Abstract
Zinc borate hydrate (Zn3B6O12·3.5H2O) was synthesized from zinc sulfate heptahydrate (ZnSO4·7H2O), anhydrous zinc chloride (ZnCl2), and boric acid (H3BO3). The product was characterized by X-ray diffraction, Fourier-transform infrared and Raman spectroscopy, and thermal gravimetry and differential thermal gravimetry. The effects of reaction time and temperature on product morphology were studied by use of scanning electron microscopy. Results from thermal analysis showed that dehydration occurred in two steps. Thermal dehydration kinetics were investigated by use of the Coats–Redfern and Horowitz–Metzger non-isothermal kinetic methods. In the Coats–Redfern method, activation energies were found to be 225.40 and 570.63 kJ/mol for the first and second steps, respectively. In the Horowitz–Metzger method, activation energies were found to be 254.33 kJ/mol for the first step and 518.22 kJ/mol for the second step.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In nature, boron occurs in combination with metal atoms as boron minerals. Boron minerals and compounds are usually classified into two categories, hydrated and non-hydrated. So-called hydrated borates, which account for most of the boron-containing minerals and synthetic borates consumed by industry, have structures containing B–OH groups (hydroxy hydrated borates) and may also contain water of crystallization. Many types of borate resemble minerals in structure, containing isolated polyborate anions or complex polyborate rings, chains, sheets, or networks [1].
Zinc borates are the most important class of boron compound, because of their high fire resistance and lack of toxicity. For this reason, they are used in the plastics and rubber industries, in particular. They are also used as char promoters, anti-arcing agents, for preservation of wood composites, as modifiers of electro-optical properties, and as after-glow-suppressant additives [2–4]. Several types of zinc borate are known, for example 2ZnO·2B2O3·3H2O, 2ZnO·3B2O3, Zn2B6O11·3H2O, Zn2B6O11·7H2O, and Zn3B10O18·14H2; of these, the most important commercially is 3ZnO·2B2O3·3.5H2O [5–7].
Ting et al. [8], prepared nano-zinc borates from zinc nitrate (Zn(NO3)2·6H2O), borax (Na2B4O7·10H2O), and ammonia (NH3); reaction times were between 6 and 15 h at 45 °C. Zheng et al. [9] synthesized zinc borate in the form 4ZnO·B2O3·H2O by addition of Zn(NO3)2·6H2O to a mixture of Na2B4O7·10H2O and a phosphate ester and heating at 70 °C for 7 h. Zn2B6O11·7H2O and Zn3B10O18·14H2O have been prepared from Na2B4O7·10H2O and a mixture of zinc oxide (ZnO) and ZnSO4·7H2O, by boiling for 11 h [6]. Tugrul et al. [10], prepared hydrophobic nanostructured zinc borate by reaction of zinc borate hydrate modified with oleic acid at 95 °C.
Thermal decomposition of metal borates usually involves dehydration, as a result of removal of water of crystallization from the structure. The characteristic features of dehydration of materials should be known to enable design of equipment and to reduce the mass of materials required, thus reducing transportation costs. The thermal dehydration behavior of boron minerals may be studied by different methods, for example supercritical drying. ZnO·B2O3·2H2O decomposes to ZnO and H3BO3 on supercritical ethanol drying at 250 °C and 6.5 MPa. In supercritical carbon dioxide drying of methanol–zinc borate mixtures, zinc borate was obtained from ZnO and H3BO3 and there was no chemical interaction with CO2. Carbonates were formed on the surface of zinc borate obtained from borax decahydrate and zinc nitrate hexahydrate [11–14]. Although structural details are known for the most commercially relevant crystalline hydrated borates, the thermal dehydration kinetics of several important zinc borates have not been described.
