Abstract
In this study, polyacrylonitrile (PAN)-based carbon fiber with high nitrogen content was activated at 800 °C with sodium carbonate and heat-treated at 950 °C to prepare activated carbon fiber (ACF), and the results of nitrate ion adsorption on the prepared ACF are presented. CHN elemental analysis, XPS measurement, and Boehm titration were used to determine the nitrogen content and surface functional groups of ACF. It is discussed that the total amount of nitrogen decreases, whereas quaternary nitrogen (N-Q) increases upon heat treatment. The decrease in adsorption capacity of the prepared activated carbon under different storage conditions is shown. It is observed that the adsorption capacity of nitrate ion at equilibrium pH (pHe) 5 is halved after 5 weeks, and the decrease in adsorption capacity at pHe 3 is suppressed. The adsorption isotherms of the prepared ACF are shown using the Langmuir equation. The effect of pH on the adsorption capacity of the prepared ACF is compared with that of ACF before heat treatment and zinc chloride-activated powdered activated carbon. The adsorption capacity of ACF without heat treatment at 950 °C decreases as the pHe of the solution increases, and the pH of the nitrate solution including ACF after heat treatment is stable at pHe 4–5.
Article highlights
-
Activated carbon fiber with high quaternary nitrogen content was prepared by Na2CO3 activation of PAN-based carbon fiber.
-
Storage of activated carbon fiber in vacuum and in 1 M hydrochloric acid solution was stable.
-
ACF with heat treatment at 950 °C had a solution equilibrium pH of 4–5 and a stable adsorption rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pollution by nitrate ion causes eutrophication, and red tides in coastal zone [1, 2]. These problems further lead to deterioration of landscape, odor, and adverse effect on ecosystem of aquatic organisms. There is a possibility that infants become methemoglobinemia by taking polluted ground water with nitrate ion [3]. Nitrate ions uptaken into the human body may also produce N-nitrosamines, which are precursors of cancer in the digestive system [4]. Therefore, removal method for nitrate ion is widely and urgently needed. There are several reports on removal methods for nitrate ion including adsorption, ion exchange [5, 6], reverse osmosis [7], and biological denitrification [8]. Adsorption is easy to operate and low cost [9, 10].
Activated carbon (AC) including activated carbon fiber (ACF) is also one of the most common adsorbents in water purification [11]. AC is insoluble in water, making it easy to recover and can be used repeatedly as an adsorbent by washing with acid and/or base. AC is characterized by its porous structure and the presence of many functional groups. The abundance of functional groups such as carboxyl, carbonyl, phenol, lactone, and quinone groups makes it particularly well suited for the removal of organic materials [12].
The adsorption performance of ACF depends on surface area, pore distribution, and surface chemistry. This study focused on improving the performance of the adsorbent by modifying the surface chemistry. The negatively charged functional groups of AC such as carboxyl, carbonyl, phenol, lactone, and quinone groups do not adsorb anions including nitrate ions. Previous studies have revealed that quaternary nitrogen (N-Q) and C-π sites can be adsorption site of activated carbon [13]. As the positively charged N-Q content on the activated carbon surface increases, the amount of nitrate ion adsorption also increases. On the other hand, pyridinic nitrogen (N-6) and pyrrolic nitrogen (N-5) are slightly negatively charged repelling nitrate ions. A proton is adsorbed on the C-π site at low pH to become positively charged surface, and the nitrate ion is adsorbed on the protonated surface. Therefore, C-π sites are easily affected by solution pH and do not work at higher pH. N-Q and C-π sites increase when activated carbon is heat-treated to release hydrogen and spread graphene-like structures. N-6 and N-5 become N-Q during the treatment at higher temperature [14].
There are various methods for doping AC with nitrogen, including the use of ammonia gas [15], nitrogen-rich biomaterials [16], and urea [17]. In this study, activated carbon fiber (ACF) is prepared by activating polyacrylonitrile (PAN) carbon fiber with high nitrogen content with sodium carbonate, based on a report by Sakamoto et al. and Machida et al. [18, 19]. PAN-based carbon fiber activated by sodium carbonate is felt-like, easy to mold, and easy to wash. Sodium carbonate was selected because it is more environmentally friendly than zinc chloride [20].
