Abstract
In this study, the nuclear radiation permeability properties of various boron minerals are evaluated because of their high neutron absorption and lowest transmission properties. Because of these properties boron minerals can be used at the area of neutron shielding. X-ray diffraction (XRD) analyses are done for the identification of the minerals, and then their B2O3 contents are determined experimentally. In addition, X-ray florescence (XRF) analyses are made for quantitative determination of calcium, iron, zinc and arsenic contents. The methods of Differential Thermal Analysis, Thermal Gravimetry (TG/DTA) and Differential Scanning Calorimeter (DSC) are used for obtaining the enthalpy and weight changes with temperature. Additionally, neutron permeability experiments are conducted. From the experimental results, the highest boron oxide content was found in clay containing colemanite. Iron, zinc and arsenic contents were not affecting the neutron shielding. The lowest permeability is provided by the kurnakovite mineral. Also it is observed that all of the minerals show an increase in their permeability in 12 years. It can be stated that boron minerals, specifically kurnakovite, is determined to yield the lowest neutron permeability value and therefore, the use of these materials for neutron shielding would be suitable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Turkey has 72.2% of the boron minerals that are present in the world, which can sufficiently meet world’s needs for many years. Approximately world’s total reserves are reported to be 1.2 billion tons. Boron is found in minerals with other elements in nature. The major usage areas of boron minerals make up a wide diversity, including glass, ceramic, nuclear, space-aviation, electricity–electronics–computers, metallurgy, enthalpy, transportation, textile, travel, cosmetics and chemistry [1, 2].
Many countries around the world, especially USA and France, use boron compounds as a shielding material in nuclear reactor technologies. A number of studies have been conducted in the area of nuclear shielding, both in Turkey and in the world, for instance Bhuiyan and Ahmed [3], manufactured a shielding material by adding boron to a polyethylene matrix in India, which they have called poly-boron. Gwaily et al. [4], produced a thermal neutron radiation shield using boron carbide and natural rubber. A neutron filter was made by Adib and Kilany [5], using Bismuth. Singh et al. [6], produced a neutron shield material that consisted of PbO–B2O3 and Bi2O3–PbO–B2O3. Sakuraia et al. [7], added various ratios of LiF and B4C to a polymer matrix in their study to produce a suitable shielding material. Ersez et al. [8], reported that a 120 mm layer made of 96% Pb and 4% Sb mounted over a concrete layer, would keep the radiation away in compliance with the security limits. A ceramic shield material containing boron carbide was developed by Cellia et al. [9], for being used in neutron scattering equipments. A phenol based neutron shield material containing 6% boron, which is resistant to temperatures as high as 300 °C, was developed by Morioka et al. [10]. Chichester and Blackburn [11], reported that bismuth or lead alone are insufficient in terms of their performance as biological shielding materials; however when bismuth was added to a polyethylene matrix or concrete, better results could be obtained.
As it can be seen, the effect of materials such as boron carbide, boron oxide, iron, lead and bismuth were investigated in shielding studies.
Several experimental studies conducted in Turkey on the addition of boron minerals, mainly colemanite into concrete. Elbeyli et al. [12], stated that Hemihydrate borogypsum may also be useful for decreasing the radioactive permeability of concrete, due to its boron content. However, the direct use of boron minerals as a raw material was not studied. Some studies regarding the direct use of boron minerals are conducted with our research group in 2009 and 2010. Borax, primary and secondary ulexite, inyoite and tincalconite minerals are investigated and characterized separately according to their neutron absorption capabilities with changing boron mineral thicknesses and their particle sizes [12–19].
The aim of this study is to determine whether boron minerals can be used as a shielding material with the purpose of nuclear radiation shielding. Also in this study, 12 year old boron minerals are used and their neutron permeability values both in 1996 and 2008 are compared.
The properties of boron minerals investigated in this study are summarized in Table 1 [13, 14].
Materials and methods
Mineral preparation and XRD analyses
Inyoite, inderite, borax and kurnakovite minerals were brought from “Kirka Boron Processing Management” in the Kirka region; colemanite and arsenic containing colemanite minerals were brought from “Emet Boron Processing Management” in the Emet region; and lastly clay containing colemanite mineral was brought from “Bigadic Boron Processing Management” in the Bigadic region.