According to the literature, combinations of ZnSO4·7H2O and ZnCl2 with NaOH and H3BO3 have not been used as starting material for synthesis of zinc borates. Long reaction times (6–15 h) are reported for synthesis of zinc borates. Also, because the thermal dehydration kinetics and effects of reaction temperature on the morphology of zinc borates have not been studied, in the work discussed in this paper we synthesized zinc borates from these raw materials and attempted to reduce reaction times, with the objective of saving energy. The pure zinc borate compounds synthesized were characterized by X-ray diffraction (XRD), Fourier-transform infrared (FT-IR) spectroscopy, Raman spectroscopy, scanning electron microscopy (SEM), and thermal gravimetry–differential thermal gravimetry (TG/DTG).
Experimental
Preparation and characterization of the raw materials
The zinc sources ZnSO4·7H2O and ZnCl2 were obtained from Sigma Aldrich; the purity was Reagent Plus® (≥99.0 % purity) and reagent grade (≥98.0 % purity), respectively. The boron source H3BO3 was obtained from Bandirma Boron Works; the minimum purity was 99.9 %. H3BO3 was pretreated by grinding and sieving to furnish a particle size <75 µm. NaOH of minimum purity of 98 % was obtained from Merck Chemicals. XRD analysis was performed with a Philips PANalytical Xpert Pro (step: 0.030°, time for step: 0.50 s, scan speed: 0.060 °/s, range 7°–90°) at 45 kV and 40 mA, using X-rays produced in a Cu-Kα tube.
Zinc borate synthesis
Preliminary experiments were conducted using different ZnSO4·7H2O–NaOH–H3BO3 (ZSNaH) and ZnCl2–NaOH–H3BO3 (ZCNaH) molar ratios. ZSNaH and ZCNaH molar ratios of 1:2:6 [15] and 1:2:7 [16], respectively, were selected for further experiments.
The reactions expected are shown in Eqs. (1) and (2):
To determine the effect of reaction temperature and reaction time on the product obtained, temperatures and reaction times were varied between 70 and 90 °C and from 1–4 h, respectively.
For experiments with ZSNaH, 0.1329 mol H3BO3 was dissolved in 25 ml pure water obtained from GFL 2004 (Gesellschaft für Labortechnik, Burgwedel, Germany), then 0.0210 mol ZnSO4·7H2O and 0.0420 mol NaOH were added to reactor. After addition of the raw materials, commercial zinc borate (Zn3B6O12·3.5H2O), which was retrieved from a local market in Turkey (0.5 % w/w as H3BO3) was added as seed agent for better crystallization. In ZCNaH experiments the amounts of ZnCl2 and NaOH were 0.0190 and 0.0380 mol, respectively.
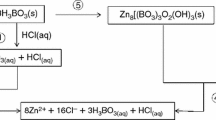
pH changes in the reaction medium were recorded by use of a Hanna Instruments HI 2211 pH/ORP meter. The pure water had a pH of 5.50 ± 0.10 (at 24 °C). On addition of H3BO3 the pH of the medium decreased to 3.60 ± 0.15 (between 70 and 90 °C). On addition of the zinc source the pH increased to 3.70 ± 0.02 (between 70 and 90 °C). On addition of NaOH the pH increased to 5.85 ± 0.20 (between 70 and 90 °C). Because the medium was acidic, semi-products of sodium borates were formed in the synthesis then the Na+ cation was exchanged with the Zn2+ cation and zinc borates were formed. The reaction scheme was, thus, as depicted in Eqs. (3) and (4):
The raw materials were reacted in a closed temperature-controlled vessel. After specific reaction times the solution was filtered through a Whatman blue ribbon filter paper and the crystallized products on the filter paper were washed three times with pure water (approximately 1,000 ml) at 50–60 °C, to remove unreacted reagents and NaSO4 and NaCl by-products, then dried in an incubator (Ecocell 111, Germany) at 105 °C for 24 h. The experimental method is shown schematically in Fig. 1.