There have been few studies on the degradation of ACF due to long-term storage. Therefore, we investigate the degradation of adsorption capacity of prepared activated carbon under different storage conditions. The prepared ACF is felt-like and can be easily collected from solution. Therefore, we examined storage method of ACF soaking in a liquid. For the liquid, we used hydrochloric acid and sodium hydroxide. Therefore, we soak ACF in a liquid whose pH was adjusted with hydrochloric acid and/or sodium hydroxide to preserve it.
In Sect. 3.1 are shown the physical properties of ACF from CHN elemental analysis, XPS measurements, and Boehm titration to indicate the physical properties of ACF. In the next section, the decrease in adsorption capacity of the prepared activated carbon under different storage conditions is displayed. It is observed that the adsorption capacity of nitrate ion at equilibrium solution pH (pHe) 5 is halved after 5 weeks, and the decrease in adsorption capacity at pHe 3 is suppressed. In Sect. 3.3, the adsorption isotherms of the prepared ACF are shown using the Langmuir equation. In Sect. 3.4, the effect of pH on the adsorption capacity of the prepared ACF is compared with that of ACF before heat treatment and zinc chloride-activated powdery activated carbon. The adsorption capacity of ACF without heat treatment at 950 °C decreases as the pHe of the solution increases, and the pH of the nitrate solution containing ACF after heat treatment is stable at pHe 4–5.
2 Materials and methods
2.1 Sample preparation
2.1.1 Sodium carbonate activation
A commercially available air-stabilized PAN-based carbon fiber, PYROMEX (Teijin Ltd, hereafter denoted as PYR), was used as the precursor for the adsorbent. PYR is a PAN-based carbon fiber with a high nitrogen content of 20% [21]. PYR was activated with Na2CO3 to increase surface area. Sodium carbonate in the same mass as PYR (8 g) was dissolved in 500 mL of distilled water, and the PYR soaked in this solution was stirred for 24 h. It was then dried in an oven at 110 °C for at least 12 h. PYR was heated in a tube furnace at 800 °C for 30 min under a nitrogen gas atmosphere. The prepared ACF was washed with 1 M hydrochloric acid and boiling distilled water to remove remaining sodium carbonate. The ACF was dried in an oven at 110 °C overnight. Based on the combination of PYR, sodium carbonate activation (SC), treatment temperature of 800 °C, and weight ratio of sodium carbonate of 1.0, the prepared sample was designated as PYR-8SC1.
2.1.2 Heat treatment
Heat treatment (annealing) was performed to convert N-5 and N-6 nitrogen on PYR-8SC1 to N-Q. PYR-8SC1 was heated in a tube furnace at 950 °C for 30 min under a pure helium gas atmosphere, in which quartz wool was placed in front of PYR-8SC1 sample to uniformly maintain the high temperature. That heat-treated ACF was designated as PYR-8SC1-9.5HT30.
2.1.3 Zinc chloride activated carbon
As a sample for comparison, zinc chloride activated carbon was also prepared according to the report by Kino et al. and Matsuzawa et al. [22, 23]. Four times the mass of ZnCl2 of PYR was dissolved in distilled water. ZnCl2 was not completely dissolved at room temperature and the solution remained turbid. The PYR was soaked in this solution and dried in an oven at 110 °C for at least 12 h. The PYR was heated in a tube furnace at 850 °C for 30 min under a nitrogen gas atmosphere in which fibrous morphology of PYR was no longer maintained. The prepared activated carbon (AC) was washed with 1 M HCl and boiling in distilled water, then thoroughly washed in a Soxhlet extractor overnight to remove any remaining zinc chloride. AC was heated at 950 °C for 10 min in a tube furnace under a helium gas atmosphere and designated PYR-8.5Z4-9.5HT10.
2.2 Elemental analysis
CHN elemental analysis was performed to determine the nitrogen content of ACF. Samples dried overnight at 110 °C were wrapped in tin foil and burned at 1000 °C to determine the content of carbon (C), hydrogen (N), and nitrogen (N) using TCD detector. Composition of ACF was assumed to be only C, H, N, and oxygen (O). The sum of the obtained compositional content of C, H, and N was subtracted from 100%, to calculate the content of O [24]. The measured samples were PYR-8SC1, PYR-8SC1-9.5HT30, PYR, and as a reference adsorbent of PYR-8.5Z4-9.5HT10.
