Abstract
The consequences of human influence can arise in vertebrates as primary, secondary, or even tertiary stressors and may be especially detrimental for slow growing species with long generation times (i.e., K-selected species). Here, we review the impacts of both direct and indirect human interactions on the reproductive biology of elasmobranchs. Within direct human influence, capture-induced stress from fisheries bycatch and poor coastal management practices leading to habitat destruction and pollution are among the most impactful on elasmobranch reproduction. Capture-induced stress has been shown to negatively influence offspring and reproductive capacity via capture-induced parturition as well as by disrupting the reproductive physiology of adults. Habitat degradation impacts essential ecosystems that are necessary for the development of young elasmobranchs. Pollutants such as heavy metals, legacy pesticides, and flame retardants have been traced through elasmobranch reproduction; however, the long-term effects of these exogenous chemicals are yet to be determined. Furthermore, within indirect human impacts, climate change-mediated influences (e.g., ocean warming and acidification) can impact development, physiological processes, and behavioral patterns necessary for essential tasks such as foraging, growth, reproduction, and ultimately survival. Here, we also present a case study, where data regarding temperature and incubation time from 28 egg-laying elasmobranch species were examined to show relevance of such data in predicting how suitable (e.g., via maximum threshold temperatures) habitats will be for skate and shark development in the coming century. Concomitantly, this information highlights areas for future research that will help inform better management as well as climate change forecasting for this threatened group of aquatic vertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elasmobranchs (subclass elasmobranchii)—sharks, rays, and skates (Compagno et al. 2005)—are crucial to aquatic ecosystems, but are currently considered one of the most vulnerable classes of vertebrates and in a global state of decline (Ward-Paige et al. 2012), with nearly a quarter of all species threatened by extinction (Dulvy et al. 2014). Currently, the main threats to elasmobranch populations include overfishing, incidental fishing capture (i.e., bycatch) (Mandelman et al. 2013), and habitat destruction (Ellis et al. 2004), with the latter being increasingly exacerbated by pollution and climate change (Ward-Paige et al. 2012). Given that most elasmobranchs are apex or meso predators, many are critically important to the health of their respective aquatic ecosystems (White et al. 2012; Hammerschlag et al. 2019), but also provide significant ecosystem services to countries, in the form of revenue and/or sustenance (e.g., eco-tourism and fisheries) (Hammerschlag and Gallagher 2011; Ward-Paige et al. 2012).
Elasmobranchs, from an ecological perspective, are K-strategists, meaning that species are typically long-lived, slow-growing, have a late age of sexual maturity, long reproductive cycles, and produce a low number of large, high-quality offspring (Conrath and Musick 2012). Elasmobranchs display, perhaps, one of the most diverse arrays of developmental modes with at least 10 unique strategies identified; for a full review of elasmobranch reproductive biology, which is beyond the scope of this review, see Conrath and Musick (2012). However, within the context of this review, it is important to note that elasmobranchs utilize internal fertilization followed by a range of oviparous (egg-laying) and viviparous (live-bearing) reproductive modes that vary in terms of maternal nutritional input and developmental location (i.e., internal vs. external) (Clark and Von Schmidt 1965; Dulvy and Reynolds 1997; Ebert et al. 2007). Each mode is associated with different costs and benefits, either to the developing embryo or to the mother, which, in turn, influences the susceptibility of certain life history stages to anthropogenic impacts.
The wide range of elasmobranch reproductive modes likely contributes to species-specific differences in their vulnerability to anthropogenic stressors during early life stages and reproduction in adults. Generally, the K-selected nature of elasmobranchs makes them extremely susceptible to population declines, as many species take 1–2 decades to reach sexual maturity (Compagno et al. 2005). Sexual maturity requires up to 150 years in the case of the Greenland shark (Somniosus microcephalus) (Nielsen et al. 2016). Thus, elasmobranchs cannot reproduce quickly enough to counteract fishing pressure or other detrimental human impacts (Gallucci et al. 2006). Indeed, understanding life history traits is vital for evaluating and forecasting population changes and can result in more effective management and conservation strategies for imperiled species (Gallucci et al. 2006). Specifically, understanding female fecundity and reproduction rates as well as identifiying essential habitats are all critical for estimating recovery rates (White et al. 2012). This information allows management authorities to account for differences between species when conducting vulnerability assessments and managing ecosystems to protect declining populations (Field et al. 2009; Hare et al. 2016). However, there remains a paucity of research on general reproductive biology and life history traits for many vulnerable elasmobranch species, which makes fundamental management difficult (Awruch et al. 2009; Simpfendorfer et al. 2011).
This review assesses the main direct (i.e., fisheries interactions and habitat degradation) and indirect (i.e., climate change) anthropogenic stressors that elasmobranch populations face globally through the lens of potential interactions with reproduction and early development while also highlighting areas for future research. Additionally, this review utilizes a case study to demonstrate the relationship between temperature and development time in oviparous elasmobranchs, thus illustrating the potential to model future impacts such as ocean warming on early development.
Influence of direct anthropogenic stressors on reproduction and development
Fisheries interactions
Both direct and indirect catch of elasmobranchs represent the largest cause for population declines worldwide (Stevens et al. 2000, 2005; Dulvy et al. 2014). Although there are many fisheries targeting specific species, bycatch is an enormous problem contributing to approximately 50% of elasmobranch global catch (Stevens et al. 2000). Fishing capture and handling can inflict considerable physiological stress and mechanical injury (Skomal and Mandelman 2012). However, such interactions affect not only general survival (Knotek et al. 2018), but also reproductive output, either directly (i.e., via increased sensitivity to mortality and capture-induced parturition), or indirectly (i.e., via post-release stress), which affects future reproduction (Guida et al. 2017; Adams et al. 2018; Wosnick et al. 2018).
Depending on the reproductive status of an elasmobranch, capture can increase sensitivity to mortality. Wosnick et al. (2018) reported that, of female shortnose guitarfish (Zapteryx brevirostris) caught in an artisanal fishery and transported to the laboratory, 25% mortality occurred in gravid females, but non-gravid females exhibited zero mortality. Over the following 48 h, 93% of the remaining gravid females aborted embryos within 10 h, and an additional 60% mortality was documented (Wosnick et al. 2018). Prado et al. (2018) also reported higher mortality rates (89%) in male shortnose guitarfish that were captured during the copulation phase of reproduction when compared to males captured outside of the reproductive season, where zero mortality was observed. To date, this is the first study to report vulnerability of the male reproductive system to capture-induced stress in an elasmobranch.