The boron minerals were grinded with Retch agate mortar and their exact identifications were determined through the X-ray diffraction method in Philips Xpert Pro. X-rays are produced with Cu Kα tube at 45 kV and 40 mA [14–19].
B2O3 analyses
The neutron shielding makes use of boron minerals since they interact with thermal neutrons. For this purpose, it is required to determine the B2O3 contents of the boron minerals. The B2O3 content of the samples were determined by the method of acid–base titration using a Metler DL-25 titrator. In the experiments, pure boric acid is used for the reference material [14–19].
Na analyses
Sodium, which is activated under the thermal neutron bundles, has a very short half-life of 15 h. Owing to this property, sodium, which is present in the boron minerals located in high enthalpy levels (1–2.7 MeV), can reach its saturation activity immediately and begins to scatter secondary gamma rays to the environment. Therefore, the sodium contents of the mineral samples are needed to be determined by using the flame photometry.
In the experiments reference calibration set is prepared with Merck NaCl and Eppendorf Geratebau Flame Photometer is used at the parameter set of standard scale was 4, gasp was 1.96 × 103 Pa, air pressure was 534.36 × 103 Pa and amplification was set to 4 [14–19].
XRF analyses
X-ray florescence spectrometer is a diverse method that can easily be used for elemental analysis. In order to identify the source of the radioactivity developing in the boron minerals under a neutron flow, the other elements present in the mineral are needed to be determined through the conducted XRF analyses. The elements of calcium, iron, zinc and arsenic were analyzed with the standardless program. For the measurements, a silicon drift detector with a separation power ranging between 4 and 30 kV was used in a PANalytical Minipal4 model instrument [14–19].
Thermal analyses
The investigation of the enthalpy and weight differences in the boron minerals in response to changes in temperature is aimed with the use of Differential Thermal Analysis and Thermal Gravimetry (DTA/TG) experiments conducted in Perkin Elmer Diamond TG-DTA Termogravimetric/Differential Thermal Analyzer instrument. Also the Differential Scanning Calorimeter (DSC) analysis was made in order to calculate the enthalpy change of the minerals while losing their water contents. The analyses were conducted in inert atmosphere with the rate of temperature gradient set to 10 °C/min ranging between 30 and 900 °C. Platinum crucibles are used in the experiments [14–19].
Thermal neutron permeability experiments
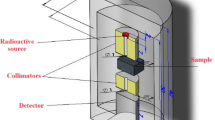
The thermal neutron permeability experiments were carried out at a room temperature between 18 and 22 °C with thermal neutrons of approximately 4 × 104 n/cm2 s which were generated from 239Pu–Be source moderated in a howitzer and BF3 detector (2.54 cm in diameter and 28 cm in length) with Canberra 85 analyzer was used. Measurements were repeated using approximately the same experimental system of the previous study in 1996.
In these measurements, the distance from the source to detector was kept constant at 5 cm and no space was left between the pellets and the detector. Neutron permeability is presented as I/I 0 where “I” is the transmitted “I 0” the incident neutron flux. To comment neutron shielding property of samples, total macroscopic cross section (Σt) values were calculated from Beer–Lambert Law:
where x is the thickness of samples. Detector setup as used in the present study is schematically depicted in Fig. 1 [14–19].
Results and discussion
XRD results
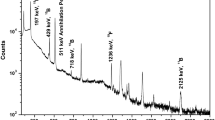
In the identification analyses of the boron minerals taken from different regions, XRD patterns are given in Fig. 2. From the XRD patterns of the six different boron minerals it is seen that the intensities and characteristic peaks of the minerals are different from each other. Their analyzed main characteristic peaks are matched with the related literature minerals and detailed information about these minerals are shown in Table 2. In the arsenic containing colemanite mineral, arsenic was not detected by XRD equipment because; the arsenic element was out of the detection limits. If the element in the mineral is less than 5%, it is not possible to detect it with XRD equipment. Also the clay containing colemanite minerals were not analyzed because of the high amounts of clay in the minerals.
