbonization process could mainly produce initial porosity and pore size, and the number determines the pore volume. This is one of the main factors influencing the adsorption efficiency of ACF. At the lower carbonization temperature, fewer initial pores were produced in the raw material. With the increase in temperature, the initial hole increases. When the temperature is lower, the initial pore may be mainly mesoporous, and with the increase in carbonization temperature, the micropores start to appear. When the carbonization temperature is too high, micropores can collapse and the polycondensation reaction results in microporous decreasing dramatically and mesoporous increasing a great deal [35]. However, micropores can play a major role in the gas adsorption, the number and volume of the micropores determining the ACF adsorption capacity. This is an important factor that affects the adsorption of CS2 over ACF.
Effect of the different CO2 activation temperature and time
The activation of ACF mainly includes physical activation and chemical activation. In this study, CO2 was used as a physical activator, and the influence of different activation temperature (600, 700, 800 and 900 °C) and time (30, 60, 90 and 120 min) on the adsorption of CS2 was investigated. As shown in Fig. 5, with the increase in activation temperature, the adsorption efficiency of CS2 firstly increased and then decreased. When the activation temperature was 900 °C, the 100% removal rate of CS2 could last for 420 min. The same phenomenon was also observed at different activation time, with the increase in activation time, the adsorption efficiency of CS2 firstly increased and then decreased. When the activation time was 90 min, the 100% removal rate of CS2 could last for 390 min. The experimentally determined breakthrough adsorption capacities of the ACF prepared by different activation temperature and time are listed in Table 3. With the increase in activation temperature and time, the breakthrough adsorption capacity first increased and then decreased. When the activation temperature was 900 °C and the activation time was 90 min, the ACF(I700)-CO2(900 + 60) and ACF(I700)-CO2(900 + 90) had the highest breakthrough adsorption capacity (50.16 mgS/g and 55.63 mgS/g, respectively).
By activating in CO2 at high temperature, micropores suitable for adsorption purpose would appear on the surface and the internal of the ACF [36]. Increasing the activation temperature and prolonging the activation time all contribute to an increase in the reactivity of the activator CO2 with the carbon fiber, accelerating the reaction speed of the CO2 and the carbon fiber, thereby increasing the volume of the micropore and increasing the specific surface area of the product to increase the adsorption amount. If the temperature is too high and the time is too long, it will cause excessive ablation of the fiber, resulting in an increase in the volume of the micropore and a decrease in the specific surface area, resulting in a decrease in the amount of adsorption and a decrease in the yield [37].
Effect of the different KOH activation conditions
Another activation process was performed by the chemical method using potassium hydroxide (KOH). This method is the most effective way of the activation and development of a porous structure in carbon materials [38]. In this study, the influence of different carbon fiber/KOH ratios (2:1, 1:1, 1:2, 1:3), activation temperatures (600, 700, 800, 900 and 1000 °C) and time (30, 60, 90 and 120 min) on the adsorption of CS2 was investigated. As shown in Fig. 6a, with the increase in carbon/KOH ratio, the adsorption efficiency of CS2 firstly increased and then decreased. When the carbon/KOH ratio was 1:1, the 100% removal rate of CS2 could last for 330 min. The experimentally determined breakthrough adsorption capacities of the ACF prepared by different KOH activation conditions are listed in Table 4. It can be found intuitively that the ACF(I700)-KOH(1:1 + 800 + 60) had the highest breakthrough adsorption capacity (48.86 mgS/g). This may be because less KOH is not enough to activate ACF, leaving no sufficiently developed pore structure on the ACF to adsorb CS2. Increasing the carbon/KOH ratio can result in a more worrying pore structure, but too much KOH will destroy the pore structure of the ACF and reduce the sulfur capacity of the ACF.
During the activation process, high temperature increases the activation energy necessary for the activation reaction, and the activation temperature and time directly affect the activation process of ACF. The influence of different activation temperatures and time on the adsorption of CS2 is shown in Fig. 6b, c. With the increase in activation temperature and time, the adsorption efficiency of CS2 firstly increased and then decreased. When the activation temperature was 900 °C, the 100% removal rate of CS2 could last for 420 min. When the activation time was 60 min, the 100% removal rate of CS2 could last for 420 min. The experimentally determined breakthrough adsorption capacities of the ACF prepared by different KOH activation temperature and time are listed in Table 4. With the increase in activation temperature and time, the breakthrough adsorption capacity first increased and then decreased. When the KOH activation temperature was 900 °C the activation time was 60 min, the ACF(I700)-KOH(1:1 + 900 + 60) had the highest breakthrough adsorption capacity (49.52 mgS/g). Low activation temperatures do not provide sufficient energy for the interaction between KOH and C, resulting in incomplete activation. However, the excessive temperature or time destroys the pore structure of the ACF to a certain extent, so that the adsorption capacity of the ACF is lowered.