Capture-induced parturition is presumed in many species of sharks and rays, where females abort embryos during or soon after capture (Rolim et al. 2016; Adams et al. 2018). This phenomenon has only been documented in viviparous species and most commonly occurs in aplacental reproductive strategies such as histotrophy (Adams et al. 2018). Loss of embryos from fisheries interactions could have major impacts on recruitment, as viviparous reproduction occurs over long cycles (1–2 years for many species) with specific timing of behaviors such as mating and parturition (Adams et al. 2018). Furthermore, even if capture-induced parturition does not occur, embryos can still be affected. Guida et al. (2017) showed that the body condition and total length of southern fiddler ray (Trygonorrhina dumerilii) embryos were significantly decreased after the mother was caught by a trawl net, indicating a reduction in neonate fitness. This finding could indicate long-term impacts on the survival of the offspring beyond the capture event (Guida et al. 2017). As evidenced by these findings for adults and embryos, species-specific spatial (e.g., mating grounds/nursery areas) and temporal (e.g., reproductive seasonality) data should continue to be investigated and applied toward bycatch reduction programs and fisheries management (Gallucci et al. 2006).
Fishing practices can also impact elasmobranchs indirectly by disturbing substrate and habitat. The majority of oviparous elasmobranchs rely on the benthic substratum to deposit their eggs, where development then ensues (Conrath and Musick 2012). Many species have a preferred substrate that reduces predation risk and offers ideal developmental conditions (Pretorius and Griffiths 2013; Trujillo et al. 2019). For example, red-spotted catsharks (Schroederichthys chilensis) select tall, thick, and stable kelp for egg deposition sites, as this structure provides temperature and salinity stability throughout the 7-month developmental periods as well as protection from potential benthic predators (Trujillo et al. 2019). Fisheries practices that interact directly with the benthos (e.g., bottom trawling) can disturb biota necessary for elasmobranch eggs (e.g., sponges, gorgonians, kelp) (McConnaughey and Syrjala 2014) and/or potentially disturb eggs that have already been deposited. Indeed, studies have noted elasmobranch egg capsules being incidentally caught by bottom trawl fishing (Hoff 2010; McConnaughey and Syrjala 2014). The impact of these trawling gear interactions, whether indirect or direct, has not yet been quantified for any oviparious species but could be substanital in areas of localized, heavy fishing pressure.
Poor coastal management
Habitat degradation
Habitat degradation resulting from overuse and development of coastal environments is a continuous threat for many elasmobranch species, as coastal areas are often used for feeding, mating, pupping, and protection from predators at early life stages (Speed et al. 2010). The anthropogenic interactions occurring in coastal environments range from physical reduction of needed biota and structure (e.g., mangrove reduction; Jennings et al. 2008) to increased runoff and altered water quality from onshore point sources (e.g., wastewater treatment and power plants; Curtis et al. 2013). Juveniles of oviparous and viviparous species alike have been documented using the benthos of specific coastal areas as nursery habitats (Ward-Paige et al. 2012; Pretorius and Griffiths 2013). For example, seagrass (Thalassia testudinum) habitat was found to be vitally important for juvenile lemon sharks (Negaprion brevirostris), such that survival decreased by 23.1% after structural complexity was compromised (Jennings et al. 2008). Contrastingly, human-altered habitats (i.e., outfall from a power plant, a boat marina, and a causeway/bridge area) appear to attract and support juvenile bull shark populations, where sharks could be opportunistically using non-natural sites that have high prey abundance (Curtis et al. 2013). In addition to nursery sites, many coastal species exhibit high philopatry, routinely using specific sites for reproduction and oviposition (e.g., Heupel 2007; Bass et al. 2017), which necessitates the preservation of intact benthos. Therefore, future research addressing the effects of habitat degradation across coastal environments should assess critical nursery areas on a species-specific level (Kinney and Simpfendorfer 2009).
Coastal pollution and nutrient run-off
To date, several studies have addressed the effects of coastal pollution and nutrient run-off from agricultural development, finding high concentrations of released pollutants in various tissues of multiple elasmobranch species (i.e., Grosell et al. 2003; Gelsleichter et al. 2005; Nakata 2005; Núñez-Nogueira 2005; Mathews and Fisher 2009; De Boeck et al. 2001, 2010; Lyons et al. 2013; Mull et al. 2013; Olin et al. 2014; Lyons and Adams 2014; Frías-Espericueta et al. 2014, 2015; Weijs et al. 2015a, b; Marler et al. 2018). Not only have contaminants been shown to accumulate in adult tissues, but contaminants have also been found in embryos and neonates, demonstrating maternal transference (Marler et al. 2018). The exact physiological impact of these exogenous materials within elasmobranch reproductive pathways is not well understood but remains a growing area of research (Lyons et al. 2013; Mull et al. 2013; Frías-Espericueta et al. 2014).
Maternal transference has been documented in several viviparous elasmobranchs, including species exhibiting placental and oophagy (where unfertilized ova are ovulated and consumed by the embryo throughout gestation) strategies (Lyons et al. 2013; Frías-Espericueta et al. 2014; Marler et al. 2018). Lyons et al. (2013) demonstrated that an adult thresher shark (Alopias vulpinus) transferred accumulated mercury and organic contaminants (PCBs, DDTs, pesticides) to its near-term embryos. From the one shark assessed in the study, Lyons et al. (2013) estimated that 29–54% of the maternal load could be transferred to embryos, likely through the initial yolk-sac and/or via ova consumption thereafter for the remainder of gestation. Similarly, modeled dietary accumulations in young of the year (YOY) white sharks (Carchardon carcharias) indicate that elevated hepatic organochlorine levels likely come from gestational maternal inputs rather than prey consumed during the short period between parturition and capture (Mull et al. 2013).
In a placental species, the Pacific sharpnose shark (Rhizoprionodon longurio), Frías-Espericueta et al. (2014) reported higher levels of copper and zinc in embryonic tissues and high levels of lead and cadmium in maternal muscle and liver tissue (Frías-Espericueta et al. 2014). Interestingly, this study also reported an inverse relationship between metal concentration of embryonic tissue and size, meaning that, as gestation progressed, metal transference seemingly decreased (Frías-Espericueta et al. 2014). Furthermore, Marler et al. (2018) determined maternal transfer of 20 polybrominated diphenyl ethers (PBDEs) and 35 non-PBDE type flame retardants in five viviparous placental shark species. They hypothesized that maternal transfer likely occurs through both the initial yolk-sac stage as well as from the bloodstream once the placenta forms (Marler et al. 2018). An unintended outcome of this study was the observed variations in maternal transfer between species, which could indicate that transfer rates differ by maternal factors such as body size, growth rates, and diet (Marler et al. 2018); however, a more directed study is needed to solidify these findings.
