Abstract
In this study, potassium pentaborate (santite: KB5O8·4H2O), with a powder diffraction number of 01-072-1688 was synthesized from potassium carbonate (K2CO3), boric acid (H3BO3) and boron oxide (B2O3) with reaction efficiencies between 84.88 and 95.11 %, through a hydrothermal route. Reaction temperatures and times were varied between 60–90 °C and 15–120 min. Synthesized minerals were characterized by X-ray diffraction, Fourier transform infrared and Raman spectroscopies, while surface morphologies were determined by scanning electron microscopy. Thermal dehydration behavior of santite was determined by thermal gravimetry and differential thermal analysis. From the results, santite lost its crystal water via a two-step process between 50 and 450 °C. Activation energies (E a) were calculated by using four non-isothermal kinetic methods and found to be 110.12 and 202.43 kJ/mol by the Coats–Redfern method, 107.77 and 304.18 kJ/mol by the Doyle method, 158.82 and 154.50 kJ/mol by the Kissinger–Akahira–Sunose method, and 158.07 and 156.76 kJ/mol by the Ozawa–Flynn–Wall method for the steps 1 and 2, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boron never appears as a free element in nature, but it can form very different compounds by comparatively small changes in the solution’s mineral content. Turkey hosts about 72.2 % (870 million tons) of the worldwide known reserves of boron. Among these reserved metal borates has been one of the groups of borate minerals subjected to continuous research, making up a wide diversity of industrial usage areas, including glass, ceramics, nuclear, space-aviation, metallurgy, transportation, cosmetics and chemistry [1, 2].
Different sub-classes can be commonly grouped, such as calcium borates, calcium–sodium borates, sodium borates and magnesium borates [3]. However, many synthetic borate compounds have been studied and used in industry apart from the ones that can be found in nature. Potassium pentaborate (KB5O8·4H2O) is widely used as one of the important borates, as it contains 4 mol of structural water per mole of crystal. It is called tetrahydrate potassium pentaborate, commonly known as santite. It has acquired importance among fields such as metal refining, welding, lubricating oil additives, cement, insulation, textiles, fiberglass and as a superior non-linear optical material (NLO) against commonly used borate compounds [4–6].
Among the literature [7–16], it is possible to find several studies on the synthesis of potassium pentaborate. However, most of these methods [8–11] focus on the solid-state field, using high temperatures (800–1400 °C) and long reaction times to produce potassium borate crystals. MacDonald et al. [8] reacted potassium carbonate (K2CO3) and boron oxide (B2O3) at 1400 °C, while Hasim et al. [11] and Alajeram et al. [9] used a reaction temperature of 1200 °C for the reaction of boric acid (H3BO3) and K2CO3. In the experiments conducted by Binu et al. [10], a mixture of K2CO3 and B2O3 was melted at 830 °C. Despite its efficiency, solid-state synthesis has corrosion and handling problems due to the melting of the potassium borates [12]. The hydrothermal type of synthesis has raised interest among these production methods, as back in 1935 Gale [7] was one of the first who attempted to create an industrial method for high efficiency preparation by reacting potassium chloride (KCl) and Na2B10O16 in a 35 °C hydrothermal mixture for the synthesis of K2B10O16. More recent studies such as those by Belokoneva et al. [13] and Wang et al. [14] were performed at higher temperatures and pressures with Belokoneva using 280 °C, 70 atm and Wang using 165 °C. Rajasekar et al. [15] produced KB5O8·4H2O by dissolving K2CO3 and B2O3 and Gurbuz et al. [16] used a 1:5 (as B2O3/K2O) molar ratio mixture to produce anhydrous potassium pentaborate. Therefore, it has been found that most of the available literature focuses on really high temperatures and long reaction times.
