Abstract
Phosphorus-containing carbons have been obtained by carbonization of porous copolymer of 4,4′-bis(maleimidodiphenyl)methane (50 mol%) and divinylbenzene (50 mol%) in presence of phosphoric acid at temperatures 400–1000 °C. Porous structure was analyzed by nitrogen adsorption isotherms while surface chemistry was investigated by potentiometric titration method. It has been shown that carbons obtained at 500–1000 °C are micro-mesoporous with pore sizes of 1–1.1, 2–3 and 5.4 nm. The most developed porosity was achieved at 600 °C reaching BET surface area 890 m2/g and total pore volume 0.45 cm3/g. Carbons obtained by carbonization of polyimide precursor in presence of phosphoric acid showed acidic character with 30–40 % of phosphate surface groups. Maximum total amount of acidic surface groups was achieved at 800 °C reaching 3.2 mmol/g. Assignment of strongly acidic surface groups to phosphates was corroborated by pK value, phosphorus content and thermal gravimetric analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Activated carbons are widely used in many fields of chemical technology. Great success of modern adsorption techniques has been facilitated significantly by the continuously increasing quality of carbons generated by improvements in their production techniques by using advanced preparation processes as well as carefully selected carbonaceous precursors.
Phosphoric acid activation is widely used for the production of activated carbons. The use of various precursors including wood, nutshells, viscose rayon and coal has been extensively investigated. It has been shown that the maximum surface area is obtained at carbonization temperatures of 350 °C for white oak wood (Jagtoyen and Derbyshire 1993), 450 °C for coconut shell and sub-bituminous coal (Laine et al. 1989) and 500 °C for bituminous coal (Jagtoyen and Derbyshire 1993). Recently, polymer precursors have also been investigated (Castro-Muñiz et al. 2011; Puziy et al. 2002a, b, c, d, 2003, 2007a; Sobiesiak et al. 2006; Suárez-García et al. 2004b).
Polyimides are thermoresistant polymers developed for use in different applications at relatively high temperatures 250–300 °C. Recently polyimides attracted attention of researchers as suitable precursor for preparation of carbons having enhanced performance in such fields as metal ion binding (Puziy et al. 2007a), gas separation (Hatori et al. 2004) and electrode material for supercapacitors (Hulicova-Jurcakova et al. 2009). The most important advantages of polyimides are the good yield and the absence of deformation during carbonization (Inagaki et al. 1999). Previous studies have shown that highly porous spherically shaped carbon having large amount of acid surface groups may be obtained by phosphoric acid activation of polyimide polymer at 800 °C (Puziy et al. 2002a, 2007a; Sobiesiak et al. 2011). Present communication describes porous structure and surface chemistry of polyimide-derived carbons obtained by phosphoric acid activation conducted at different temperatures.
2 Experimental
Parent porous copolymer of 4,4′-bis(maleimidodiphenyl)methane (50 mol%) and divinylbenzene (50 mol%) was obtained by the suspension polymerization method using mixture of 1-decanol and benzyl alcohol as pore-forming agents (Gawdzik and Sobiesiak 2003; Matynia et al. 1996; Sobiesiak et al. 2011).
Parent copolymer (BM-DVB) was impregnated with 60 % phosphoric acid to impregnation ratio 1.1, dried in air and then carbonized in flow of argon (0.5 L/min) at specified temperatures in the range 400–1000 °C for 30 min. After carbonization carbons were extensively washed with hot water in Soxhlet extractor until neutral pH. Carbons were abbreviated as PXXX where XXX stands for carbonization temperature. For comparison purpose a carbon C800 was obtained by carbonization of the same polymer precursor at 800 °C without addition of phosphoric acid.