Characterization of zinc borate
Zinc borate was characterized by use of XRD, and FT-IR and Raman spectroscopy. For XRD, the conditions were the same as for characterization of the raw materials. The range was between 10° and 70°, in accordance with literature reports of the location of the characteristic peaks of zinc borate hydrates.
The characteristic peaks of borate compounds are in the range 500–1,500 cm−1 [17]; thus, the spectral ranges for FT-IR and Raman were set to 1,800–650 and 1,800–250 cm−1, respectively. No peak was observed above 1,800 cm−1 in either FT-IR or Raman analysis.
Analysis of the surface morphology and particle size of the synthesized zinc borates was conducted by use of a CamScan Apollo 300 field-emission SEM (FESEM) at 15 kV. A backscattering electron detector (BEI) was used, and the magnification was set to 10.000.
Yield analysis was performed by use of the procedures of Derun et al. [18] and Kipcak et al. [19–22] with ZnSO4·7H2O and ZnCl2 defined as the key components for ZSNaH and ZCNaH, respectively. Three identical experiments were performed and average yields were calculated.
To calculate the overall yield, Y D, the number of moles of the final product, N D, was divided by the number of moles of the key reactant, A, consumed. The number of moles of A consumed was calculated from the initial (N A0) and the final (N A) numbers of moles of reactant, by use of Eq. (5) [23].
Thermal dehydration and kinetic study
The thermal properties of zinc borate samples were analyzed in the temperature range 105–620 °C with heating rates of 2, 5, 10, 15, 20 °C/min, under a nitrogen atmosphere, by use of Perkin Elmer Diamond DTA/TG. The kinetic data activation energy (E a) and exponential factor (A o) were calculated by use of the Coats–Redfern and Horowitz–Metzger methods.
In the Coats–Redfern non-isothermal kinetic method, a plot of ln[−ln(1 − α)] as a function of 1/T for n = 1.0 or a plot of ln[(1 − (1 − α)1−n)/(1 − n)] as a function of 1/T for n ≠ 1 gives the value of E a from the slope and the value of A o from the intercept.
where α is the degree of dehydration or conversion, R is the gas constant, n is the order of the reaction, T is the absolute temperature, and β is the heating rate.
In the Horowitz–Metzger method, a plot of ln[−ln(1 − α)2] as a function of θ for n = 1.0 or a plot of ln[(1 − (1 − α)1−n)/(1 − n)2] as a function of θ for n ≠ 1 gives the value of E a from the slope and value of A o from the intercept. T S is the peak of the DTG curve and θ = T − T S.
After the thermal analysis, zinc borate samples were heated in a Protherm MOS 180/4 high-temperature furnace set to increase at 10 °C/min to a temperature of 620 °C under a nitrogen atmosphere flowing at 5 ml/min. XRD analysis was then used to investigate the characteristics of the calcined zinc borate.
Experimental results
Results from characterization of the raw material
According to the XRD results obtained from the raw materials, the source of zinc, ZnSO4·7H2O, was a mixture of two phases, bianchite (ZnSO4·6H2O), with the powder diffraction file (pdf) number 01-075-0949, and goslarite (ZnSO4·7H2O), with the pdf number 00-009-0395. Another source of zinc, ZnCl2, was a mixture of two different forms of zinc chloride with powder diffraction file (pdf) numbers 01-074-0519 and 01-070-1284, respectively. The boron source, H3BO3, was determined to be sassolite (H3BO3), pdf number 01-073-2158, and the boron and sodium source Na2B4O7·5H2O was determined to be tincalconite (Na2B4O7·5H2O), pdf number 00-007-0277. The commercial zinc borate raw material was found to be zinc oxide borate hydrate (Zn3B6O12·3.5H2O), pdf number 00-035-0433.