Dehydration is the process of removing water from a hydrate structure at a certain temperature. It can be used for different technological and economical purposes, in research for thermal properties, decreasing the mass of hydrate minerals during transport, or it can be a necessary stage of a chemical process [17]. Dehydration reactions are strongly endothermic and many are reversible. In some dehydration examples, the process may occur in several steps, including water elimination and structural recrystallization of the residual product (lower hydrate or anhydrous salt) [18]. Thermal dehydration behaviors of hydrate borate minerals have been studied widely due to the notable properties of boron minerals. There are some studies of thermal kinetics for the different types of hydrated borates such as H3BO3 [19], ulexite (NaCaB5H16O17) [17], sodium borate hydrate (NaB(OH)4·2H2O) [20], mcallisterite (Mg2(B6O7(OH)6)2·9(H2O)) [21], admontite (MgO·3B2O3·7H2O) [22], inderite (MgB3O3(OH)5·5H2O) [23], zinc borate hydrate (Zn3B6O12·3.5H2O) [24], lithium borate [25], howlite (Ca2B5SiH5O14) [26] and tunellite (SrB6H8O14) [26, 27].
As can be seen in the literature for the hydrothermal route, potassium borates can be synthesized at very high reaction temperatures and times so the first novelty of this paper is to synthesize a pure KB5O8·4H2O (santite) mineral with very low reaction temperatures and reaction times by comparison with the literature using the hydrothermal method. The reactor type and crystallization temperature used in the experiments were the same as in the studies of both Derun and Senberber [21] and Derun et al. [22]. Synthesis parameters were selected to focus on the wide ranges of 60–90 °C and 15–120 min, together with the achievement of high reaction yields from the samples. Following the synthesis, the characterization of the minerals have been done by X-ray diffraction (XRD), Fourier transform infrared (FT-IR) and Raman spectroscopies, while surface morphologies were determined by scanning electron microscopy (SEM). The second novelty of this paper is the determination of the thermal dehydration behavior, such as: reaction order, activation energy and exponential factor for dehydration steps of santite, which have not previously been reported in the literature. Thermal dehydration behavior is determined by the equipment of thermal gravimetry and differential thermal analysis (TG/DTA).
Materials and methods
Raw material preparation
In synthesis, H3BO3 and B2O3 were used as boron sources, and were obtained from Bandırma Boron Works (Eti Maden, Balıkesir, Turkey) and sieved through a Fritsch analysette 3 Spartan pulverisette 0 vibratory sieve-shaker (Fritsch, Idar-Oberstein, Germany) (particle size < 75 μm) after being subjected to crushing and grinding by a Retsch RM 100 (Retsch, Haan, Germany). The potassium source was K2CO3 which was supplied by Sigma-Aldrich, St. Louis, USA (CAS Number 584-08-7, with a minimum purity of 99 %) and used without any pretreatment. Identification studies of raw materials were carried out by a Philips PANalytical XPert Pro (PANalytical, Almelo, The Netherlands) X-ray diffractometer (XRD) at 45 kV and 40 mA (λ = 1.53 cm−1) by using Cu-Kα radiation in the 2θ range of 7°–90°.
Hydrothermal synthesis
Two different sets were conducted to synthesize potassium borates, using different sources of boron minerals. For set 1, the used boron source was H3BO3, while B2O3 was used for set 2. In both sets, K2CO3 was used as the potassium source. The reaction temperature and time ranges selected were 60–90 °C and 15–120 min, respectively.
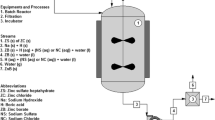
The nucleation cell used during the experiments was a 105-ml glass reactor, covered with the proper lid. The nucleation cell was kept on a thermal platform and the temperature was controlled using a thermocouple with an accuracy of 0.1 °C. In order to achieve a homogeneous temperature and a proper concentration in the entire reactor, a magnetic stirrer located in the thermal platform was used.
In the set 1 and set 2 experiments, the optimum raw material ratio of elemental potassium to elemental boron was determined as 1:5 and 1:6, respectively. The resulting crystal formations were found to be quite different depending both on the molar ratio and on the different temperatures and reaction times used. Generally, low molar ratios and low temperatures lead to a more amorphous crystal and a less homogeneous pattern [28]. For set 1 in the optimum molar ratio, 12.94 × 10−3 mol of H3BO3 was dissolved in 50 mL of deionized water obtained from a GFL 2004 (Gesellschaft für Labortechnik, Burgwedel, Germany) water purification system in a reactor at a determined reaction temperature. Then, 1.29 × 10−3 mol of K2CO3 was added. In set 2, in order to maintain the same stoichiometric amount of boron in set 1, 6.46 × 10−3 mol of B2O3 was used along with 1.08 × 10−3 mol K2CO3. After the required reaction time, the mixture was removed from the nucleation cell and placed in a crystallizer glass and allowed to evaporate in a controlled atmosphere of 40 °C for 48 h, which is enough time for crystalization to occur.