The majority of reproductive toxicity research in elasmobranchs focuses on viviparous species, where embryos have long periods of maternal contact. In comparison, single oviparous elasmobranch species only retain an egg capsule for a few days to a week; however, the yolk-sac deposited within the egg capsule could still contain transferred contaminants, as yolk has been shown to contain high toxin loads in viviparous species (Weijs et al. 2015a, b). Additionally, water conditions external to the egg could serve as a second pathway for exposure to pollutants. For example, spotted dogfish (Scyliorhinus canicula) embryos cutaneously absorbed a host of radio-isotopes (241Am, 109Cd, 57Co, 134Cs, 54Mn, and 65Zn) through the egg case and apertures after short-term exposure to irradiated seawater (Jeffree et al. 2006).
As with metal contaminants, other toxins such as those from algal blooms could pose similar threats to elasmobranch reproduction. In 2000, for example, a red algae (Karenia brevis) bloom caused massive die-offs of blacktip (Carcharhinus limbatus) and Atlantic sharpnose (Rhizoprionodon terraenovae) sharks in northwest Florida (Flewelling et al. 2010). From samples collected, it was demonstrated that these species transferred brevotoxins from the algal bloom to embryos via the placenta (Flewelling et al. 2010). Although observations of these mass die-offs are anomalous in sharks, these events demonstrate the maternal transference of not only heavy metals, but also other naturally occurring environmental toxins.
Although maternal transference is occurring for a host of environmental contaminants, there is no definitive understanding as to the effects on adults or offspring. For example, Merly et al. (2019) documented extremely high levels of circulating mercury, arsenic, and cadmium in adult white sharks, but no impact on the body condition was observed. Furthermore, high circulating concentrations of copper were positively associated with body condition, and the authors referenced copper as a co-factor for enzymatic activity with potential to protect against oxidative stress, thereby increasing body condition (Merly et al. 2019). So, while there is evidence that metals and other toxins are present in adults and transferred via maternal inputs, the effects of such contaminants, particularly on developing elasmobranch embryos, is not yet understood. It is still unknown for example how exposure may affect development, survivorship, and fitness after birth or hatching; however, the abundant presence of these contaminants in elasmobranch embryonic tissues illustrates the need to continue research within this area.
Indirect anthropogenic reproductive stressors
Over the past century, climate change driven by greenhouse gas emissions has begun to change the marine environment by causing large-scale changes in ocean temperatures and acidity (Pörtner et al. 2014). Due to the inherent logistical issues around studying most viviparous species in a laboratory setting (i.e., size and life history), studies assessing climate change on reproduction and development are currently limited to oviparous species (Rosa et al. 2014; Di Santo 2015; Pistevos et al. 2015; Gervais et al. 2016, 2018; Johnson et al. 2016; Pegado et al. 2019a, b). As most oviparous species deposit their early stage embryos directly into the environment, developing embryos must therefore possess the necessary mechanisms to tolerate local environmental conditions until they hatch, which could be anywhere from a few months to years (Hoff 2008).
Climate change-mediated acidification
With atmospheric CO2 expected to rise to 900 ppm by the end of the century, the ocean is an increasingly large sink, absorbing up to 30% of the atmospheric carbon (Bindoff et al. 2019). Changes of this magnitude represent a 0.30–0.32 unit change in pH, which, in combination with changes in speciation of dissolved inorganic carbon (DIC), is collectively termed ocean acidification—OA (Bindoff et al. 2019). OA is an unprecedented and unknown change for modern times with large predicted consequences on marine ecosystems (Gattuso et al. 2015).
With respect to elasmobranch development, OA is an increasing area of interest; thus far, a handful of laboratory studies have demonstrated varying effects of exposure to elevated pCO2 (Green and Jutfelt 2014; Rosa et al. 2014; 2016a, b, 2017; Di Santo 2015; Dixson et al. 2015; Pistevos et al. 2015; Johnson et al. 2016; Lopes et al. 2018; Pegado et al. 2019a, b). During embryonic development, ocean acidification in isolation does not pose measurable, negative effects on development time, hatching success, or hatching condition/size in any elasmobranch species studied thus far (Rosa et al. 2014; Di Santo 2015; Pistevos et al. 2015; Johnson et al. 2016). Studies have found mixed impacts on neonate and juveniles, where both DiSanto (2015) and Johnson et al. (2016) reported no significant change in neonate survival after exposure to simulated OA throughout embryonic development for little skates (Leucoraja erinacea) and epaulette sharks (Hemiscyllium ocellatum), respectively. However, Rosa et al. (2014) found OA conditions to decrease survival, metabolic rates, ventilation rates, and body condition of 30-day post-hatch bamboo sharks (Chiloscyllium punctatum). Pegado et al. (2019a) reported decreases in somatic growth for juvenile whitespotted bamboo sharks (Chiloscyllium plagiosum); although, body condition and specific growth rates were not significantly impacted.
Other studies have explored the underlying physiological effects of elevated pCO2 on neonate (i.e., ≤ 45 days post hatch), juvenile, and adult elasmobranchs. OA conditions did not cause oxidative damage in neonate whitespotted bamboo sharks (Lopes et al. 2018); however, a study that exposed this same species to higher levels of pCO2 in the embryonic stages did observe neuro-oxidative damage (Rosa et al. 2016a). Furthermore, Pegado et al. (2019a) found that some juvenile whitespotted bamboo sharks exposed to high pCO2 had a decrease of acetylcholinesterase activity in their brains, an enzyme vital to nervous system regulation. In the temperate spotted catshark (Scyliorhinus canicula), hematological parameters (e.g., blood cell counts) were not impacted in juveniles reared under OA conditions during embryogenesis (i.e., approximately 4 months) or for an additional 5 months after hatching (Pegado et al. 2019b). In adults of the same species, individuals were similarly unaffected for metrics such as growth rate, resting metabolic rate, and aerobic scope; however, plasma HCO3− and Na+ were elevated after a long acclimation period, providing evidence of buffering in response to internal acidosis (Green and Jutfelt 2014). A similar buffering response was elicited in sub-adult epaulette sharks exposed to simulated OA conditions (Heinrich et al. 2014). Indeed, there is much room for further investigation, as some physiological pathways are altered under ocean acidification conditions, while survival is, by and large, unaffected.
Changes in physiological processes associated with simulated OA conditions may impact elasmobranch reproduction via altered behaviors. Spotted catsharks changed their nocturnal swimming patterns and increased lateralization upon exposure to elevated pCO2 conditions (Green and Jutfelt 2014). Similar effects have been observed in juvenile whitespotted bamboo sharks, where exposure to elevated pCO2 conditions related to less time spent swimming (Pegado et al. 2019a). Additionally, increased acidity has been found to profoundly affect some sensory modalities. Both the Port Jackson shark (Heterodontus portusjacksoni) (Pistevos et al. 2015) and smooth dogfish (Mustelus canis) (Dixson et al. 2015) were negatively affected by elevated pCO2 exposure at the level of olfaction, which could impact hunting behaviors. For Port Jackson sharks, the inability to find food under elevated pCO2 conditions may have contributed to the 70% reduction in growth rates reported (Pistevos et al. 2015).