B2O3 results
The high thermal neutron absorption influence profile of the boron element is one of the most significant factors in the selection of boron minerals as a shielding material. For this reason, the boron oxide content of the boron minerals are determined and found to be varying between 35 and 52% by weight.
As presented in Table 3, colemanite mineral has the highest boron oxide content, with the mutual agreement of both the experimental results and the theoretical values. The ratio of differences (D) between theoretical (T) and experimental (E) B2O3 contents are calculated from the following equation:
However, the boron oxide contents of kurnakovite, inderite, borax and inyoite are so close to each other that it is normal to see experimental differences in this order. Generally, it is observed that the experimental and theoretical values of the boron oxide contents of the raw minerals differ in 1–5%. This difference results from the purities of the boron minerals used in the experiments. For arsenic and clay containing minerals B2O3 contents can be changed with the arsenic and clay inside them so their comparison was not possible.
Na and XRF results
A flame photometer was used to measure the sodium content of the borax mineral and it was determined as 13.06%. The calcium content (%), the iron, zinc and arsenic concentrations (ppm) of the borax mineral are given in Table 4. The iron, zinc and the arsenic content of the boron minerals were predicted not to affect the neutron permeability and the neutron permeability was only thought to decrease with increasing Ca content. But from the neutron permeability results that will be evaluated in the following section, inyoite mineral, which has a lesser content of Ca than the colemanite mineral, yielded a lower neutron permeability value. Nevertheless, other variables such as B2O3 and water contents should also be considered before making any decisions regarding the effect of Ca content on neutron permeability. Because Hydrogen is a very important key element because of its high total neutron cross section of neutron-proton interaction and it is extremely efficient neutron enthalpy moderator.
Thermal analyses results
The thermal analyses and the thermal gravimetric analyses of the inyoite, inderite, borax, kurnakovite and clay containing colemanite minerals are presented in Fig. 3. Colemanite and arsenic containing colemanite could not yield any recordable results, due to the expansion and explosion of the studied colemanite mineral samples with increasing temperature.
-
(1)
Inyoite mineral: It can be observed from the DTA/TG curve of the inyoite mineral that initially two endothermic peaks occurred at 110.35 and 131.47 °C. The enthalpy difference at the first peak was ΔH 1 = 7.71 J/g, whereas the ΔH value of the second peak was ΔH 2 = 51.62 J/g. These peaks were followed by a third endothermic peak, with a ΔH value of ΔH 3 = 3.16 J/g. Finally, an exothermic reaction was observed at 785.97 °C, with an enthalpy value of ΔH 4 = −13.39 J/g. When the TG curve of inyoite mineral between 30 and 900 °C was investigated, total weight loss of 47.70% was observed to occur in two steps. In the first step a decrease of 32.75% in weight was observed between 30 and 250 °C. A sudden weight loss of 15.02% was observed at 400 °C in the second step. The reason for these weight losses were thought to be the evaporation of the water present in the structure of the mineral. The curve was fixed at a steady value 550 °C. Since the inyoite mineral has 4 mol of H2O and 5 mol of OH−, totally it can be said that 7.5 mol of H2O content theoretically. Experimental water loss in the mineral was 6.8 mol so the mineral was lost all its water content at 550 °C and at the beginning of the experiment the mineral had some moisture at about 0.7 mol.
-
(2)
Inderite mineral: As it can be observed from the DTA/TG curve of the inderite mineral, the only peak had a vertex at 135.87 °C. An endothermic peak occurred between 73.00 and 375.91 °C and when the DSC curve for this peak was investigated, the change in enthalpy was calculated as ΔH = 1237.80 J/g. The TG analysis of inderite showed that the weight decrease of 44.78% occurred in a single step.
Inderite mineral has 5 mol of H2O and 5 mol of OH−, totally it can be said that 7.5 mol of H2O content theoretically. Experimentally water lost in the mineral was 6.9 mol so the mineral lost nearly all its water content.