The resulting crystals were removed from the crystallizer, ground, and then purified by ethanol to remove the excess of H3BO3. The samples were allowed to dry at 40 °C in an incubator (Ecocell LSIS-B2V/EC55; MMM Medcenter Einrichtungen, Planegg, Germany) and then weighed and analyzed. Expected reaction equations are shown in Eqs. (1) and (2). The reaction scheme is shown in Fig. 1.
Characterization
After the synthesis processes, the synthesized compounds were identified by XRD, FT-IR and Raman spectroscopy techniques. The XRD parameters used as same as in “Raw material preparation” except 2θ range was selected between 10º– 90º. The characteristic peaks of the borate compounds were in the range 1500–500 cm−1 [28, 29]. Based on the literature, for FT-IR and Raman spectroscopies, the spectrum ranges were determined as 1800–650 and 1800–250 cm−1, respectively. FT-IR analyses were carried out by a PerkinElmer Spectrum One FT-IR (PerkinElmer, MA, USA) with a universal attenuation total reflectance (ATR) sampling accessory with a diamond/ZnSe crystal. A Perkin Elmer brand Raman Station 400F (PerkinElmer) was used for Raman spectroscopy, which, coupled with vibrational (FT-IR) spectroscopy, enabled observations on the molecular structure of the potassium borate mineral.
The surface morphologies and particle properties of the synthesized compounds were investigated by using a CamScan Apollo 300 field-emission SEM (CamScan, Oxford, UK) at 20 kV. The detector used was back scattering electron (BEI) and the magnification was set to 10,000.
Reaction yields
The reaction yields were calculated using the method given by Derun et al. [22] and Kipcak et al. [1, 30–32]. In this method, K2CO3 were identified as the key component in the first and second sets of experiments.
Specifically, the number of moles of product at the final stage, N D , was divided by the number of consumed moles of the key reactant, A, to calculate the overall yield, Y D (3). The number of moles of A that were consumed was calculated using the initial (N A0) and final (N A ) moles of the reactant. For a batch system, the equation becomes [33]
The calculations of the reaction yields were completed in triplicate.
Thermal dehydration kinetics
The thermal dehydration behavior of potassium borate mineral was studied between temperatures of 50 and 550 °C with a PerkinElmer Diamond TG/DTA (PerkinElmer). The thermal behavior of the synthesized potassium borate mineral was subjected to different heating rates (β) of 5, 15, and 20 °C/min in an inert (N2) atmosphere. Kinetic parameters such as the activation energy (E a) and exponential factor (k 0) were calculated by Coats–Redfern, Doyle, Kissinger–Akahira–Sunose (KAS) and Ozawa–Flynn–Wall (OFW) non-isothermal kinetic methods.
In the Coats–Redfern kinetic method, data from the TG curve in the dehydration range were used to determine the kinetic parameters. These were calculated using the plot of conversion (α) as a function of temperature (T). The Coats–Redfern kinetic method equations are given by (4) and (5):
In the Doyle kinetic method, kinetic parameters were calculated by using the peak temperatures of the DTA curves. The values of –log β were plotted against 1/T m . The slope of line was 0.4567 × E/R. The equation for the Doyle kinetic method is given by (6) and (7):
The KAS kinetic model uses the same approximation asthe Coats–Redfern method and determines kinetic parameters from the plots which are drawn ln (β/T 2) versus 1/T where k 0 is the exponential factor, g(α) is the integral conversion kinetic function, R is gas constant and E a is activation energy (8).
The OFW kinetic model (9) gives the kinetic parameters from a plot of the logarithm of heating rates versus 1/T for a given value of conversion at various heating rates (β).
The highest scored potassium borate was calcined in a Protherm MOS 180/4 high-temperature furnace under a nitrogen atmosphere flowing at 5 mL/min in order to investigate the thermal conversion of the potassium borate mineral. Prior to thermal analysis, calcination temperatures were determined as 450 and 550 °C. Crystal lattice structures of calcined minerals were identified by XRD and changes in morphologies were studied by SEM.