Indeed, ocean acidification, in isolation, may have some effects on elasmobranch reproduction indirectly, where changes in blood chemistry and behavior indicate that individuals are at least partially physiologically compromised and likely stressed from these conditions. Surviving these conditions long-term could be metabolically taxing to adults, where energy required for reproductive processes (e.g., creating a nutrient-rich yolk) could be greatly reduced. Additionally, changes in sensory modalities could affect reproduction in terms of locating and selecting mates (Tricas et al. 1995). To date, there are no studies directly assessing OA on reproductive biology in an adult elasmobranch, which provides an interesting area of research for the future.
Climate change-mediated ocean warming
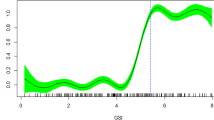
Increasing ocean temperatures from climate change are already having massive impacts on marine ecosystems worldwide (e.g., Hughes et al. 2018). Temperature affects the rate of nearly all physiological and biochemical reactions in ectothermic organisms and as such, temperature will likely have some of the most profound effects on elasmobranchs as climate change continues. In the context of reproduction and development, the reproductive mode that may be most susceptible to changes in environmental temperatures is oviparity, as embryos are confined to one location on the benthos throughout early development (Chen and Liu 2006; Pretorius and Griffiths 2013). A clear relationship (exponential decay) exists between water temperature and incubation time in oviparous species, as reported by Hoff (2008) (Fig. 1). After updating and thereby doubling the sample size of Hoff’s 2008 analysis from 13 to 28 species (Table S1), the model fits are remarkably similar (Fig. 1; Table S2). The similarity in the exponential models and the strong relationship between the log transformation of these variables (Fig. 2; Table S3) indicates that a small subsample of species could predict important biological relationships applicable to the whole of oviparous elasmobranchs (~ 400 species; Ebert et al. 2013; Last et al. 2016). Models such as those included here could be extrapolated to predict effects of ocean warming with species-specific maximum thermal limits for development.
The relationship between rearing temperature and incubation time (in days) of 28 oviparous chondrichthyan species. A nonlinear least squares (nls) regression was fit and compared to the exponential relationship reported in Hoff (2008) (see Table S2). See the Supplemental Material for the full model fit and associated data
The best fit linear mixed-effects model of the relationship between the log(rearing temperature) and log(incubation days) for 28 oviparous chondrichthyan species (df = 21.44, cAIC − 2.03; Table S3). The blue shaded area indicates the 95% prediction interval of the fixed and random effects. The data and full model fit can be found in the supplemental section
Given that temperature acts as a master factor for oviparous development in ectotherms, ocean warming stands to shift elasmobranch development drastically. Both tropical and temperate species exhibit decreased survival 4–5 °C above ambient (Rosa et al. 2014; Di Santo 2015). On the contrary, in the temperate Port Jackson shark, + 3 °C above ambient conditions increased embryonic development rates but did not decrease survival (Pistevos et al. 2015). With only a handful of studies, it is difficult to determine if there is a difference in tolerance to ocean warming scenarios between tropical and temperate embryonic elasmobranchs. Based on the climate variability hypothesis, however, temperate species should tolerate a wider range of temperature (Stevens 1989); yet, more targeted studies that test wider thermal ranges are needed to elucidate whether this hypothesis holds true for embryonic elasmobranch development.
Beyond embryonic impacts, increased temperature decreases survival (Rosa et al. 2014; Di Santo 2015) and growth rates in neonate and juvenile lifestages (Gervais et al. 2018). Of course, in juvenile life stages, unlike embryonic stages, individuals can thermoregulate by moving to cooler areas, which may prove to be physiologically advantageous (Gervais et al. 2018). Futhermore, recent work has reported that, although Port Jackson shark embryonic survival was reduced by 41.7% with a 3 °C increase in temperature above ambient, hatchlings from elevated temperatures were able to learn a task more quickly, which could aid in survival under non-ideal thermal conditions (Vila Pouca et al. 2019).
Finally, reproduction in adult elasmobranchs will also be affected by warming ocean temperatures, but the impacts may be hard to quantify. Elasmobranchs are suspected to use temperature as a cue during the reproductive season (e.g., Heupel et al. 1999; Elisio et al. 2019). Some studies have suggested that adult elasmobranchs may use behavioral thermoregulation to reduce gestation times (Hight and Lowe 2007; Speed et al. 2012; Sulikowski et al. 2016). For example, in a laboratory experiment, gravid Atlantic stingrays (Hypanus sabina) preferred temperatures that were 1 °C higher than those of their non-gravid conspecifics; this slight increase in temperature was enough to reduce gestation times of gravid females by approximately 2 weeks (Wallman and Bennett 2006). Similarly, it has been hypothesized that the oviparous Pacific white skate (Bathyraja spinosissima) deposits their eggs in close proximity to black smoker hydrothermal vents, presumably using the elevated temperatures to reduce embryo development times (Salinas-de-León et al. 2018). To date, no studies have investigated the thermal limits of reproduction in elasmobranchs, which could be particularly important data for oviparous species that are not likely to undertake range shifts due to ocean warming.
Climate change-mediated interacting effects
When OA has been studied in combination with ocean warming scenarios in embryonic elasmobranchs, mixed effects on growth and development have been observed (e.g., Rosa et al. 2014, 2016a, b; Di Santo 2015; Pistevos et al. 2015). In the brownbanded bamboo shark, elevated pCO2 and temperatures in combination did not significantly change survival or development time when compared to the effects of elevated temperatures alone (Rosa et al. 2014). However, the resting metabolic rate of embryos throughout development and after hatching was affected by elevated pCO2 under the increased temperature treatment, illustrating that OA conditions may negatively impact the energetic needs of the embryos beyond the expected changes elicited by elevated temperatures alone (Rosa et al. 2014). At hatching, neonates were heavily impacted by the combination of elevated pCO2 and temperatures with low survival and physiological fitness (Rosa et al. 2014). In a similar study with the temperate little skate, results showed that two functionally separate populations had varying developmental and energetic responses to OA and warming scenarios, where OA conditions exacerbated the effects of elevated temperatures (Di Santo 2015). These findings provide evidence that local adaptation may be occurring within this species, with results indicating that the more poleward population had an increased tolerance to OA conditions, likely because it experiences more variable conditions (Di Santo 2015).