2.3 Scanning electron microscopy
The morphology of ACF activated with sodium carbonate and AC activated with zinc chloride was compared by SEM images. The images were taken by scanning electron microscopy (SEM, JSM-6510 A, Japan).
2.4 X-ray photoelectron spectroscopy
Surface characterization of ACF was conducted by X-ray photoelectron spectroscopy (XPS). The N1s signal obtained by XPS measurements was deconvoluted to pyridine nitrogen (N-6, 398.7 ± 0.3 eV), pyrrole nitrogen (N-5, 400.4 ± 0.3 eV), quaternary nitrogen (N-Q, 401.1 ± 0.3 eV), and pyridine N-oxide (N-X, 402–404 eV) [14, 25].
2.5 The surface area and pore size distribution
The surface area and pore size distribution of the prepared ACF were determined from N2 adsorption-desorption isotherms at − 196 °C using a BELSORP-mini II surface analyzer (MicrotracBEL, Japan). Surface area (SBET), average pore size (Davg) and total pore volume (Vtotal) were obtained by the BET method. Micropore volume (Vmicro) was obtained by the t-plot method. Mesopore volume (Vmeso) was calculated by subtracting Vmicro from Vtotal.
2.6 Boehm titration
Boehm titration was carried out to determine the acidic functional groups including N-Q on the ACF. Boehm titration is a method to calculate the functional groups on a surface using the difference in acidity between the adsorbent and the adsorbate [26]. An 80 mg of ACF was added to 40 mL of 0.05 M base solution (Na2CO3, NaHCO3, NaOH) or 0.05 M HCl. They were shaken at 100 rpm for 72 h. After shaking, 5 mL of each solution was sampled and titrated with 0.05 mol/L HCl. As for 0.1 mol/L HCl, back titration was performed by adding 10 mL of 0.1 mol/L NaOH. The concentration difference of NaHCO3 (pKa 6.37) before and after the reaction indicates the number of carboxy groups (pKa 3–6), and the number of carboxy and lactone groups (pKa 7–9) can be calculated from the concentration difference of Na2CO3 (pKa 10.25). Similarly, the number of carboxy, lactone, and phenol groups (pKa 8–11) can be calculated as the total acidic functional groups from the NaOH concentration difference (pKa 15.74). Previous studies have reported that acidic functional groups containing oxygen are decomposed by heat treatment [27], and the temperature at which the decomposition begins is reported to be 100–700 °C [28]. In other words, most of the acidic functional groups in the ACF prepared in this study must be decomposed by sodium carbonate activation at 800 °C. The carboxy groups detected by Boehm titration could be considered as N-Q.
2.7 Nitrate ion adsorption
In this study, nitrate ion adsorption was executed in batch system. A 15 mL of 200 mg-NO3−/L sodium nitrate solution was added to 30 mg of sample. The solution was stirred at room temperature for at least 24 h. The concentration of nitrate ions before and after adsorption was measured by ion chromatography (ICS-1100, Nippon Dionex KK, Japan). Equilibrium adsorption was determined by Eq. (1).
where, C0 and Ce indicate the initial and the equilibrium nitrate concentration (mmol/L), respectively, V depicts solution volume (mL), and W represents the weight of adsorbent (mg).
2.8 Effect of storage conditions on degradation
Differences in degradation of adsorbent over time due to different storage methods were examined. Samples were stored for 5 weeks, during the period the equilibrium adsorption capacity for nitrate ions was examined with 1-week intervals, using the same procedure as mentioned in Sect. 2.5. Five preservation methods were used; air and vacuum preservation, and preservation being impregnated with 1 M HCl, NaCl, and NaOH. Air and vacuum storage were carried out in an Erlenmeyer flask and in a cubic shaped desiccator for the vacuum conditions, respectively. Comparison was made between pHe 3, which adsorbs an optimal amount of nitrate ions, and pHe 5, similar to groundwater and the other environmental water.