Porous structure of carbons was characterized by nitrogen adsorption measured at 77 K using Autosorb-6 gas adsorption analyzer (Quantachrome, USA). Prior to measurements samples were outgassed overnight at 200 °C under vacuum. Pore size distribution was calculated by Autosorb-1 software (Quantachrome, USA) using QSDFT method and slit/cylindrical pore model (Gor et al. 2012; Neimark et al. 2009). BET surface area (SBET) was calculated by the standard BET method (Brunauer et al. 1938) using nitrogen adsorption data in the relative pressure range from 0.01 to 0.10 since deviations of the BET plot from linearity was observed for relative pressures <0.01 and >0.10. Total pore volume (Vtot) was calculated by converting the amount of nitrogen adsorbed at a maximum relative pressure to the volume of liquid adsorbate. Micropore volume (Vmi) and mesopore volume (Vme) were calculated from cumulative pore size distribution as volume of pores having size <2 nm and from 2 to 50 nm respectively.
Thermogravimetric (TGA) analysis was carried out using thermal analyzer Netzsch STA 449 F1 Jupiter (Netzsch) in the range from 50 to 1000 °C with a heating rate 10 °C/min. About 5 mg of sample was placed into Al2O3 crucible and analyzed under helium atmosphere. The flow rate of helium was 40 mL/min.
Phosphorus content was measured by energy-dispersive X-ray fluorescence method using ElvaX analyzer (Elvatech, Ukraine).
Surface groups of polyimide-derived carbons were investigated by potentiometric titration conducted at 25 °C in thermostatic vessel under argon flow (Puziy et al. 2001, 2005, 2010). 0.1 g of carbon was placed in 20 mL of 0.1 M NaCl solution, equilibrated for at least 8 h and then titrated with 0.1 M NaOH (base titration part) or 0.1 M HCl (acid titration part) using a 672 Titroprocessor combined with 655 Dosimat (Metrohm, Switzerland). Proton concentration was monitored by means of an LL pH glass electrode (Metrohm, Switzerland). Prior to experiments, the electrode electromotive force was calibrated to proton concentration by blank titration. The amount of protons adsorbed at each titration point was calculated using equation:
where V 0 and V t are the volumes of background electrolyte and volume of titrant added, and m is the mass of adsorbent. Subscripts i and e refer to initial and equilibrium ion concentrations respectively. Proton affinity distributions were calculated by solving adsorption integral equation using CONTIN method (Puziy et al. 1999, 2001, 2002b, 2003, 2005, 2007b):
where Qexp is the experimentally measured proton binding, Qloc is kernel function describing local proton binding to surface center with certain pK, F(pK) is sought for distribution function, called Proton Affinity Distribution (PAD), and pKmin and pKmax are the limits of integration. The distribution function, F(pK), describes the site concentration as a function of proton binding constant, and is a unique characteristic of the adsorbent material. To reproduce both positive and negative parts of proton binding isotherm a combination of two Langmuir equations was used as a local isotherm:
where PZC is point of zero charge.
3 Results and discussion
Carbonization of BM-DVB copolymer impregnated with phosphoric acid resulted in formation of carbonaceous residue with 70 % yield that decreased to 40 % with increasing heat treatment temperature from 400 to 1000 °C (Table 1). It should be noted that addition of phosphoric acid to polymer precursor increased the yield by a factor of 1.7 (compare carbons C800 and P800 obtained at equivalent temperatures).
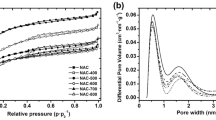
Figure 1 shows nitrogen adsorption isotherms on parent copolymer BM-DVB and polyimide-derived carbons. As expected, there is no steep rise at low relative pressures on the nitrogen adsorption isotherm on the parent copolymer BM–DVB. The isotherm belongs to type IV of the IUPAC classification (Sing et al. 1985) with the H3 hysteresis loop characteristic of capillary condensation in mesopore structures.