Through a physiological lens, Rosa et al. (2016b) assessed pancreatic trypsin and intestinal alkaline phosphatase across simulated warming and OA conditions in 30-days post-hatch brownbanded bamboo sharks. These enzymes are common metrics used to assess metabolic processes and the overall physiological state of fish during development (Rosa et al. 2016b). Interestingly, under ocean warming scenarios, these enzyme activity levels increased, and under ocean acidification scenarios, these enzymes decreased. However, in the concomitant treatment, these enzymes did not differ from the control group, suggesting antagonistic effects of elevated temperature and pCO2 levels on the physiological response (Rosa et al. 2016b).
Overall, the effects of human-induced climate change on elasmobranch reproduction and development may be extensive and cause knock-down effects. As temperature, in particular, regulates most biochemical and physiological processes, including gestation and development time, species may redistribute, following conditions that are more preferred in response to elevated temperatures. However, species that display philopatry and/or those with limited mobility, such as many neonates and/or species that develop in external eggs for extended periods of time, may be severely impacted. Ocean acidification has been shown to exacerbate the effects of ocean warming on growth and development of some oviparous embryos and post-hatch neonates studied in captivity thus far (Rosa et al. 2014, 2016a, b; Di Santo 2015; Pistevos et al. 2015; Gervais et al. 2016, 2018). More research is needed to elucidate whether these trends also apply to viviparous species.
Synthesis and future research
Both indirect and direct anthropogenic stressors can cause shifts in reproduction and development in elasmobranchs; although this is an immensely understudied area. Targeted research outlined in this review will give a better understanding as to the holistic changes in reproductive output and consequent population shifts in elasmobranchs now and into the future. However, many logistical issues complicate this area of research (e.g., K-selected life history, migratory behavior of some species, large size, relative lack of basic biological information for many species), which will ultimately require innovative experimental design. In the case of direct anthropogenic stressors (e.g., incidental fisheries interactions, habitat degradation, algal blooms, and pollution), quantitative research will greatly strengthen management decisions. For example, Adams et al. (2018) outlines the reproductive modes that are most affected by incidental catch; however, the exact long-term quantitative impacts of capture stress on an individual’s reproductive output are unknown. Laboratory studies assessing changes in female egg production based on simulated catch as well as climate change relevant stressors (e.g., ocean warming and acidification) could be a good starting point.
In the case of studies on indirect anthropogenic stressors on growth and development of oviparous elasmobranchs (Rosa et al. 2014, 2016a, b; Di Santo 2015; Pistevos et al. 2015, 2017; Gervais et al. 2016, 2018; Johnson et al. 2016), the results can only forecast biological changes on a short time scale. There is a growing body of literature discussing transgenerational acclimation and phenotypic plasticity with respect to century-long climate change for many teleost fish species, which has been possible due to their relatively fast generation times and growth rates (e.g., Donelson et al. 2018); however, the slow nature of the elasmobranch life history does not lend to this type of research. Indeed, an innovative approach would be needed to obtain these types of data without relying on multi-decadal studies. Perhaps a more thorough comprehension as to the interaction between reproduction, stress physiology, and endocrinology in elasmobranchs would allow predictions as to how long-term stress affects reproductive output.
Beyond oviparity, most viviparous elasmobranchs do not lend well to a laboratory setting and cannot realistically be studied in a controlled environment. There is no current methodology for studying changes in reproductive output in mobile, wild elasmobranchs, which represents a massive limitation moving forward with research in this field. There is potential for a scaling model from small to large elasmobranchs (i.e., oviparity to viviparity); however, it would need to account for variables such as size, reproduction, movement patterns, and metabolic differences between species. Given that these types of life history data are limited for a plethora of species (Dulvy et al. 2014), the likelihood of an accurate scaling model, with our current understanding, is low.
Of all the stressors outlined, overfishing is undoubtedly the most imminent threat to elasmobranchs globally, where, for example, many pelagic sharks have extensive overlaps spatially and temporally with industrial fisheries (Queiroz et al. 2019). As a case study, Indonesia has the largest elasmobranch fishery worldwide, with many shark and ray species considered overfished, mainly driven by shark fin exportation (Blaber et al. 2009). In this case, assessing current stocks and implementing informed management is a top priority, as stocks could be decimated long before climate change effects completely unfold. However, these climate change effects many have a different relative importance in well managed systems. As a comparison, the United States shark fisheries are considered some of the most sustainable globally (Shiffman and Hueter 2017), and therefore other anthropogenic stressors should now gain more scientific attention. Moving forward, it is important in the context of understudied areas like reproduction and development that we prioritize research needs based on the system.
It is clear that anthropogenic stressors not only affect elasmobranchs directly, but also the broader ecosystem. Short and long-term changes to habitat and food web structure can critically affect apex and meso predators like elasmobranchs (Hempson et al. 2017). For example, trophic cascades could decrease prey availability, thereby negatively impacting elasmobranch reproduction and development because metabolic needs are not met (Field et al. 2009; Hempson et al. 2017). The stressors discussed in this review will not be experienced by elasmobranchs in isolation, thus highlighting the importance of future study design that includes synergistic effects of direct and indirect human interactions with elasmobranchs. Species-specific data, such as sensitivity to OA conditions and elevated temperatures, as well as reproductive complexity and early life history survival will be instrumental moving forward, as these data inform climate vulnerability assessments that are used as a fisheries management tool (Hare et al. 2016). Overall, environmental shifts from anthropogenic interactions hold major implications for marine ecosystem health, resilience, and global biodiversity. For maximum effectiveness, policy and conservation measures must account for shifting baselines that define marine environments as well as the effects of acute and chronic climate-related changes. Given that the slow reproductive and growth rates of elasmobranchs underpin the group’s ability to recover from overexploitation, further research within the context of anthropogenic stressors will be paramount to management and conservation both now and into the future.