Characterization results of ACF
SEM
The scanning electron microscope (SEM) image of activated sample ACF(I700)-CO2(900 + 90) is shown in Fig. 7. From Fig. 7, the ACF elementary filaments have a diameter around 15 μm. Their relative small diameter enhances the adsorption kinetics by reducing the mass transfer limitations (between the outer surface and the active sites for adsorption inside the pores). Moreover, such thin filaments render the macrostructure of the ACF very open, avoiding high pressure drop at fast gas passage during adsorption process [39]. Hence, ACF adsorbent bed allows a higher gas throughput as compared to a bed composed of granular adsorbents such as activated carbon, zeolite or silica. This is the reason why as-prepared ACF has such a good CS2 adsorption activity.
CO2-TPD
The basic sites on the ACF surface are beneficial for the adsorption of CS2. To better explain the influence of some preparation conditions (preoxidation process, carbonization temperature and CO2 activation temperature) on the basic sites of ACF, the chemisorption characterization: CO2-temperature-programmed desorption (CO2-TPD) was conducted to investigate the basic sites on the surface. As shown in previous adsorption activity tests, the ACF(I700)-CO2(800 + 60) and ACF(I700)-CO2(900 + 60) adsorbents have better adsorption activity than other ACF. Theoretically speaking, it should provide strengthened basic sites, thus improving the CS2 adsorption activity. The CO2-TPD characterization results are presented in Fig. 8. Some ACF samples present two desorption peeks, which correspond with weak and intermediate strength, respectively. Meanwhile, some ACF samples present three desorption peeks, which correspond with weak, intermediate strength and strong basic sites, respectively. From Fig. 8, it is obvious that the ACF(I700)-CO2(800 + 60) with better CS2 adsorption activity had obvious peaks at the strong basic sites, while the other catalysts did not. However, we can find that the intermediate strength basic sites on the ACF(I700)-CO2(900 + 60) surface with better CS2 adsorption activity were strengthened. There is speculation that the strengthened intermediate strength and strong basic sites are helpful for the adsorption of acid CS2, because of the abundance of the OH group. Therefore, appropriate preparation conditions can provide strengthened basic sites, thus improving the CS2 adsorption activity of ACF.
FTIR
To reveal the different functional groups on the catalyst surface, the FTIR spectra of all ACF samples which were prepared by different conditions were obtained, as shown in Figs. 9–11. It could be easily seen that there were certain differences among their wave number bands, due to the preparing conditions. As shown in Fig. 9, several IR-active vibrations were detected. The band at 3440 cm−1 was -OH group [40]. The weak bands from 1500 cm−1 to 1630 cm−1 could be assigned to bidentate nitrate and monodentate nitrite vibrations, respectively [41]. The band at 1382 cm−1 was the molecular vibration of -C = O groups [42]. It is obvious that the amount of -OH group (3440 cm−1) on ACF(I700)-CO2(800 + 60) surface is much higher than other ACF. The abundant -OH groups from ACF could provide the basic sites on the catalyst surface, which is favored for the adsorption CS2. Therefore, these ACF samples showed an excellent adsorption activity.
Meanwhile, we can see from Fig. 10 that the amount of bidentate nitrate, monodentate nitrite (from 1500 cm−1 to 1630 cm−1) and –C=O groups (1382 cm−1) is the highest on the ACF(I700)-CO2(900 + 60) and ACF(I700)-CO2(900 + 90) surface. Besides, ACF(I700)-CO2(900 + 90) has the highest breakthrough adsorption capacity of CS2 (55.63 mgS/g) according to above adsorption activity test results. It is well known that the most important step in the preparation of carbon-based adsorbents is undoubtedly activation, whether physical or chemical. Combining with this information, it can be speculated that bidentate nitrate, monodentate nitrite and –C=O groups play a very important role in the adsorption CS2 process.