XRD results for the synthesized zinc borates
XRD scores for zinc borates synthesized at different temperatures for different reaction times are listed in Table 1. Synthesized zinc borate was found to be zinc oxide borate hydrate (Zn3B6O12·3.5H2O), pdf number 00-035-0433. The different XRD scores for synthesized zinc borates in Set-1 and Set-2, as a result of different reaction time and temperature, are shown in Fig. 2. In Fig. 2, drawn by use of Statsoft Statistica software, the y-axis shows the XRD score (with a perfect crystal score set to 100), the z-axis shows the reaction temperature, and the x-axis shows the reaction time. The results from XRD indicate that with increasing time and temperature the XRD scores of the zinc borates increased.
In Set-1 experiments, zinc borate was prepared by use of a reaction temperature of 70 °C and reaction times of 3 and 4 h. The zinc borates obtained were coded in the format “set code-reaction temperature-reaction time”, where the set codes were “ZSNaH” for the set ZnSO4·7H2O–NaOH–H3BO3 and “ZCNaH” for the set ZnCl2–NaOH–H3BO3. For example the product synthesized at 70 °C for 4 h by use of ZnSO4·7H2O–NaOH–H3BO3 was coded as “ZSNaH-70-4”. For reaction times of 1 and 2 h, some expected zinc borate peaks were seen but formation was not complete. In Set-2 experiments, amorphous product(s) were obtained after reaction for 1 h. After reaction for 2 and 3 h some expected zinc borate peaks were seen but formation was not complete. After reaction for 4 h formation of zinc borates was achieved. After reaction at 80 °C for 1 h an amorphous product was obtained and zinc borate formation was not complete in 2 h in Set-1 experiments. In contrast, in Set-2 experiments formation of zinc borate was successful after 2 h. After 3 h and 4 h in both sets of experiments zinc borate with high XRD scores was obtained. At 90 °C zinc borates were obtained after reaction for 2 h, after 1 h some zinc borate peaks and amorphous products were observed in Set-2 and Set-1, respectively. Comparison of all the XRD results revealed that the products obtained from Set-1 had better crystal scores than those from Set-2.
XRD patterns of selected zinc borates are shown in Fig. 3. According to Fig. 3, the characteristic peaks and respective miller indexes (h k l) and d spacing [Å] of synthesized zinc borates were determined as 17.7° (1 1 −1) (5.88 Å), 18.1° (0 2 0) (4.90 Å), 20.6° (1 0 1) (4.30 Å), 21.8° (1 2 0) (4.08 Å), 23.7° (1 2 −1) (3.74 Å), 25.8° (2 1 0) (3.44 Å), 28.8° (0 1 2) (3.10 Å), 30.2° (2 2 1) (2.95 Å), and 36.8° (2 3 0) (2.44 Å).
Results from FT-IR and Raman spectral analysis of the synthesized products
FT-IR and Raman spectra of the synthesized zinc borates were given in Figs. 4 and 5, respectively.
In FT-IR spectra (Fig. 4), asymmetrical and symmetrical stretching bands of three-coordinate boron to oxygen (ν as(B(3)–O)) were observed in the ranges 1,407–1,251 and 924–886 cm−1, respectively. The bands between 1,067–980 and 856–855 cm−1, correspond to asymmetrical (νas(B(4)–O)) and symmetrical (νs(B(4)–O)) stretching, respectively, of four-coordinate boron to oxygen. Bands ascribed to bending of boron–oxygen hydrogen bonds (δ(B–O–H)) and bending of three-coordinate boron to oxygen (γ(B(3)–O)) were seen between 1,221 and 1,108 and between 657 and 655 cm−1, respectively. The ν p(B(OH)4)− band was in the range 793–718 cm−1.
In the Raman spectra (Fig. 5), ν as(B(3)–O) and ν s(B(3)–O) bands were seen in the ranges 1,294–1,292 and 920–919 cm−1, respectively. Bands between 1,049 and 1,037 and between 849 and 755 cm−1 correspond to ν as(B(4)–O) and ν s(B(4)–O), respectively. δ(B–O–H) and γ(B(3)–O) bands were seen between 1,190 and 1,187 and between 665 and 664 cm−1, respectively. The last two regions, which correspond to δ(B(3)–O)/δ(B(4)–O) and δ(B(4)–O), were between 596 and 578 and between 437 and 296 cm−1.