Results and discussion
Results of the raw material characterization
According to the XRD results of raw materials, the potassium source was identified as the mixture of three different crystal structures of “potassium carbonate (K2CO3)” with the powder diffraction file numbers (pdf nos.) of: 00-049-1093, 00-016-0820 and 01-087-0730. The XRD results of the boron sources showed that H3BO3 was defined as sassolite (H3BO3) with pdf no. of 01-073-2158 and B2O3 was determined to be a mixture of two types of boron oxide with the pdf nos. of 00-006-0297 and 01-088-2485.
XRD results of the synthesized potassium borates
The synthesized potassium borate XRD results are given in Table 1, from which it can be seen that, at all reaction temperatures and reaction times, santite (KB5O8·4H2O) mineral, which is a special kind of potassium borate, was synthesized. Its pdf no. is 01-072-1688 and crystallized in the orthorhombic crystal structure. The detailed crystallographic data for santite are given in Table 2.
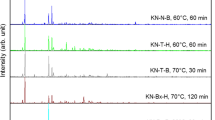
Synthesized products were coded as; “set code—reaction temperature—reaction time” and “K–H” for the set of “K2CO3:H3BO3” and “K–B” for the set of “K2CO3:B2O3”. For example, the product synthesized with the conditions of 90 °C reaction temperature and 15 min of reaction time using K2CO3:H3BO3 was coded as “K-H-90-15”. The highest XRD score—when all the peak intensities (%) and peak locations are matched perfectly with the reference card, the XRD score of the analyzed mineral is equal to 100—are obtained at the reaction temperature of 60 °C and 15 min of reaction time in set 1 with a value of 74. On the other hand, in set 2, the highest XRD score was found to be 65 at 90 °C reaction temperature and 15 min of reaction time. From the results, generally higher XRD scores were obtained at lower reaction temperatures and reaction times. Also, it can be seen that higher XRD scores were obtained in set 1 than in set 2. Optimum products (with higher XRD scores) were obtained in the parameters of K-H-90-15, K-H-80-30, K-H-70-30 and K-H-60-15 in set 1 and K-B-90-15, K-B-80-30, K-B-70-30 and K-B-60-30 in set 2. These optimum XRD patterns of the products are given in Fig. 2, from which the characteristic peaks [h k l (d spacing)] of santite can be observed at the 2θ positions of 14.92° [1 1 1 (5.93 Å)], 15.85° [0 2 0 (5.59 Å)], 25.32° [0 2 2 (3.51 Å)], 26.59° [1 2 2 (3.35 Å)], 32.35° [4 0 0 (2.77 Å)] and 41.52° [4 2 2 (2.17 Å)].
FT-IR and Raman spectral analysis results of the synthesized products
FT-IR spectra of optimum potassium borate compounds are shown in Fig. 3. For all parameters, FT-IR spectra of synthesized potassium borates are similar.
For IR peaks between 1332 and 1328 cm−1 are ascribed to asymmetric stretching of three coordinate boron to oxygen bands [ν as(B(3)–O)], whereas the bending mode of boron–oxygen–hydrogen [δ(B–O–H)] are fitted in the range of 1247–1245 cm−1. The peaks between 1095 and 1021 cm−1 are assigned to asymmetric stretching of four coordinate boron to oxygen bands [ν as(B(4)–O)]. The symmetric stretching of three coordinate boron to oxygen bands [ν s(B(3)–O)] is observed at around 913 cm−1. The frequency at 780 cm−1 belongs to symmetric stretching of four coordinate boron to oxygen bands [ν s(B(4)–O)]. Bending of three-coordinate boron [δ(B(3)–O)] is observed at 690 cm−1. Figure 4 shows the Raman spectra of optimum potassium borates. According to the Raman spectra, around 918 cm−1 the vibrations are caused by ν s(B(3)–O), when the frequencies between 766 and 764 cm−1 are symmetric stretching of ν s(B(4)–O). The peak around 557 cm−1 is assigned to the symmetric pulse vibration frequency of the pentaborate anion [(B5O6(OH)4)−]. The Raman frequencies between 510 and 297 cm−1 belong to bending of four-coordinate boron δ(B(4)–O). The obtained FT-IR and Raman bands were in accordance with the results obtained by Jun et al. [29].