References
Adams KR, Fetterplace LC, Davis AR, Taylor MD, Knott NA (2018) Sharks, rays and abortion: the prevalence of capture-induced parturition in elasmobranchs. Biol Conserv 217:11–27. https://doi.org/10.1016/j.biocon.2017.10.010
Awruch CA, Pankhurst NW, Frusher SD, Stevens JD (2009) Reproductive seasonality and embryo development in the draughtboard shark Cepaloscyllium laticeps. Mar Freshw Res 60:1265–1272. https://doi.org/10.1071/MF09030
Bass NC, Mourier J, Knott NA, Day J, Guttridge T, Brown C (2017) Long-term migration patterns and bisexual philopatry in a benthic shark species. Mar Freshw Res 68:1414–1421. https://doi.org/10.1071/MF16122
Bindoff NL, Cheung WWL, Kairo JG et al (2019) Changing ocean, marine ecosystems, and dependent communities. In: Pörtner HO, Roberts DC, Masson-Delmotte V et al (eds) IPCC Special Report on the ocean and cryosphere in a changing climate. https://www.ipcc.ch/srocc/chapter/chapter-5/. Accessed 30 Jan 2020
Blaber SJM, Dichmont CM, White W, Buckworth R, Sadiyah L, Iskandar B, Nurhakim S, Pillans R, Andamari R, Dharmadi Fahmi (2009) Elasmobranchs in southern Indonesian fisheries: the fisheries, the status of the stocks and management options. Rev Fish Biol Fisher 19:367–391. https://doi.org/10.1007/s11160-009-9110-9
Chen WK, Liu KM (2006) Reproductive biology of whitespotted bamboo shark Chiloscyllium plagiosum in northern waters off Taiwan. Fish Sci 72:1215–1224. https://doi.org/10.1111/j.1444-2906.2006.01279.x
Clark E, Von Schmidt K (1965) Sharks of the central gulf coast of Florida. Bull Mar Sci 15:13–83
Compagno LJV, Dando M, Fowler S (2005) A field guide to the sharks of the world. Harper Collins Publishers Ltd, London
Conrath CL, Musick JA (2012) Reproductive biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Boca Raton, pp 291–311
Curtis TH, Parkyn DC, Burgess GH (2013) Use of human-altered habitats by bull sharks in a Florida nursery area. Mar Coast Fish 5:28–38. https://doi.org/10.1080/19425120.2012.756438
De Boeck G, Grosell M, Wood C (2001) Sensitivity of the spiny dogfish (Squalus acanthias) to waterborne silver exposure. Aquat Toxicol 54:261–275. https://doi.org/10.1016/S0166-445X(00)00180-6
De Boeck G, Eyckmans M, Lardon I, Bobbaers R, Sinha AK, Blust R (2010) Metal accumulation and metallothionein induction in the spotted dogfish Scyliorhinus canicula. Comp Biochem Physiol Part A Mol Integr Physiol 155:503–508. https://doi.org/10.1016/j.cbpa.2009.12.014
Di Santo V (2015) Ocean acidification exacerbates the impacts of global warming on embryonic little skate, Leucoraja erinacea (Mitchill). J Exp Mar Biol Ecol 463:72–78. https://doi.org/10.1016/j.jembe.2014.11.006
Dixson DL, Jennings AR, Atema J, Munday PL (2015) Odor tracking in sharks is reduced under future ocean acidification conditions. Glob Change Biol 21:1454–1462. https://doi.org/10.1111/gcb.12678
Donelson JM, Salinas S, Munday PL, Shama LNS (2018) Transgenerational plasticity and climate change experiments: where do we go from here? Glob Change Biol 24:13–34. https://doi.org/10.1111/gcb.13903
Dulvy NK, Reynolds JD (1997) Evolutionary transition among egg-laying, live-bearing and maternal inputs in sharks and rays. Proc R Soc B Biol Sci 264:1309–1315. https://doi.org/10.1098/rspb.1997.0181
Dulvy NK, Fowler SL, Musick JA et al (2014) Extinction risk and conservation of the world’s sharks and rays. Elife 3:1–35. https://doi.org/10.7554/eLife.00590
Ebert DA, Compagno LJV, Cowley PD (2007) Aspects of the reproductive biology of skates (Chondrichthyes: Rajiformes: Rajoidei) from southern Africa. ICES J Mar Sci 65:81–102. https://doi.org/10.1093/icesjms/fsm169
Ebert DA, Fowler S, Compagno L (2013) Sharks of the world. Wild Nature Press, Plymouth
Elisio M, Awruch CA, Massa AM, Macchi GJ, Somoza GM (2019) Effects of temperature on the reproductive physiology of female elasmobranchs: the case of the narrownose smooth-hound shark (Mustelus schmitti). Gen Comp Endocr 284:113242. https://doi.org/10.1016/j.ygcen.2019.113242
Ellis JR, Cruz-Martínez A, Rackham BD, Rogers SI (2004) The distribution of chondrichthyan fishes around the British Isles and implications for conservation. J Northw Atl Fish Sci 35:195–213. https://doi.org/10.2960/J.v35.m485
Field IC, Meekan MG, Buckworth RC, Bradshaw CJ (2009) Chapter 4. Susceptibility of sharks, rays and chimaeras to global extinction. Adv Mar Biol 56:275–363. https://doi.org/10.1016/S0065-2881(09)56004-X
Flewelling LJ, Adams DH, Naar JP, Atwood KE, Granholm AA, O’Dea SN, Landsberg JH (2010) Brevetoxins in sharks and rays (Chondrichthyes, Elasmobranchii) from Florida coastal waters. Mar Biol 157:1937–1953. https://doi.org/10.1007/s00227-010-1463-z
Frías-Espericueta MG, Cardenas-Nava NG, Márquez-Farías JF, Osuna-López JI, Muy-Rangel MD, Rubio-Carrasco W, Voltolina D (2014) Cadmium, copper, lead and zinc concentrations in female and embryonic pacific sharpnose shark (Rhizoprionodon longurio) tissues. Bull Environ Contam Toxicol 93:532–535. https://doi.org/10.1007/s00128-014-1360-0
Frías-Espericueta MG, Zamora-Sarabia FKG, Márquez-Farías JF, Osuna-López JI, Ruelas-Inzunza J, Voltolina D (2015) Total mercury in female Pacific sharpnose sharks Rhizoprionodon longurio and their embryos. Lat Am J Aquat Res 43:534–538. https://doi.org/10.3856/vol43-issue3-fulltext-14
Gallucci VF, Taylor IG, Erzini K (2006) Conservation and management of exploited shark populations based on reproductive value. Can J Fish Aquat Sci 63:931–942. https://doi.org/10.1139/f05-267
Gattuso JP et al (2015) Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 349:aac4722. https://doi.org/10.1126/science.aac4722
Gelsleichter J, Manire CA, Szabo NJ, Cortés E, Carlson J, Lombardi-Carlson L (2005) Organochlorine concentrations in bonnethead sharks (Sphyrna tiburo) from four Florida estuaries. Arch Environ Contam Toxicol 48:474–483. https://doi.org/10.1007/s00244-003-0275-2
Gervais C, Mourier J, Rummer JL (2016) Developing in warm water: irregular colouration and patterns of a neonate elasmobranch. Mar Biodivers 46:743–744. https://doi.org/10.1007/s12526-015-0429-2