The results obtained from FT-IR and Raman spectroscopy were in good agreement with literature data [24, 25].
Surface morphology and particle size of the synthesized zinc borates
The synthesized zinc borate hydrate specimens were subjected to SEM analysis to determine how reaction time and temperature affected the products. The surface morphology and particle size of zinc borate hydrates are shown in Fig. 6. Heterogeneous mixtures of round and rectangular crystals were obtained from Set-1 experiments performed at 70 °C. In contrast, thin cylindrical crystals were obtained from the Set-2 experiments. In both sets, proper particle formation was observed when reaction temperatures higher than 70 °C were used. Better rectangular crystals were obtained from Set-2 experiments than from Set-1 experiments. There were minor differences between the particle sizes obtained in each set. The best crystals were obtained by synthesis at 90 °C for 240 min in Set-2.
The particle sizes of the zinc borate hydrates obtained by use of different reaction conditions were on the micron scale for both sets. For the ZSNaH set, the shapes and particle sizes of the crystals were irregular at the lower temperatures. After reaction at 70 °C, crystal sizes ranged from 315 nm to 694 nm. After reaction at 80 °C, the minerals obtained had rectangle-like particle shapes and the crystal sizes ranged from 280 nm to 1.15 µm. The particles synthesized at 90 °C had more uniform distributions and the crystal sizes ranged from 303 nm to 1.29 µm.
In the ZCNaH set, temperature affected the morphology of synthesized compounds. At 70 °C, the particles formed as one dimensional micro-rods of length 1.40–3.08 µm and width 346–445 nm. With increasing temperature, rectangle-like particles were obtained in the range of 387 nm–1.48 µm. Proper crystal shape formation was observed after reaction at 90 °C, and particle sizes were between 336 nm and 2.06 µm.
Calculation of the yield of synthesized zinc borate hydrates
Figure 7 shows the reaction yields of the synthesized zinc borate hydrates; reaction yields increased with increasing temperature and reaction times for both sets. In the set ZCNaH, higher reaction yields were observed at 90 °C and lower reaction yields were observed at 80 °C and 70 °C. Reaction yields were calculated to be between 88.1–96.7 and 76.5–98.9 % for the sets ZSNaH and ZCNaH, respectively.
Highest reaction yields were 96.7 ± 1.1 and 98.9 ± 0.8 % for the sets ZSNaH and ZCNaH, respectively.
Thermal analysis and dehydration kinetic results
TG and DTG analysis curves for pure synthesized Zn3B6O12·3.5H2O are shown in Fig. 8.
The curves show that Zn3B6O12·3.5H2O lost its water of crystallization in two steps, represented by two endothermic peaks. At a heating rate of 2 °C/min, in the first step of the dehydration process zinc borate hydrate lost 7.03 % of its weight in the range 261.61–391.86 °C. The peak temperature of DTG was at 382.72 °C. In the second step the initial, peak, and final temperatures were 391.86, 412.53, and 601.69 °C, respectively. The weight decrease in the second step was 6.37 %, for a total mass loss of 13.40 %. The initial, peak, and final temperatures on the TG and DTG curves and total weight losses are given in Table 2. In Table 2, the mass losses at each heating rate varied between 7.03 and 9.14 and between 4.78 and 6.38 % in the first and second steps, respectively.
The kinetic data E a and A o were calculated for conversion values (α) of 0.03–0.995 for the first step and 0.2–0.95 for the second step, by use of the Coats–Redfern and Horowitz–Metzger kinetic methods. Calculated values are given in Table 3. Correlation coefficients (R 2) and the similarity of the results obtained show that these kinetic methods were suitable for study of the kinetics of dehydration of zinc borate.