Carbonization at low temperature (400 °C) of BM-DVB copolymer impregnated with phosphoric acid has led to almost non-porous carbon material, while increasing heat treatment temperature dramatically increased nitrogen uptake with significant adsorption at low relative pressures characteristic to microporous materials. Carbons obtained at 500–1000 °C are essentially microporous (60 %) with appreciable contribution of mesopores (Table 1). To study the changes in the pore structure in more detail, the pore size distributions (PSD) from nitrogen adsorption isotherms were calculated using QSDFT method using slit/cylindrical pore model (Gor et al. 2012; Neimark et al. 2009). Porous structure of parent copolymer BM-DVB is mainly presented by relatively large pores (<10 nm) with a maximum pore size at 17 nm (Fig. 2). Mesoporous structure is preserved in carbon C800 obtained by carbonization of parent copolymer BM-DVB without phosphoric acid. Pore size distribution for carbon C800 does not show any peak in micropore region due to lack of points on the isotherm. However, micropore volume, calculated from cumulative distribution, amounts 0.14 cm3/g which constitutes a half of total pore volume (Table 1). Pore size distributions (Fig. 2) shows that main porosity of carbons obtained at 500–1000 °C is represented by micropores of size 1–1.1 nm and mesoporous structure of size 2–3 and 5.4 nm. The developed mesoporosity present only in carbons treated with phosphoric acid at temperatures 500–1000 °C suggests that the development was caused by activating action of phosphoric acid. Increasing carbonization temperature from 400 to 600 °C led to drastic increasing BET surface area and pore volumes (Table 1). Heat treatment at 700 °C resulted in decreasing porosity with leveling off at higher temperatures. Slight increase in BET surface area and total pore volume for carbon P900 as compared to P800 could be ascribed to unblocking pore space by elimination of phosphorus compounds due to thermal decomposition and reduction of phosphates to elemental phosphorus (Puzii 2011; Puziy and Tascón 2012). Similar tendency to increasing porosity at 900 °C was observed for carbons obtained from styrene–divinylbenzene copolymer (Puziy et al. 2002c, 2003). Further increasing of heat treatment temperature to 1000 °C has led to slight decreasing BET surface area and total pore volume due to contraction of carbon material.
It is interesting to note that carbon C800 obtained by carbonization of BM-DVB copolymer without addition of phosphoric acid retained mesoporous structure of polymer precursor in the range of 10–50 nm induced by suspension polymerization (Fig. 2). The porosity in this region is completely absent when polymer precursor was impregnated with H3PO4 before carbonization. This fact may be explained by cleaving weak connecting bridges in polymer molecules at sub-pyrolysis temperatures under the attack of phosphoric acid. Subsequently phosphoric acid can catalyze the alkylation of aromatic structures or oligomerization of alkenes (Jagtoyen et al. 1993; Jagtoyen and Derbyshire 1998). Thus, the attack of phosphoric acid led to rearrangement of polymer material and to destruction of porosity initially present in the precursor.
Carbons obtained with addition of phosphoric acid contain significant amount of phosphorus (Table 2). With increasing heat treatment temperature up to 800 °C phosphorus content increased with gradual decreasing at higher temperatures. Similar trend was observed for carbons obtained from both styrene–divinylbenzene (Puziy et al. 2002b, 2003) and lignocellulosic (Puziy et al. 2005, 2007b) precursors. It should be noted that maximum phosphorus content in carbon from polyimide precursor is 1.5 times higher than for carbons from styrene–divinylbenzene and lignocellulosic precursors. This fact could be ascribed to more strong interaction of phosphoric acid with nitrogen-containing polyimide polymer.
Surface groups of polyimide-derived carbons were investigated by potentiometric titration method (Puziy et al. 2001). Proton binding isotherms (Fig. 3) shows positive part (proton adsorption) and negative part (proton desorption or dissociation) with a point of zero charge (PZC) where adsorption and desorption of protons are equal. PZC shifts from neutral to acid region for carbons obtained in presence of phosphoric acid as compared to carbon C800 obtained without H3PO4 (Table 2). The shift is due to formation of surface groups of acidic character. All phosphoric acid activated carbons have much higher total amount of acid surface groups (Qa) as compared to carbon C800 obtained without addition of acid. With increasing carbonization temperature up to 800 °C the total amount of acid surface groups increases and gradually decreases with further rise of heat treatment temperature (Fig. 3; Table 2).