SEM morphologies
SEM surface morphologies of optimum potassium borates are given in Fig. 5a, b for set 1 and set 2, respectively. According to the SEM images, the particle sizes of the synthesized potassium borates were changed between 667.67 nm and 5.98 μm. For the K–H set, the particle size increases with increasing reaction time; also, at the low temperatures, the particle size distribution is seen to be more uniform than the higher temperatures. At both 60 and 70 °C, the product particles have multiangular and overlapped shapes. In the second set of K–B, when the reaction temperature was set to 60 °C, a layered and dust-like particle texture is the obtained while for the other reaction temperature the potassium borate particles have irregular particle morphologies and less sharp edges.
Reaction yield results
Figure 6 shows the reaction yields of the synthesized potassium borates. In both sets, the reaction yields increased with increasing temperature and reaction times. In the sets, higher reaction yields were observed at 90 °C and lower reaction yields at 60 °C. Reaction yields were calculated to be between 93.49 and 95.11 and 84.88 and 89.27 %, for the sets of K–H and K–B, respectively. Highest reaction yields were found to be 95.11 ± 0.5 and 89.27 ± 0.6 % for the sets of K–H and K–B, respectively.
Thermal dehydration behavior
Thermal analysis results for dehydration behavior of KB5O8·4H2O are given in Table 3 and Figs. 7 and 8. The mineral lost its crystal water via a two-step process between the temperatures of 50 and 450 °C. The first two endothermic peaks seen in the DTA curves were in compatible with the peaks in the DTG curves. Although the dehydration process ended at approximately 450 °C, there was a third peak in the DTA curves in the range of 450–550 °C which indicates the recrystallization of the dehydrated structure.
The probable reactions for the dehydration process are given in Eqs. (10) and (11). According to the thermal analyses results (Table 3), the first step of dehydration had the approximate initial temperature of 105 °C while the final temperature varied in the range of 175.16–207.11 °C. The average weight loss was 15.22 % for the first step, equal to a value of 2.5 mol of water.
The second step started at the final temperature of the first step and ended at a temperature around 450 °C. The average weight loss was 8.98 % for the second step, equal to a value of 1.5 mol of water. The total average weight loss was 24.19 %.
From the thermal conversion results, calcined santite at 450 and 550 °C lost approximately 24.50 % of its initial weight. This result was in mutual agreement with the TG analyses, which is equal to 4 molar equivalents of structural water content. The loss in the structural water content indicates that the santite lost all of its structural water at 450 °C and transformed into a dehydrated type of potassium borate-type compound with a pdf no. of 01-070-1402. After that temperature, its lattice parameters were changed and it transformed to another type of dehydrated potassium borate mineral with a pdf no. of 01-076-1367 at 550 °C, which has the same formula. The probable equation at the recrystallization step (around 500 ºC) is given in Eq. (12).
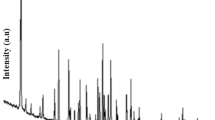
The crystallographic data and XRD patterns for the calcined products are shown in Table 4 and Fig. 9, respectively.
From Fig. 9, the characteristic peaks [h k l (d spacing)] of 550 °C calcined (01-076-1367) potassium borate were observed at the 2θ positions of 20.24° [1 2 1 (4.38 Å)], 23.97° [2 0 0 (3.71 Å)], 24.16° [0 0 4 (3.68 Å)], 25.16° [2 1 0 (3.53 Å)], 26.50° [1 3 1 (3.36 Å)], 31.15° [2 1 3 (2.87 Å)] and 34.29° [2 0 4 (2.61 Å)]. Whereas the characteristic peaks [h k l (d spacing)] of 450 °C calcined (01-070-1402) potassium borate were observed at the 2θ positions of 17.03° [1 1 2 (5.20 Å)], 21.18° [2 0 0 (4.19 Å)], 21.50° [0 2 1 (4.13 Å)], 22.66° [0 2 2 (3.92 Å)], 22.75° [2 0 2 (3.91 Å)], 24.50° [0 2 3 (3.63 Å)] and 26.86° [0 2 4 (3.31 Å)].