2.9 Adsorption isotherm
To investigate the adsorption characteristics of ACF, adsorption isotherms were prepared. The adsorption experiments for nitrate ions were conducted using sodium nitrate solutions with the initial concentrations of 10-1000 mg-NO3−/L at pH 3, using the same procedure as described in Sect. 2.4. The equilibrium concentration Ce (mmol/L) and equilibrium adsorption amount Qe (mmol/g) at each initial concentration were applied to the following Langmuir equation to calculate maximum adsorption capacity Xm (mmol/g) and Langmuir adsorption affinity Ke (L/mmol) [29].
3 Results
3.1 Characterization of prepared samples
The results of CHN elemental analysis are shown in Table 1. Comparing PYR-8SC1-9.5HT30 with PYR-8SC1, the N content was reduced from 2.3 to 1.2 after the heat treatment. On the other hand, the amount of nitrate ions adsorbed increased suggesting that nitrogen such as N-5 and N-6, which have negative charges that might repel nitrate ions, was removed from ACF in the form of ammonia or quaternized on the surface by heat treatment at 950 °C. Oxygen (O) also decreased, indicating that acidic functional groups such as carbonyl groups and lactones that repel nitrate ions were removed from ACF in the form of CO and/or CO2.
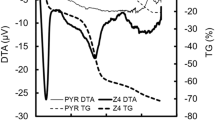
Figure 1 shows SEM images of the prepared ACF and AC. In PYR-8.5Z4-9.5HT10 activated with zinc chloride, it was observed that the fibers stuck to each other due to the strong dehydration effect of zinc chloride. On the other hand, PYR-8SC1-9.5HT30, which was activated with sodium carbonate, was found to retain its fibrous form.
XPS data are exhibited in Fig. 2 and Table 2. As expected from the elemental analysis, the amount of nitrogen decreased but that of N-Q increased, suggesting that the heat treatment enhanced the quaternization of nitrogen. Compared to the relatively stable N-6, the amount of N-5 was more reduced by the heat treatment. Therefore, the inhibition of nitrate ion adsorption by N-5 also decreased and the amount of adsorbed ion increased.
The surface area and pore size distributions are shown in Table 3. The surface area of ACF increased after heat treatment at 950 °C, while pore size distributions were slightly decreased. This would be due to the fact that the fiber itself became thinner, rather than to the formation of pores, as a result of the heat treatment.
The results of Boehm titration are displayed in Table 4. Since the carboxyl group is considered to be released from ACF as carbon dioxide by the heat treatment [28, 30], it is expected that only N-Q corresponds to NaHCO3 adsorption. This result also supports the fact that N-Q is increased by the heat treatment.
3.2 Effect of storage conditions on degradation
The effect of storage conditions on the degradation of the prepared ACF was examined, and the results are shown in Fig. 3. At pHe 5, the C-π sites are affected by the decrease in adsorption capacity due to the lack of protons. This suggests that N-Q, which is less sensitive to solution pH, is more susceptible to degradation over time. It is unlikely that nitrogen naturally disappears from ACF. Therefore, the degradation of N-Q would be due to oxidation by oxygen in the air.
The decrease in adsorption capacity of ACF stored in vacuum and 1 M HCl was relatively small and 75% of the average decrease for all storage methods. Vacuum preservation prevented N-Q from oxidation because ACF was less exposed to air. On the other hand, 1 M HCl preservation made it easier for nitrate ions to adsorb by charging protons to the C-π sites in ACF. Therefore, a large amount of protons may be present on the surface, leading to the inhibition of oxidation. Comparing nitrate and chloride ions, the latter is more easily adsorbed [31], so it is possible that the adsorption sites for nitrate ions is protected by chloride ions during ACF storage.