Values of A o obtained by use of the Coats–Redfern method were in the range 1.03 × 1017–4.09 × 1018 for the first step of dehydration and 7.43 × 1036–8.61 × 1053 for the second step. E a values were determined as 225.40 and 570.63 kJ/mol for the first and second steps, respectively. Values of A o obtained by use of the Horowitz–Metzger kinetic method were between 1.67 × 1019 and 3.68 × 1020 for the first step of dehydration and between 3.17 × 1037 and 2.24 × 1055 for the second step. E a values were determined as 254.33 and 518.22 kJ/mol for the first and second steps, respectively. Because of the presence 3.5 mol water of crystallization in the structure of synthesized Zn3B6O12·3.5H2O, removal of water could be explained by a dehydration mechanism. In contrast with some types of boron mineral, the structure of Zn3B6O12·3.5H2O does not contain hydroxyl ions, so dehydroxylation was not observed.
The reaction schemes expected for the dehydration steps are given in Eqs. (10) and (11).
The thermal conversion results showed that calcined Zn3B6O12·3.5H2O lost approximately 13.6 % of its weight. This result, which is equal to 3.5 molar equivalents of water, was in accord with TG analysis. This indicates that the zinc borate hydrate lost all of its structural water and was transformed into a dehydrated zinc borate type of compound. The XRD result for calcined zinc borate hydrate revealed two different types of dehydrated zinc borate, ZnB4O7 (pdf no 01-071-0634) and Zn4O(BO2)6 (pdf no 01-076-0917), were formed. The reaction scheme for the calcination is given in Eq. (12).
Crystallographic data for synthesized and calcined zinc borate hydrate are listed in Table 4. The XRD pattern of calcined zinc borate hydrate is shown in Fig. 9.
According to the Fig. 9, the five major characteristic peaks and their respective Miller indexes (h k l) and d spacing (Å) of ZnB4O7 and Zn4O(BO2)6 were determined as 16.9° (2 1 0) (5.23 Å), 21.6° (1 0 2) (4.12 Å), 21.9° (0 2 0) (4.06 Å), 28.4° (3 0 2) (3.14 Å), 31.3° (3 2 1) (2.86 Å) and 29.2° (2 1 1) (3.05 Å), 38.0° (3 1 0) (2.36 Å), 45.3° (3 2 1) (2.00 Å), 48.7° (4 0 0) (1.87 Å), 51.8° (3 3 0) (1.76 Å), respectively.
Conclusions
In this study a Zn3B6O12·3.5H2O-type zinc borate was synthesized under moderate reaction conditions with very high yield from the starting zinc sources ZnSO4·7H2O and ZnCl2. XRD results showed that the zinc borates could be synthesized by reaction at 70 °C for 4 h, at 80 °C for 3 h, and at 90 °C for 2 h. The highest yields were obtained by reaction at 90 °C for 4 h for both ZSNaH and ZCNaH, with yields of 96.7 and 98.9 %, respectively. SEM results showed that homogeneous products were obtained at the highest temperatures. Thermal studies showed that Zn3B6O12·3.5H2O lost its water of crystallization in two steps, forming the dehydrated zinc borate compounds ZnB4O7 and Zn4O(BO2)6. Kinetic studies were conducted by use of the Coats–Redfern and Horowitz–Metzger methods; average activation energies were in the ranges 225.40–254.33 and 570.63–518.22 kJ/mol, for the first and second steps, respectively.