The SEM morphologies of the calcined potassium borates are given in Fig. 10, from which it can be seen that round overlapped particles and some rectangular particles were obtained from both the calcination temperatures of 450 and 550 °C, with the increase in the temperature leading to a more uniformly distributed and smooth particles. At 450 °C, the round particles were seen between 394.20 and 1.07 μm, and rectangular particles length were seen at approximately 1.17 μm. At 550 °C, the particle sizes of round articles were found between 684.31 and 1.13 μm, and rectangular particles length were seen at approximately 1.98 μm. So the increase in the calcination temperature leads to bigger particle sizes than 450 °C.
Kinetic results
Coats–Redfern, Doyle, KAS and OFW kinetic methods were applied for the calculation of kinetic parameters such as reaction order (n), activation energy (E a) and exponential factor (k 0). Calculated parameters for these kinetic methods are given in Table 5.
In the Coats–Redfern method, the n value was determined as 1, the average E a was 110.12 kJ/mol and k 0 values were in the range of 3.79 × 1012–7.06 × 1013 for the first step of dehydration. At the second step of dehydration, n value was determined as 2, average E a was 202.43 kJ/mol and k 0 values were in the range of 1.76 × 1020–1.17 × 1024.
In the Doyle method, k 0 values were in the range of 9.59 × 10−7–1.42 × 10−7 for the first step of dehydration. At the second step of dehydration, k 0 values were in the range of 1.42 × 10−7–9.59 × 10−7. On the other hand, E a values were determined as 107.77 and 304.18 kJ/mol for the first and second steps of dehydration, respectively.
In the KAS method, E a values were calculated as 158.82 and 154.50 kJ/mol for the first and second steps of dehydration, respectively. Similar with the KAS method, in the OFW method calculated E a values were in the range of 158.07 and 156.76 kJ/mol for the first and second steps of dehydration, respectively.
Kinetic parameters of different borate compounds which are found in the literature are given in Table 6, according to which, hydrated borate minerals generally lose their crystal water in one or two steps except for sodium metaborate hydrate. In general, total activation energies are in the range of 80–120 kJ/mol. The zinc borate hydrate and admontite minerals have higher activation energies than the other types of borate hydrates. Their total activation energies for the dehydration process are approximately 780 and 250 kJ/mol, respectively. Compared to the other borate hydrates, santite mineral’s total activation energy is 310 kJ/mol. This high activation energy leads to better heat resistance than the other types of hydrated borates except for zinc borate hydrate and admontite.
Conclusions
In this paper, a potassium borate mineral of santite (KB5O8·4H2O) was synthesized from different raw materials at various reaction temperatures (60–90 °C) and reaction times (15–120 min). According to the XRD results, at lower temperatures the crystallinity of the produced KB5O8·4H2O is higher when the reaction yield increases allied with increasing reaction temperature. For the reactions that were taking place with H3BO3 and B2O3, the reaction yields reached 95.11 and 89.20 %, respectively. FT-IR and Raman spectra of the products were in conformity with typical peaks of potassium borate in both the infrared and visible regions. On the basis of SEM observations of the specimen, the particle size of synthesized compounds for different reaction sets were found between 666.7 nm and 5.98 µm. Following the synthesis step, dehydration kinetics of the santite mineral were studied and thermogravimetric analyses showed that dehydration of santite occurred in two steps. Coats–Redfern and Doyle non-isothermal kinetic methods were used for the determination of activation energies (E a) and found to be 110.12 and 202.43 kJ/mol in the Coats–Redfern method for steps 1 and 2. On the other hand, in the method of Doyle, E a was found as 107.77 and 304.18 kJ/mol for steps 1 and 2. The differences between the E a can be explained by the variation of the data obtained from different kinetic methods which are compatible with the other study given in the literature [34].