For KOH activation process, same IR-active vibrations were detected (Fig. 11). Compared to the FTIR spectra of different ACF samples activated by different carbon/KOH ratio, KOH activation temperature and time, remarkable differences were observed in the band at 3440 cm−1. Specifically, more -OH groups were present on the surface of ACF(I700)-KOH(1:1 + 900 + 60). As mentioned earlier, the abundant -OH groups from ACF could provide the basic sites on the catalyst surface, which is favored for the adsorption CS2. This is consistent with the results of adsorption activity test.
The adsorption efficiency of the prepared ACF to CS2 is obvious. Except for physical adsorption based on van der Waals force, there must be chemisorption between ACF and CS2. It is well known that alkalinity is good for the removal of CS2 [43]. According to the CO2-TPD and FTIR characterizations results, ACF surface with high adsorption activity contains a large amount of –OH groups, which inferences that –OH groups play an important role in the adsorption of CS2. The -OH groups on the ACF surface may react with CS2 as follows to fix it to ACF:
Conclusion
The PAN-based ACF adsorbents were prepared by CO2 and KOH activation method, respectively, and were used for the adsorption of CS2 at room temperature. The best breakthrough adsorption capacity of CS2 was 55.63 mgS/g when the ACF was activated by CO2 [ACF(I700)-CO2(900 + 90)]. The best breakthrough adsorption capacity of CS2 was 49.52 mgS/g when the activator is KOH [ACF(I700)-KOH(1:1 + 900 + 60)]. The SEM results indicated that thin filaments render the macrostructure of the ACF allows a higher gas throughput. The CO2-TPD and FTIR results showed that the abundance of the -OH group was present on the surface of ACF under the optimal preparation conditions, and these -OH groups could provide the basic sites on the catalyst surface, which is favored for the adsorption CS2. The -OH groups on the ACF surface may react with CS2 to form -SH, which fixes CS2 to ACF. Future research includes studies on the adsorption mechanism and regeneration of ACF adsorbent.
References
X. Song, P. Ning, C. Wang, K. Li, L. Tang, X. Sun, H. Ruan, Chem. Eng. J. 314, 418 (2017)
D. He, H. Yi, X. Tang, P. Ning, K. Li, H. Wang, S. Zhao, J. Mol. Catal. A: Chem. 357, 44 (2012)
T. Toops, M. Crocker, Appl. Catal. B: Environ. 82, 199 (2008)
D.E. Sparks, T. Morgan, P.M. Patterson, S.A. Tackett, E. Morris, M. Crocker, Appl. Catal. B: Environm. 82, 190 (2008)
K. Li, H. Ruan, P. Ning, C. Wang, X. Sun, X. Song, S. Han, Res. Chem. Intermed. 44, 1209 (2018)
X. Chen, B. Shen, H. Sun, G. Zhan, Z. Huo, Ind. Eng. Chem. Res. 56, 6499 (2017)
Q. Li, H. Yi, X. Tang, S. Zhao, B. Zhao, D. Liu, F. Gao, Chem. Eng. J. 284, 103 (2016)
F. Wang, X. Wang, P. Ning, X. Jing, Y. Ma, P. Wang, W. Chen, Adsorption 21, 401 (2015)
H. Yi, S. Zhao, X. Tang, C. Song, F. Gao, B. Zhang, Z. Wang, Y. Zuo, Fuel 128, 268 (2014)
P. Ning, K. Li, H. Yi, X. Tang, J. Peng, D. He, H. Wang, S. Zhao, J. Phys. Chem. C 116, 17055 (2012)
X.Q. Wang, F. Wang, W. Chen, P. Ning, Y.X. Ma, L.L. Wang, Z.T. Bian, Ind. Eng. Chem. Res. 53, 13626 (2014)