-
(3)
Borax mineral: An endothermic peak between 47.09 and 243.63 °C with a peak vertex at 80.36 °C and a second endothermic peak between 615.93 and 659.60 °C with a peak vertex at 650.25 °C were observed when the DTA/TG curve of the borax mineral was studied. The change in enthalpy was ΔH 1 = 728.08 J/g in the first endothermic peak and ΔH 2 = 10.33 J/g in the second endothermic peak. When the TG curve was investigated, the weight loss of 46.50% was observed to occur in a single step. The reason for this weight loss is thought to be the evaporation of water present in the structure of the mineral.
In Borax mineral 8 mol of H2O and 4 mol of OH−, totally it can be said that 10 mol of H2O is present theoretically. Experimentally the water loss in the mineral was 9.4 mol so the mineral lost nearly all its water content.
-
(4)
Kurnakovite mineral: The investigation of the DTA/TG and DSC curves of kurnakovite mineral between 30 and 900 °C yielded an endothermic reaction with a peak vertex at 138.59 °C with a ΔH value of 1271.01 J/g. The water present in its structure was lost in a single step, yielding a weight loss of 44.58%.
Since Kurnakovite mineral has 5 mol of H2O and 5 mol of OH−, totally 7.5 mol of H2O is present theoretically. Experimentally water loss in the mineral was 6.9 mol so the mineral lost nearly all its water content.
-
(5)
Colemanite and arsenic containing colemanite minerals exploded at temperatures about 350 °C and therefore their DTA/TG curves could not be evaluated.
-
(6)
Clay containing colemanite mineral: Due to the huge amounts of clay inside, Clay containing colemanite Mineral also exploded a little and its DTA curve was sufficient for evaluation. Two main enthalpy changes were determined from the DTA/TG curve of the clay containing colemanite. The first peak had a vertex at 385.87 °C, with an enthalpy change of ΔH 1 = 222.46 J/g. The second peak had a vertex at 788.20 °C, with an enthalpy change of ΔH 2 = 6.41 J/g. A small peak between the two main peaks was observed at 671.38 °C. However, since it did not show up in the DSC curve, it was not included for evaluation.
As a result of the thermal analyses that were conducted, it was seen that the borax mineral lost its structural H2O content at temperatures lower than 100 °C and this value could be named as the critical temperature. The critical temperatures of inyoite, inderite and kurnakovite were 150 °C and clay containing colemanite mineral was 350 °C. Since the hydrogen atoms are important in terms of neutron shielding, these temperatures should be taken into account in the design of the shielding material. Even after losing water molecules, boron minerals still contained OH− groups for neutron shielding performance.
Thermal neutron permeability results
Thermal neutron permeability experiments were carried out by using boron minerals that were used in a previous study, which was completed in 1996. The results of the previous study, combined with the results obtained from the current study are presented in Table 5.
In the study conducted in 1996, the lowest permeability was obtained using the kurnakovite mineral, and colemanite, clay containing colemanite, arsenic containing colemanite, borax, inderite and inyoite followed this mineral.
In the current study, the lowest permeability is again provided by the kurnakovite mineral. Generally, it is observed that all of the minerals show an increase in their permeabilities.
The reason for the increase in the permeability for the pellet samples used in the Howitzer experiments would be the formation of structural micro cracks in the samples during the past 12 years. In fact, the increase in permeability was an expected outcome especially if the fact that the samples were kept in open air conditions for 12 years is taken into consideration. Also these minerals might be lose some of their water contents. The storage of the boron minerals, which are kept with the purpose of re-investigation for neutron permeability in the future, should take place in an environment, in which no or very little interaction with air would take place, so that the possible decreases in permeability would be minimized.
Conclusions
Neutron shielding properties of concretes consisting of boron was both studied by Mollah et al. [20] and Yarar and Bayulken [21]. Korkut et al. studied the fast neutron shielding properties of MgB2, NaBH4 and KBH4 [22]. But the usability of the boron minerals as a raw material against neutron radiation was not studied before. In Addition in our study, 12 years of neutron performances of used boron minerals were also measured.