References
D.M. Schubert, F. Alam, M.Z. Visi, C.B. Knobler, Chem. Mater. (2003). doi:10.1021/cm020791z
K.K. Shen, D.J. Ferm, Boron Compounds as Fire Retardants, Flame Retardants—101: Basic Dynamics. (Fire Retardant Chemicals Association, Pennsylvania, 1996), p.139
Y. Tian, Y. Guo, M. Jiang, Y. Sheng, B. Hari, G. Zhang, Y. Jiang, B. Zhou, Y. Zhu, Z. Wang, Mater. Lett. (2006). doi:10.1016/j.matlet.2006.01.108
X. Shi, Y. Xiao, M. Li, L. Yuan, J. Sun, Powder Technol. (2008). doi:10.1016/j.powtec.2007.12.014
M. Kılınc, G.O. Cakal, S. Yesil, G. Bayram, I. Eroglu, S. Ozkar, J. Cryst. Growth (2010). doi:10.1016/j.jcrysgro.2010.05.048
Y.H. Gao, Z.H. Liu, Thermochim. Acta (2009). doi:10.1016/j.tca.2008.11.013
Y. Tian, Y. He, L. Yu, Y. Deng, Y. Zheng, F. Sun, Zh Liu, Z. Wang, Colloid Surf. A (2008). doi:10.1016/j.colsurfa.2007.06.029
C. Ting, D.J. Cheng, W.L. Shuo, F. Gang, J Mater Process Technol. (2009). doi:10.1016/j.jmatprotec.2008.09.029
Y. Zheng, Y. Tian, H. Ma, Y. Qu, Z. Wang, D. An, S. Guan, X. Gao, Colloid Surf. A (2009). doi:10.1016/j.colsurfa.2009.02.018
N. Tugrul, M. Bardakci, E. Ozturk, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1538-4
A.K. Galwey, Thermochim. Acta (2000). doi:10.1016/S0040-6031(00)00448-2
E.M. Derun, F.T. Senberber, Sci. World J. (2014). doi:10.1155/2014/985185
M. Gonen, D. Balkose, S. Ulku, J. Supercrit. Fluids (2011). doi:10.1016/j.supflu.2011.08.004
M. Gonen, D. Balkose, R.M. Gupta, S. Ulku, Ind. Eng. Chem. Res. (2009). doi:10.1021/ie9003046
N. Tugrul, A.S. Kipcak, E.M. Derun, S. Piskin, Int. J. Chem. Nucl. Metall. Mater. Eng. 8, 7 (2014)
M. Piskin, A.S. Kipcak, M. Yildirim, T. Senberber, N. Tugrul, E.M. Derun, S. Piskin, Zinc chloride, sodium hydroxide and boric acid molar ratio determination for the production of zinc borates. TMS 2015 Annual Meeting & Exhibition, Orlando, FL, USA., 15–19 March 2015
A. Chen, P. Gu, S. Xu, Z. Ni, Adv Mater Res 399, 343 (2012)
E.M. Derun, A.S. Kipcak, F.T. Senberber, M.S. Yilmaz, S. Piskin, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1237-6
A.S. Kipcak, E.M. Derun, S. Piskin, J. Chem. (2013). doi:10.1155/2013/329238
A.S. Kipcak, D.Y. Baysoy, E.M. Derun, S. Piskin, Adv. Mater. Sci. Eng. (2013). doi:10.1155/2013/747383
A.S. Kipcak, E.M. Derun, S. Piskin, Turk. J. Chem. (2014). doi:10.3906/kim-1307-61
A.S. Kipcak, M. Yildirim, S.A. Yuksel, E.M. Derun, S. Piskin, Adv. Mater. Sci. Eng. (2014). doi:10.1155/2014/819745
H.S. Fogler, Element of Chemical Reaction Engineering (Prentice-Hall Press, New Jersey, 1999)
J. Yongzhong, G. Shiyang, X. Shuping, L. Lun, Spectrochim. Acta Part A (2000). doi:10.1016/S1386-1425(99)00227-9
R.L. Frost, J. Raman Spectrosc, (2010). doi:10.1002/jrs.2745
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kipcak, A.S., Senberber, F.T., Moroydor Derun, E. et al. Characterization and thermal dehydration kinetics of zinc borates synthesized from zinc sulfate and zinc chloride. Res Chem Intermed 41, 9129–9143 (2015). https://doi.org/10.1007/s11164-015-1952-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-1952-2