Proton affinity distributions show five types of acid surface groups in phosphoric acid activated carbons (Fig. 4). By comparing dissociation constants of surface groups with that of known simple compounds the surface groups could be assigned to phosphate (pK 1.5–2.3), carboxylic (pK 4.1–5.1), lactone or keto-enol (pK 6.5–7.1, 7.9–8.8) and phenolic (pK 10.1–10.4) groups. Phosphate surface groups appeared in carbons that were obtained at relatively high temperatures (500–1000 °C). The absence of phosphate groups in carbon carbonized at 400 °C is most likely due to absence of porosity, which prevents contact of phosphate groups with solution. With increasing heat treatment temperature the attack of phosphoric acid is advances and results in developing porous structure of carbon.
Assignment of strongly acidic surface groups to phosphates is supported by thermal gravimetric analysis and phosphorus content. DTG curves measured in helium atmosphere (Fig. 5) showed appreciable mass loss in the temperature range of 800–900 °C for all carbons, except P400. Most probably, the mass loss was due to thermal degradation of C–O–P bonding and volatilization of phosphorus compounds. Mass loss in the same temperature range was also observed by other researchers and has been assigned to the rupture of C–O–P bonds (Castro-Muñiz et al. 2011; Labruquère et al. 1998; Oh and Rodriguez 1993; Olivares-Marín et al. 2006; Suárez-García et al. 2004a). The intensity of the mass loss at 800–900 °C followed the same trend as phosphorus content and concentration of phosphate groups—increase with increasing carbonization temperature up to 800 °C followed by decrease at higher temperatures. Figure 6 demonstrates a good linear relationship between concentration of strongly acidic surface groups and both intensity of the mass loss at 800–900 °C and phosphorus content. This fact favors assignment of the most acidic groups to phosphate.
4 Conclusion
The investigation revealed the variations of the porous structure and surface chemistry of carbons obtained by carbonization at different temperatures of BM–DVB copolymer impregnated with phosphoric acid. It has been shown that increasing heat treatment temperature up to 600 °C caused developing porosity of carbons reaching BET surface area 890 m2/g and total pore volume 0.45 cm3/g carbons obtained at higher temperatures had 25–32 % lower specific surface area and total pore volume. All carbons, except those obtained at 400 °C, were micro-mesoporous with pore sizes of 1–1.1, 2–3 and 5.4 nm. Chemical activation of BM–DVB copolymer produced phosphorus-containing carbons with strongly acidic character. About 30–40 % of total amount were strongly acidic with pK 1.5–2.3. These groups were assigned to phosphates. Both total amount and strongly acidic surface groups were at maximum for carbon obtained at 800 °C reaching 3.2 and 0.99 mmol/g respectively. Assignment of strongly acidic surface groups to phosphates was supported by pK value, phosphorus content and by thermal gravimetric analysis.