From the results obtained in this study, it can be stated that it is suitable to use boron minerals as a component for neutron shielding materials. Since kurnakovite mineral has the lowest neutron radiation permeability value among the studied minerals, its use would yield a better performance. Kurnakovite mineral does not have high boron oxide content but the hydrogen atoms inside the mineral helps the neutron radiation shielding performance. From this point of view, inderite mineral can also be concluded to employ good neutron radiation shielding characteristics, since it has the same structural formula with the kurnakovite mineral. However the experimental results indicate that their neutron radiation permeabilities are different because of the variation in their bond structures.
Among all investigated minerals, the inyoite mineral yielded the worst performance in terms of neutron radiation shielding.
References
Helvaci C (2004) Proceedings of the fifth industrial raw material symposium, Izmir, Turkey, 13–14 May 2004 (in Turkish)
Kilinc E, Mordogan H, Tanriverdi M (2001) Proceedings of the fourth industrial raw material symposium, Izmir, Turkey, 18–19 October 2001 (in Turkish)
Bhuiyan SI, Ahmed FU (1989) Health Phys 57-5:819–824
Gwaily SE, Badawy MM, Hassan HH, Madani M (2002) Polym Test 21:129–133
Adib M, Kilany M (2003) Radiat Phys Chem 66:81–88
Singh N, Singh KJ, Singh K, Singh H (2004) Nucl Instrum Methods B 225:305–309
Sakuraia Y, Sasakib A, Kobayashi T (2004) Nucl Instrum Methods A 522:455–461
Ersez T, Braoudakis G, Osborn JC (2006) Physica B 385–386:1268–1270
Cellia M, Grazzia F, Zoppia M (2006) Nucl Instrum Methods A 565:861–863
Morioka A, Sakurai A, Okuno K, Sato A, Verzirov Y, Kaminaga A, Nishitani T, Tamai H, Kudo Y, Yoshida S, Matsukawa M (2007) J Nucl Mater 367–370:1085–1089
Chichester DL, Blackburn BW (2007) Nucl Instrum Methods B 261:845–849
Elbeyli IY, Derun EM, Gulen J, Piskin S (2003) Cem Concr Res 33:1729–1735
TR Prime Ministry State Planning Organization, Chemical Industry Private Expertise Commission (2006) Boron Operations Group Report: ninth development plan (2007–2013) (in Turkish)
Kipcak AS (2009) MSc thesis, Yildiz Technical University (in Turkish)
Kipcak AS, Moroydor Derun E, Piskin MB, Tugrul N (2009) Proceedings of the third Balkan mining congress, Izmir, Turkey, 1–3 October 2009
Kipcak AS, Moroydor Derun E, Tugrul N, Piskin MB (2010) 2009 Annual bulletin of the Australian Institute of High Energetic Materials, vol 1. USBN: 978-0-9806811-3-0
Kipcak AS, Moroydor Derun E, Tugrul N, Piskin MB (2010) Ireche 1–2:149–156
Kipcak AS, Moroydor Derun E, Tugrul N, Piskin S (2010) Proceedings of the second conference on Chemical Engineering and Advanced Materials (CEAM), Naples, Italy, 15–26 November 2010
Kipcak AS, Moroydor Derun E, Tugrul N, Piskin S (2010) Proceedings of the 2nd international conference on nuclear and renewable enthalpy resources, Ankara, Turkey, 4–7 July 2010
Mollah AS, Ahmad GU, Husain SR (1992) Nucl Eng Des 135(3):321–325
Yarar Y, Bayulken A (1994) J Nucl Mater 212–215:1720–1723
Korkut T, Karabulut A, Budak G, Korkut H (2010) J Radioanal Nucl Chem 286:61–65
Acknowledgments
The authors wish to express their deepest gratitude and appreciation to Prof. Dr. Sabriye Piskin and also thank to Derya Y. Baysoy for her valuable help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Derun, E.M., Kipcak, A.S. Characterization of some boron minerals against neutron shielding and 12 year performance of neutron permeability. J Radioanal Nucl Chem 292, 871–878 (2012). https://doi.org/10.1007/s10967-011-1528-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1528-6