3.3 Adsorption isotherms
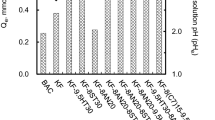
Figure 4 shows the Langmuir isotherms for each sample when the initial concentration was adjusted to 10–1000 mg-NO3−/L and equilibrium solution pH (pHe) 3. Table 5 shows the values of each parameter calculated from the experimental data. Comparing PYR-8SC1 before heat treatment with PYR-8SC1-9.5HT after the treatment, the maximum adsorption Xm increased by ca. 1.5 times, suggesting that the heat treatment may have quaternized the nitrogen. Comparing PYR-8SC1-9.5HT and PYR-8SC1-9.5HT(HCl), there was almost no change in Xm. However, for PYR-8SC1-9.5HT(HCl), the equilibrium adsorption Qe increased from the Langmuir equation when the equilibrium concentration Ce was around 5 mmol/L. This would be due to the adsorption of nitrate ions on protonated surface in the pores of ACF that did not enter when the concentration was low [19].
3.4 Effect of equilibrium pH
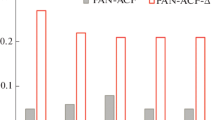
Figure 5 shows the effect of equilibrium solution pH (pHe) on the adsorption capacity for nitrate ions. The nitrate adsorption capacity of PYR-8SC1-9.5HT30 was stable at around pH 5, which is the same as that of environmental water such as groundwater. At around pH 5, nitrate adsorption by C-π sites did not occur, and nitrate adsorption by N-Q sites works. PYR-8SC1-9.5HT30 showed similar adsorption capacity to PYR-8.5Z4(30)-9.5HT10 at solution pHe above 5. Zinc chloride is so strong activated agent that AC cannot retain its fibrous morphology probably due to severe dehydration effect. PYR-8SC1-9.5HT30 is useful because it had a similar adsorption capacity for nitrate ions at pH close to that of ambient water and retained its fibrous quality. The effect of solution pH on the adsorption of PYR-8SC1-9.5HT30 and ACF (PYR-8SC1-9.5HT30(HCl)) stored in 1 M HCl was also unchanged. On the other hand, at high pH, the adsorption of nitrate ions decreased due to the competitive adsorption of nitrate and hydroxide ions.
The nitrate adsorption uptake capacities of PYR-8SC1-9.5HT30 are shown in Table 6, along with other reported adsorbents. The results show that PYR-8SC1-9.5HT30 can effectively adsorb and remove nitrate in water.
4 Conclusion
In this study, PAN-based fibers were activated with sodium carbonate at 800 °C for 30 min and thermal treatment at 950 °C for 30 min to produce activated carbon fiber (PYR-8SC1-9.5HT30). The fiber-like shape of PYR-8SC1-9.5HT30 allows for easy processing and recovery of the adsorbent after adsorption. The adsorption properties of nitrate ions were investigated by adsorption of nitrate ions on the prepared activated carbon fibers. Acidic functional groups that repel nitrate ions such as –COOH and –COO– were decreased by heat treatment resulting in the increase of the adsorption capacity of nitrate ions. The degradation of ACF over time was also examined to determine a suitable storage method and to study the effect of equilibrium solution pH (pHe) on the adsorption capacity. The effect of degradation by long time storage on adsorption at pHe 3 was small. Even if adsorption occurs at pHe 5, degradation can be prevented by vacuum storage or hydrochloric acid storage. At pHe 4–5, which is close to groundwater, the adsorption capacity of the prepared ACF for nitrate ions is stable and has potential for application in actual environmental water.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Amano H, Nakagawa K, Berndtsson R (2018) Surface water chemistry and nitrate pollution in Shimabara, Nagasaki, Japan, Environ. Earth Sci 77:354. https://doi.org/10.1007/s12665-018-7529-9
Abascal E, Gómez-Coma L, Ortiz I, Ortiz A (2022) Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci Total Environ 810:152233. https://doi.org/10.1016/j.scitotenv.2021.152233
Fewtrell L (2004) Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect 112:1371–1374
Gulis G, Czompolyova M, Cerhan JR (2002) An ecologic study of nitrate in municipal drinking water and cancer incidence in Trnava district. Slovakia Environ Res 88:182–187. https://doi.org/10.1006/enrs.2002.4331