Gervais CR, Nay TJ, Renshaw G, Johansen JL, Steffensen JF, Rummer JL (2018) Too hot to handle? Using movement to alleviate effects of elevated temperatures in a benthic elasmobranch, Hemiscyllium ocellatum. Mar Biol 165:162. https://doi.org/10.1007/s00227-018-3427-7
Green L, Jutfelt F (2014) Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol Lett 10:20140538. https://doi.org/10.1098/rsbl.2014.0538
Grosell M, Wood CM, Walsh PJ (2003) Copper homeostasis and toxicity in the elasmobranch Raja erinacea and the teleost Myoxocephalus octodecemspinosus during exposure to elevated water-borne copper. Comp Biochem Physiol Toxicol Pharmacol 135:179–190. https://doi.org/10.1016/S1532-0456(03)00089-9
Guida L, Awruch C, Walker TI, Reina RD (2017) Prenatal stress from trawl capture affects mothers and neonates: a case study using the southern fiddler ray (Trygonorrhina dumerilii). Sci Rep 7:1–10. https://doi.org/10.1038/srep46300
Hammerschag N, Schmitz OJ, Flecker AS, Lafferty KD, Sih A, Atwood TB, Gallagher AJ, Irschick DJ, Skubel R, Cooke SJ (2019) Ecosystem function and services of aquatic predators in the Anthropocene. Trends Ecol Evol 34:369–383. https://doi.org/10.1016/j.tree.2019.01.005
Hammerschlag N, Gallagher AJ (2011) Global shark currency: the distribution, frequency, and economic value of shark ecotourism. Curr Issues Tour 14:797–812. https://doi.org/10.1080/13683500.2011.585227
Hare JA, Morrison WE, Nelson MW et al (2016) A vulnerability assessment of fish and invertebrates to climate change on the northeast U.S. continental shelf. PLoS ONE 11:1–30. https://doi.org/10.1371/journal.pone.0146756
Heinrich DDU, Rummer JL, Morash AJ et al (2014) A product of its environment: the epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conserv Physiol 2:cou047. https://doi.org/10.1093/conphys/cou047
Hempson TN, Graham NAJ, Macneil MA, Williamson DH, Jones GP, Almany GR (2017) Coral reef mesopredators switch prey, shortening food chains, in response to habitat degradation. Ecol Evol 6:2626–2635. https://doi.org/10.1002/ece3.2805
Heupel MR (2007) Exiting Terra Ceia Bay: examination of cues stimulating migration from a summer nursery area. In: McCandless CT, Kohler NE Jr, Pratt HL (eds) Shark nursery grounds of the Gulf of Mexico and east coast waters of the United States, vol 50. Am Fish Soc Symp, Bethesda, pp 265–280
Heupel MR, Whittier JM, Bennett MB (1999) Plasma steroid hormone profiles and reproductive biology of the epaulette shark, Hemiscyllium ocellatum. J Exp Zool 284:586–594
Hight BV, Lowe CG (2007) Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: evidence for behavioral thermoregulation? J Exp Mar Biol Ecol 352:114–128. https://doi.org/10.1016/j.jembe.2007.07.021
Hoff GR (2008) A nursery site of the Alaska skate (Bathyraja parmifera) in the eastern Bering Sea. Fish Bull 106:233–244. https://doi.org/10.1111/j.1095-8649.2008.02137.x
Hoff GR (2010) Identification of skate nursery habitat in the eastern Bering Sea. Mar Ecol Prog Ser 403:243–254. https://doi.org/10.3354/meps08424
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT et al (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83. https://doi.org/10.1126/science.aan8048
Jeffree RA, Warnau M, Oberhansli F, Teyssie JL (2006) Bioaccumulation of heavy metals and radionuclides from seawater by encased embryos of the spotted dogfish Scyliorhinus canicula. Mar Pollut Bull 52:1278–1286. https://doi.org/10.1016/j.marpolbul.2006.03.015
Jennings D, Gruber S, Franks B, Kessel S, Robertson A (2008) Effects of large-scale anthropogenic development on juvenile lemon shark (Negaprion brevirostris) populations of Bimini, Bahamas. Environ Biol Fish 83:369–377. https://doi.org/10.1007/s10641-008-9357-3
Johnson MS, Kraver DW, Renshaw GMC, Rummer JL (2016) Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv Physiol 4:1–11. https://doi.org/10.1093/conphys/cow003
Kinney MJ, Simpfendorfer CA (2009) Reassessing the value of nursery areas to shark conservation and management. Conserv Lett 2:52–60. https://doi.org/10.1111/j.1755-263X.2008.00046.x
Knotek RJ, Rudders DB, Mandelman JW, Benoît HP, Sulikowski JA (2018) The survival of rajids discarded in the New England scallop dredge fisheries. Fish Res 198:50–62. https://doi.org/10.1016/j.fishres.2017.10.015
Last PR, White WT, de Carvalho MR, Séret B, Stehmann MF, Naylor GJP (2016) Rays of the world. CSIRO Publishing, Clayton
Lopes AR, Sampaio E, Santos C, Couto A, Pegado MR, Diniz M, Munday PL, Rummer JL, Rosa R (2018) Absence of cellular damage in tropical newly hatched sharks (Chiloscyllium plagiosum) under ocean acidification conditions. Cell Stress Chaperon 23:837–846. https://doi.org/10.1007/s12192-018-0892-3
Lyons K, Adams DH (2014) Maternal offloading of organochlorine contaminants in the yolk-sac placental scalloped hammerhead shark (Sphyrna lewini). Ecotoxicology 24:553–562. https://doi.org/10.1007/s10646-014-1403-7
Lyons K, Lowe CG, Gillanders BM (2013) Mechanisms of maternal transfer of organochlorine contaminants and mercury in the common thresher shark (Alopias vulpinus). Can J Fis Aquat Sci 70:1667–1672. https://doi.org/10.1139/cjfas-2013-0222
Mandelman JW, Cicia AM, Ingram GW, Driggers WB, Coutre KM, Sulikowski JA (2013) Short-term post-release mortality of skates (family Rajidae) discarded in a western North Atlantic commercial otter trawl fishery. Fish Res 139:76–84. https://doi.org/10.1016/j.fishres.2012.09.020
Marler H, Adams DH, Wu Y, Nielsen CK, Shen L, Reiner EJ, Chen D (2018) Maternal Transfer of Flame Retardants in Sharks from the Western North Atlantic Ocean. Environ Sci Technol 52:12978–12986. https://doi.org/10.1021/acs.est.8b01613
Mathews T, Fisher NS (2009) Dominance of dietary intake of metals in marine elasmobranch and teleost fish. Sci Total Environ 407:5156–5161. https://doi.org/10.1016/j.scitotenv.2009.06.003
McConnaughey RA, Syrjala SE (2014) Short-term effects of bottom trawling and a storm event on soft-bottom benthos in the eastern Bering Sea. ICES J Mar Sci 71:2469–2483. https://doi.org/10.1093/icesjms/fss153