R.D. Milton, R. Cai, S. Abdellaoui, D. Leech, A.L. De Lacey, M. Pita, S.D. Minteer, Angewandte Chemie Int. Edn. 56, 2680 (2017)
A. Puziy, O. Poddubnaya, M. Sobiesiak, B. Gawdzik, Adsorption 19, 717 (2013)
M.B. López, A. Martınez-Alonso, J. Tascón, Microporous Mesoporous Mater. 34, 171 (2000)
K. Torchała, K. Kierzek, G. Gryglewicz, J. Machnikowski, Electrochimica Acta 167, 348 (2015)
C. Ma, J. Chen, Q. Fan, J. Guo, W. Liu, E. Cao, J. Shi, Y. Song, J. Mater. Sci. 53, 4527 (2018)
S. Zhao, C. Tao, A. Zhang, X. Zhang, Z. Yang, C. Zhang, Y. Wan, X. Zhao, New Chem. Mater. 46, 87 (2018)
S. Han, L. Xu, W. Cao, H. Yao, L. Bai, New Carb. Mater. 21, 54 (2006)
Y.J. Su, T.H. Ko, J.H. Lin, J. Appl. Polym. Sci. 108, 3610 (2008)
A. Gopinath, K. Kadirvelu, Environ. Chem. Lett. 16, 1137 (2018)
C. Faur, H. Métivier-Pignon, P. Le Cloirec, Adsorption 11, 479 (2005)
H. Fallou, N. Cimetiere, S. Giraudet, D. Wolbert, P. Le Cloirec, J. Environ. Manag. 166, 544 (2016)
K. Kadirvelu, C. Faur-Brasquet, P.L. Cloirec, Langmuir 16, 8404 (2000)
C. Lorimier, A. Subrenat, L. Le Coq, P. Le Cloirec, Environ. Technol. 26, 1217 (2005)
I. Mochida, Y. Korai, M. Shirahama, S. Kawano, T. Hada, Y. Seo, M. Yoshikawa, A. Yasutake, Carbon 38, 227 (2000)
A.S. Subrenat, P.A. Le Cloirec, Chem. Eng. Commun. 193, 478 (2006)
J. Yang, P. Juan, Z. Shen, R. Guo, J. Jia, H. Fang, Y. Wang, Carbon 44, 1367 (2006)
A. Sedghi, R.E. Farsani, A. Shokuhfar, J. Mater. Process. Technol. 198, 60 (2008)
M.S.A. Rahaman, A.F. Ismail, A. Mustafa, Polym. Degrad. Stab. 92, 1421 (2007)
J. Sun, C. He, S. Zhu, Q. Wang, J. Appl. Polym. Sci. 106, 470 (2007)
E. Gliścińska, K. Babeł, Fibres Textiles in Eastern Europe, (2013)
Y. Yang, Z. Mei-Hua, X. Gang, J. Zheng-Xiong, J. Porous Mater. 18, 379 (2010)
J. Sun, G. Wu, Q. Wang, J. Appl. Polym. Sci. 97, 2155 (2005)
H.-Y. Hsiao, C.-M. Huang, M.-Y. Hsu, H. Chen, Separat. Purif. Technol. 82, 19 (2011)
O. Ioannidou, A. Zabaniotou, Renew. Sustain. Energy Rev. 11, 1966 (2007)
J. Sun, Q. Wang, J. Appl. Polym. Sci. 100, 3778 (2006)
C. Gong, H. Dai, S. Wang, Carb. Tech. 31, a9 (2012)
E. Gliścińska, K. Babeł, Fibres Text. East. Eur. 3, 1230 (2013)
G.B. Baur, J. Spring and L. Kiwi-Minsker Adsorption 24, 725 (2018)
P. Lu, C. Li, G. Zeng, L. He, D. Peng, H. Cui, S. Li, Y. Zhai, Appl. Catal. B: Environ. 96, 157 (2010)
G. Qi, R.T. Yang, R. Chang, Appl. Catal. B: Environ. 51, 93 (2004)
S. Velu, V. Ramkumar, A. Narayanan, C. Swamy, J. Mater. Sci. 32, 957 (1997)
X. Sun, H. Ruan, X. Song, L. Sun, K. Li, P. Ning, C.J.R.A. Wang, 8, 6996 (2018)
Acknowledgements
This work was supported by National Natural Science Foundation of China [51968034, 21667015, 41807373 and 51708266], National Key R&D Program of China [2018YFC0213400] and the Analysis and Testing Foundation of Kunming University of Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, K., Li, K., Wang, C. et al. Preparation of polyacrylonitrile-based activated carbon fiber for CS2 adsorption. Res Chem Intermed 46, 3459–3476 (2020). https://doi.org/10.1007/s11164-020-04156-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04156-1