Boumediene M, Achour D (2004) Denitrification of the underground waters by specific resin exchange of ion. Desalination 168:187–194. https://doi.org/10.1016/j.desal.2004.06.186
Chabani M, Amrane A, Aïcha B (2006) Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem Eng J 125:111–117. https://doi.org/10.1016/j.cej.2006.08.014
Zhu X, Choo KH, Park JM (2005) Nitrate removal from contaminated water using polyelectrolyte-enhanced ultrafiltration. Desalination 193:350–360. https://doi.org/10.1016/j.desal.2005.06.067
Archna, Sharma SK, Sobti RC (2012) Nitrate removal from ground water: a review. J Chem 9:1667–1675. https://doi.org/10.1155/2012/154616
Tofighy MA, Mohammadi T (2012) Nitrate removal from water using functionalized carbon nanotube sheets. Chem Eng Res Des 90:1815–1822. https://doi.org/10.1016/j.cherd.2012.04.001
Adeleye AS, Conway JR, Garner K, Huang Y, Su Y, Keller AA (2016) Engineered nanomaterials for water treatment and remediation: costs, benefits, and applicability. Chem Eng J 286:640–662. https://doi.org/10.1016/j.cej.2015.10.105
Mazarji M, Aminzadeh B, Baghdadi M, Bhatnagar A (2017) Removal of nitrate from aqueous solution using modified granular activated carbon. J Mol Liq 233:139–148. https://doi.org/10.1016/j.molliq.2017.03.004
Bhatnagara A, Hoglanda W, Marquesab M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511. https://doi.org/10.1016/j.cej.2012.12.038
Tsuchiya Y, Yamaya Y, Amano Y, Machida M (2021) Effect of two types of adsorption sites of activated carbon fibers on nitrate ion adsorption. J Environ Manage 289:112484. https://doi.org/10.1016/j.jenvman.2021.112484
Pels JR, Kapteijn F, Moulijn JA, Zhu Q, Thomas KM (1995) Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 33:1641–1653. https://doi.org/10.1016/0008-6223(95)00154-6
Iida T, Amano Y, Machida M, Imazeki F (2013) Effect of surface property of activated carbon on adsorption of nitrate ion. Chem Pharm Bull 61:1173–1177. https://doi.org/10.1248/cpb.c13-00422
Gao F, Qu J, Zhao Z, Wang Z, Qiu J (2016) Nitrogen-doped activated carbon derived from prawn shells for high-performance supercapacitors. Electrochim Acta 190:1134–1141. https://doi.org/10.1016/j.electacta.2016.01.005
Shi W (2016) Preparation of coralline-like nitrogen-doped porous carbon by urea-assisted pyrolysis of low-cost and environmental friendly polyaniline. Environ Prog Sustain Energy 35:840–846. https://doi.org/10.1002/ep.12331
Sakamoto T, Amano Y, Machida M (2021) Phosphate ion adsorption properties of PAN-based activated carbon fiber prepared with Na2CO3 activation. J Water Chem Technol 43:298–304. https://doi.org/10.3103/S1063455X21040111
Machida M, Tsuchiya Y, Yuan J, Amano Y (2021) Efficient nitrate adsorbent applicable to wide pH range derived from polyacrylonitrile (PAN) fiber. Res Eng 11:100276. https://doi.org/10.1016/j.rineng.2021.100276
Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpää M (2020) Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18:393–415. https://doi.org/10.1007/s10311-019-00955-0
Machida M, Sakamoto T, Sato K, Goto T, Amano Y (2018) Adsorptive removal of nitrate from aqueous phase using steam activated and thermal treated polyacrylonitrile (PAN) fiber. J Fiber Sci Technol 74:158–164https://doi.org/10.2115/fiberst.2018-0023
Kino K, Sakamoto T, Yuan J, Amano Y, Machida M (2022) Quaternary nitrogen functionalized carbonaceous adsorbents to remove nitrate from aqueous phase. Catal Today 388–389:269–273. https://doi.org/10.1016/j.cattod.2020.06.036
Matsuzawa F, Amano Y, Machida M (2021) Phosphate ion adsorption characteristics of PAN-based activated carbon prepared by zinc chloride activation. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03695-3
Sakamoto T, Amano Y, Machida M (2020) Phosphate ion adsorption properties of PAN-based activated carbon fibers prepared with K2CO3 activation. SN Appl Sci 2:702. https://doi.org/10.1007/s42452-020-2465-1