References
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938). doi:10.1021/ja01269a023
Castro-Muñiz, A., Suárez-García, F., Martínez-Alonso, A., Tascón, J.M.D.: Activated carbon fibers with a high content of surface functional groups by phosphoric acid activation of PPTA. J. Colloid Interface Sci. 361, 307–315 (2011). doi:10.1016/j.jcis.2011.05.064
Gawdzik, B., Sobiesiak, M.: Chemical composition of plasma treated polyimide microspheres. Appl. Surf. Sci. 214, 52–57 (2003). doi:10.1016/S0169-4332(03)00353-2
Gor, G.Y., Thommes, M., Cychosz, K.A., Neimark, A.V.: Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 50, 1583–1590 (2012). doi:10.1016/j.carbon.2011.11.037
Hatori, H., Takagi, H., Yamada, Y.: Gas separation properties of molecular sieving carbon membranes with nanopore channels. Carbon 42, 1169–1173 (2004). doi:10.1016/j.carbon.2003.12.051
Hulicova-Jurcakova, D., Puziy, A.M., Poddubnaya, O.I., et al.: Highly stable performance of supercapacitors from phosphorus-enriched carbons. J. Am. Chem. Soc. 131, 5026–5027 (2009). doi:10.1021/ja809265m
Inagaki, M., Takeichi, T., Hishiyama, Y., Oberlin, A.: High quality graphite films produced from aromatic polyimides. In: Thrower, P.A., Radovic, L.R. (eds.) Chemistry and Physics of Carbon, pp. 245–334. Marcel Dekker, New York, Basel (1999)
Jagtoyen, M., Derbyshire, F.: Some considerations of the origins of porosity in carbons from chemically activated wood. Carbon 31, 1185–1192 (1993). doi:10.1016/0008-6223(93)90071-H
Jagtoyen, M., Groppo, J., Derbyshire, F.: Activated carbons from bituminous coals by reaction with H3PO4: the influence of coal cleaning. Fuel Process. Technol. 34, 85–96 (1993). doi:10.1016/0378-3820(93)90093-J
Jagtoyen, M., Derbyshire, F.: Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 36, 1085–1097 (1998). doi:10.1016/S0008-6223(98)00082-7
Labruquère, S., Pailler, R., Naslain, R., Desbat, B.: Oxidation inhibition of carbon fibre preforms and C/C composites by H3PO4. J. Eur. Ceram. Soc. 18, 1953–1960 (1998). doi:10.1016/S0955-2219(98)00135-6
Laine, J., Calafat, A., Labady, M.: Preparation and characterization of activated carbons from coconut shell impregnated with phosphoric acid. Carbon 27, 191–195 (1989). doi:10.1016/0008-6223(89)90123-1
Matynia, T., Gawdzik, B., Chmielewska, E.: Synthesis and properties of porous copolymers of 4, 4′-bismaleimido diphenyl methane and styrene. J. Appl. Polym. Sci. 60, 1971–1975 (1996). doi:10.1002/(SICI)1097-4628(19960613)60:11<1971:AID-APP21>3.0.CO;2-2
Neimark, A.V., Lin, Y., Ravikovitch, P.I., Thommes, M.: Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 47, 1617–1628 (2009). doi:10.1016/j.carbon.2009.01.050
Oh, S.G., Rodriguez, N.M.: In situ electron microscopy studies of the inhibition of graphite oxidation by phosphorus. J. Mater. Res. 8, 2879–2888 (1993)
Olivares-Marín, M., Fernández-González, C., Macías-García, A., Gómez-Serrano, V.: Thermal behaviour of lignocellulosic material in the presence of phosphoric acid. Influence of the acid content in the initial solution. Carbon 44, 2347–2350 (2006). doi:10.1016/j.carbon.2006.04.004
Puzii, A.M.: Methods of production, structure, and physicochemical characteristics of phosphorylated carbon adsorbents. Theor Exp Chem 47, 277–291 (2011). doi:10.1007/s11237-011-9216-8
Puziy, A.M., Matynia, T., Gawdzik, B., Poddubnaya, O.I.: Use of CONTIN for calculation of adsorption energy distribution. Langmuir 15, 6016–6025 (1999). doi:10.1021/la981369t
Puziy, A.M., Poddubnaya, O.I., Ritter, J.A., et al.: Elucidation of the ion binding mechanism in heterogeneous carbon-composite adsorbents. Carbon 39, 2313–2324 (2001). doi:10.1016/S0008-6223(01)00048-3