References
A.S. Kipcak, E. Moroydor Derun, J. Piskin, J. Chem. 2013, 329238 (2013)
E.M. Derun, A.S. Kipcak, J. Radioanal. Nucl. Chem. 292, 871 (2012)
L.D. Pye, V.D. Frechette, N.J. Kreidl, Borate Glasses: Structure, Properties, Applications, 1st edn. (Plenum, New York, 1978)
G. Yang, Z. Li, Y. Zhang, Chem. Eng. Process. 44, 1216 (2005)
K. Thamizharasan, S.X. Jesu Raja, F.P. Xavier, P. Sagayaraj, J. Cryst. Growth 218, 323 (2000)
S. Merlino, F. Sartori, Contr. Mineral. Petrol. 27, 159 (1970)
W.A. Gale, Method of preparing potassium pentaborate. Patented Oct. 5, 1937 (No. 45, 57)
W.M. MacDonald, A.-C. Anderson, J. Schoreder, Phys. Rev. B 32, 1208 (1985)
Y.S.M. Alajerami, S. Hashim, W.M.S. Wan Hassan, A.T. Ramli, J. Mol. Struct. 1026, 159 (2012)
P.R. Binu, C.M. Joseph, C.S. Menon, K. Shreekrishnakumar, Mater. Lett. 52, 323 (2002)
S. Hashim, Y.S.M. Alajerami, A.T. Ramli, S.K. Ghoshal, M.A. Saleh, A.B. AbdulKadir, M.I. Saripan, K. Alzimami, D.A. Bradley, M.H.A. Mhareb, Appl. Radiat. Isotopes 91, 126 (2014)
O. Sahin, E.S. Cennetkusu, H. Dolas, M. Ozdemir, Thermochim. Acta 440, 7 (2005)
E.L. Belokoneva, S.Y. Stefanovich, O.V. Dimitrova, J. Solid State Chem. 195, 79 (2012)
G. Wang, Y.G. Sun, S. Zheng, G. Yang, Z. Anorg, Allg. Chem. 632, 1586 (2006)
S.A. Rajasekar, K. Thamizharasan, A. Joseph Arul Pragasam, J. Packiam Julius, P. Sagayaraj, J. Cryst. Growth 247, 199 (2003)
H. Gurbuz, G. Badem, A.N. Bulutcu, J. Cryst. Growth 283, 222 (2005)
M. Tunc, H. Ersahan, S. Yapici, S. Colak, J. Therm. Anal. 48, 403 (1997)
A.K. Galwey, Thermochim. Acta 355, 181 (2000)
F. Sevim, F. Demir, M. Bilen, H. Okur, Korean J. Chem. Eng. 23, 736 (2006)
A. Kantürk, M. Sarı, S. Piskin, Korean J. Chem. Eng. 6, 1331 (2008)
E.M. Derun, F.T. Senberber, Sci. World J. 2014, 985185 (2014)
E.M. Derun, A.S. Kipcak, F.T. Senberber, M.S. Yilmaz, Res. Chem. Intermed. 41, 853 (2015)
A.K. Figen, M.S. Yılmaz, S. Piskin, Mater. Charact. 6(61), 640–647 (2010)
A.S. Kipcak, F.T. Senberber, E.M. Derun, N. Tugrul, S. Piskin, Res. Chem. Intermed. 41, 9129 (2015)
M. Touboul, E. Betourne, Solid State Ionics 84, 189–197 (1996)
Y. Erdogan, A. Zeybek, A. Sahin, A. Demirbas, Thermochim. Acta 326, 99 (1999)
M.S. Yılmaz, S. Piskin, J. Chem. Soc. Pak. 34(3), 526 (2012)
J. Yongzhong, G. Shiyang, X. Shuping, L. Lun, Spectrochim. Acta A 56, 1291 (2000)
L. Jun, X. Shuping, G. Shiyang, Spectrochim. Acta 51A, 519 (1995)
A.S. Kipcak, D.Y. Baysoy, E.M. Derun, S. Piskin, Adv. Mater. Sci. Eng. 2013, 747383 (2013)
A.S. Kipcak, E.M. Derun, S. Piskin, Turk. J. Chem. 38, 792 (2014)
A.S. Kipcak, M. Yildirim, S.A. Yuksel, E.M. Derun, S. Piskin, Adv. Mater. Sci. Eng. 2014, 819745 (2014)
H.S. Fogler, Element of Chemical Reaction Engineering, 3rd edn. (Prentice-Hall, New Jersey, 1999)
M.S. Yilmaz, A.K. Figen, S. Piskin, Res. Chem. Intermed. 41, 1893 (2015)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asensio, M.O., Yildirim, M., Senberber, F.T. et al. Thermal dehydration kinetics and characterization of synthesized potassium borates. Res Chem Intermed 42, 4859–4878 (2016). https://doi.org/10.1007/s11164-015-2326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2326-5