Leng L, Xu S, Liu R, Yu T, Zhuo X, Leng S, Xiong Q, Huang H (2020) Nitrogen containing functional groups of biochar: An overview. Bioresour Technol 298:122286. https://doi.org/10.1016/j.biortech.2019.122286
Boehm HP (2002) Surface oxides on carbon and their analysis: a critical assessment. Carbon 40:145–149. https://doi.org/10.1016/S0008-6223(01)00165-8
Goto T, Amano Y, Machida M, Imazeki F (2015) Effect of polarity of activated carbon surface, solvent and adsorbate on adsorption of aromatic compounds from liquid phase. Chem Pharm Bull 63:726–730. https://doi.org/10.1248/cpb.c15-00039
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143–151. https://doi.org/10.1016/j.jaap.2010.07.006
Tsuchiya Y, Amano Y, Machida M (2020) Nitrate ion adsorption characteristics of activated carbon fibers with and without quaternary nitrogen effective for anion adsorption. Chem Pharm Bull 68:1001–1007. https://doi.org/10.1248/cpb.c20-00364
Sato S, Yoshihara K, Moriyama K, Machida M, Tatsumoto H (2007) Influence of activated carbon surface acidity on adsorption of heavy metal ions and aromatics from aqueous solution. Appl Surf Sci 253:8554–8559. https://doi.org/10.1016/j.apsusc.2007.04.025
Mubita TM, Dykstra JE, Biesheuvel PM, van der Wal A, Porada S (2019) Selective adsorption of nitrate over chloride in microporous carbons. Water Res 164:114885. https://doi.org/10.1016/j.watres.2019.114885
Nassar H, Zyoud A, El-Hamouz A, Tanbour R, Halayqa N, Hilal S H S (2020) Aqueous nitrate ion adsorption/desorption by olive solid waste-based carbon activated using ZnCl2. Sustainable Chem Pharm 18:100335. https://doi.org/10.1016/j.scp.2020.100335
Nunell GV, Fernandez ME, .Bonelli PR, Cukierman AL (2015) Nitrate uptake improvement by modified activated carbons developed from two species of pine cones. J Colloid Interface Sci 440:102–108. https://doi.org/10.1016/j.jcis.2014.10.058
Khan MA, Ahn YT, Kumar M, Lee W, Min B, Kim G, Cho DW, Park WB, Jeon BH (2011) Adsorption studies for the removal of nitrate using modified lignite granular activated carbon. Sep Sci Technol 46:2575–2584. https://doi.org/10.1080/01496395.2011.601782
Demiral H, Gunduzoglu G (2010) Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour Technol 101:1675–1680. https://doi.org/10.1016/j.biortech.2009.09.087
Xia F, Yang HF, Li L, Ren Y, Shi DZ, Chai HX, Ai HN, He Q, Gu L (2019) Enhanced nitrate adsorption by using cetyltrimethylammonium chloride pre-loaded activated carbon. Environ Technol 41:3562–3572. https://doi.org/10.1080/09593330.2019.1615133
Acknowledgements
This study was funded in part by the Japan Society for the Promotion of Science (JSPS) under Grants-in-aid for Scientific Research (C) (KAKENHI Grant No. JP20K05187) and by JST, the establishment of university fellowships towards the creation of science technology innovation (Grant No. JPMJFS2107). The authors would like to express thanks to Chiba Iodine Resource Innovation Center (CIRIC) for the XPS measurements and Center for Analytical Instrumentation of Chiba University for supporting elemental analysis. The authors are also grateful to Shizuka Ishibashi for her dedicate technical assistance with the experiments. Gratitude is greatly extended to Prof. Dr. Reiko Uruma, the head of Safety and Health Organization, Chiba University, for her encouragement on our study.
Funding
This study was funded in part by the Japan Society for the Promotion of Science (JSPS) under Grants-in-aid for Scientific Research (C) (KAKENHI Grant No. JP20K05187) and by JST, the establishment of university fellowships towards the creation of science technology innovation (Grant No. JPMJFS2107).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest directly relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sato, N., Amano, Y. & Machida, M. Adsorption characteristics of nitrate ion by sodium carbonate activated PAN-based activated carbon fiber. SN Appl. Sci. 4, 315 (2022). https://doi.org/10.1007/s42452-022-05191-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05191-w