Puziy, A.M., Poddubnaya, O.I., Gawdzik, B., et al.: Heterogeneity of synthetic carbons obtained from polyimides. Appl. Surf. Sci. 196, 89–97 (2002a). doi:10.1016/S0169-4332(02)00037-5
Puziy, A.M., Poddubnaya, O.I., Martínez-Alonso, A., et al.: Synthetic carbons activated with phosphoric acid I. Surface chemistry and ion binding properties. Carbon 40, 1493–1505 (2002b). doi:10.1016/S0008-6223(01)00317-7
Puziy, A.M., Poddubnaya, O.I., Martínez-Alonso, A., et al.: Synthetic carbons activated with phosphoric acid. II. Porous structure. Carbon 40, 1507–1519 (2002c). doi:10.1016/S0008-6223(01)00318-9
Puziy, A.M., Poddubnaya, O.I., Martínez-Alonso, A., et al.: Characterization of synthetic carbons activated with phosphoric acid. Appl. Surf. Sci. 200, 196–202 (2002d). doi:10.1016/S0169-4332(02)00883-8
Puziy, A.M., Poddubnaya, O.I., Martínez-Alonso, A., et al.: Synthetic carbons activated with phosphoric acid. III. Carbons prepared in air. Carbon 41, 1181–1191 (2003). doi:10.1016/S0008-6223(03)00031-9
Puziy, A.M., Poddubnaya, O.I., Martínez-Alonso, A., et al.: Surface chemistry of phosphorus-containing carbons of lignocellulosic origin. Carbon 43, 2857–2868 (2005). doi:10.1016/j.carbon.2005.06.014
Puziy, A.M., Poddubnaya, O.I., Gawdzik, B., et al.: Phosphoric acid activation—functionalization and porosity modification. Appl. Surf. Sci. 253, 5736–5740 (2007a). doi:10.1016/j.apsusc.2006.12.034
Puziy, A.M., Poddubnaya, O.I., Martínez-Alonso, A., et al.: Oxygen and phosphorus enriched carbons from lignocellulosic material. Carbon 45, 1941–1950 (2007b). doi:10.1016/j.carbon.2007.06.014
Puziy, A.M., Poddubnaya, O.I., Kochkin, Y.N., et al.: Acid properties of phosphoric acid activated carbons and their catalytic behavior in ethyl-tert-butyl ether synthesis. Carbon 48, 706–713 (2010). doi:10.1016/j.carbon.2009.10.015
Puziy, A.M., Tascón, J.M.D.: Adsorption by phosphorus-containing carbons. In: Tascón, J.M.D. (ed.) Novel Carbon Adsorbents, pp. 245–267. Elsevier, Amsterdam (2012)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., et al.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985). doi:10.1351/pac198557040603
Sobiesiak, M., Gawdzik, B., Puziy, A.M., Poddubnaya, O.I.: Phosphoric acid and steam as activation agents for carbonized porous polymer surfaces. Adsorpt. Sci. Technol. 24, 167–175 (2006). doi:10.1260/026361706778529209
Sobiesiak, M., Gawdzik, B., Puziy, A.M., Poddubnaya, O.I.: Thermal properties of porous copolymers of BM–DVB and their carbonization products. New Carbon Mater. 26, 137–144 (2011). doi:10.1016/S1872-5805(11)60072-3
Suárez-García, F., Villar-Rodil, S., Blanco, C.G., et al.: Effect of phosphoric acid on chemical transformations during nomex Pyrolysis. Chem. Mater. 16, 2639–2647 (2004a). doi:10.1021/cm0349654
Suárez-García, F., Martínez-Alonso, A., Tascón, J.M.D.: Nomex polyaramid as a precursor for activated carbon fibres by phosphoric acid activation. Temperature and time effects. Micropor Mesopor Mater 75, 73–80 (2004b). doi:10.1016/j.micromeso.2004.07.004
Acknowledgments
The research was made possible in part by support from National Academy of Sciences of Ukraine (projects 0110U001330 and 0110U004545).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puziy, A.M., Poddubnaya, O.I., Sobiesiak, M. et al. Structural and surface heterogeneity of phosphorus-containing polyimide-derived carbons: effect of heat treatment temperature. Adsorption 19, 717–722 (2013). https://doi.org/10.1007/s10450-013-9497-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9497-4