Merly L, Lange L, Meÿer M, Hewitt AM, Koen P, Fischer C, Muller J, Schilack V, Wentzel M, Hammerschlag N (2019) Blood plasma levels of heavy metals and trace elements in white sharks (Carcharodon carcharias) and potential health consequences. Mar Pollut Bull 142:85–92. https://doi.org/10.1016/J.MARPOLBUL.2019.03.018
Mull CG, Lyon K, Blasius ME, Winkler C, O’Sulliva JB, Lowe CG (2013) Evidence of maternal offloading of organic contaminants in white sharks (Carcharodon carcharias). PLoS ONE 8:e62886. https://doi.org/10.1371/journal.pone.0062886
Nakata H (2005) Occurrence of synthetic musk fragrances in marine mammals and sharks from Japanese coastal waters. Environ Sci Technol 39:3430–3434. https://doi.org/10.1021/es050199l
Nielsen J, Hedeholm RB, Heinemeier J, Bushnell PG, Christiansen JS, Olsen J, Bronk Ramsey C, Brill RW, Simon M, Steffensen KF, Steffensen JF (2016) Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 353:702–704. https://doi.org/10.1126/science.aaf1703
Núnez-Nogueira G (2005) Concentration of essential and non-essential metals in two shark species commonly caught in Mexican (Gulf of Mexico) coastline. In: Botello AV, Rendón-von Osten J, Gold-Bouchot G, Agraz-Hernandéz C (eds) Golfo de México Contaminación e impacto ambiental: Diagnóstico y tendencias. Univ. Autón. de Campeche, Univ. Nal. Autón. de México, Instituto Nacional de Ecologia, Tlacopac, pp 451–474
Olin JA, Beaudry M, Fisk AT, Paterson G (2014) Age-related polychlorinated biphenyl dynamics in immature bull sharks (Carcharhinus leucas). Environ Toxicol Chem 33:35–43. https://doi.org/10.1002/etc.2402
Pegado MR, Santos C, Couto A et al (2019a) Reduced impact of ocean acidification on growth and swimming performance of newly hatched tropical sharks (Chiloscyllium plagiosum). Mar Freshw Behav Physiol 51:347–357. https://doi.org/10.1080/10236244.2019.1590120
Pegado MR, Santos CP, Pimentel M, Cyrne R, Maulvaut AL, Raffoul D, Diniz M, Bispo R, Rosa R (2019b) Effects of elevated carbon dioxide on hematological parameters of a temperate catshark. J Exp Zool A Ecol Genet Physiol 333:126–132. https://doi.org/10.1002/jez.2333
Pistevos JCA, Nagelkerken I, Rossi T, Olmos M, Connell SD (2015) Ocean acidification and global warming impair shark hunting behaviour and growth. Sci Rep 5:16293. https://doi.org/10.1038/srep16293
Pistevos JCA, Nagelkerken I, Rossi T, Connell SD (2017) Antagonistic effects of ocean acidification and warming on hunting sharks. Oikos 126:241–247. https://doi.org/10.1111/oik.03182
Pörtner HO, Karl D, Boyd P et al (2014) Ocean systems. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TF et al (eds) Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 411–484
Prado AC, Wosnick N, Freire C (2018) Capture stress in Zapteryx brevirostris (Muller and Henle, 1841) (Elasmobranchii, Trygonorrhinidae) males during the reproductive period: Nutritional and reproductive consequences and conservation impacts. Sharks International Meeting, João Pessoa, Paraíba, Brazil, p 339
Pretorius C, Griffiths CL (2013) Patterns of Egg Deposition and Egg Development in the Catsharks Poroderma pantherinum and Haploblepharus pictus. Afr Zool 48:115–124. https://doi.org/10.1080/15627020.2013.11407574
Queiroz N, Humphries NE, Couto A et al (2019) Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572:461–466. https://doi.org/10.1038/s41586-019-1444-4
Rolim FA, Rotundo MM, Vaske-Júnior T (2016) Notes on the reproductive biology of the Brazilian electric ray Narcine brasiliensis (Elasmobranchii: Narcinidae). J Fish Biol 89:1105–1111. https://doi.org/10.1111/jfb.12778
Rosa R, Baptista M, Lopes VM et al (2014) Early-life exposure to climate change impairs tropical shark survival. Proc R Soc B Biol Sci 281:20141738. https://doi.org/10.1098/rspb.2014.1738
Rosa R, Paula JR, Sampaio E et al (2016a) Neuro-oxidative damage and aerobic potential loss of sharks under elevated CO2. Mar Biol 163:119. https://doi.org/10.1007/s00227-016-2898-7
Rosa R, Pimentel M, Galan JG et al (2016b) Deficit in digestive capabilities of bamboo shark early stages under climate change. Mar Biol 163:1–5. https://doi.org/10.1007/s00227-016-2840-z
Rosa R, Rummer JL, Munday PL (2017) Biological responses of sharks to ocean acidification. Biol Lett 13:20160796. https://doi.org/10.1098/rsbl.2016.0796
Salinas-De-León P, Phillips B, Ebert D, Shivji M, Cerutti-Pereyra F, Ruck C, Fisher CR, Marsh L (2018) Deep-sea hydrothermal vents as natural egg-case incubators at the Galapagos Rift. Sci Rep 8:1–7. https://doi.org/10.1038/s41598-018-20046-4
Shiffman DS, Hueter RE (2017) A United States shark fin ban would undermine sustainable shark fisheries. Mar Policy 85:138–140. https://doi.org/10.1016/j.marpol.2017.08.026
Simpfendorfer CA, Heupel MR, White WT, Dulvy NK (2011) The importance of research and public opinion to conservation management of sharks and rays: a synthesis. Mar Freshw Res 62:518–527. https://doi.org/10.1071/MF11086
Skomal GB, Mandelman JW (2012) The physiological response to anthropogenic stressors in marine elasmobranch fishes: a review with a focus on the secondary response. Comp Biochem Phys A 162:146–155. https://doi.org/10.1016/j.cbpa.2011.10.002
Speed CW, Field IC, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293. https://doi.org/10.3354/meps08581
Speed CW, Meekan MG, Field IC, McMahon CR, Bradshaw CJA (2012) Heat-seeking sharks: support for behavioral thermoregulation in reef sharks. Mar Ecol Prog Ser 463:231–244. https://doi.org/10.3354/meps09864
Stevens GC (1989) The latitudinal gradient in geographical range: how many species coexist in the tropics. Am Nat 133:240–256
Stevens JD, Bonfil R, Dulvy NK, Walker PA (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci 57:476–494. https://doi.org/10.1006/jmsc.2000.0724
Stevens JD, Walker TI, Cook SF, Fordham SV (2005) Threats faced by chondrichthyan fish. In: Fowler SL, Cavanagh RD, Camhi M et al (eds) Sharks, rays and chimeras: the status of chondrichthyan fishes. IUCN/SSC Shark Specialist Group. IUCN, Gland and Cambridge, pp 48–54
Sulikowski JA, Wheeler CR, Gallagher AJ, Prohaska BK, Langan JA, Hammerschlag N (2016) Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female-dominated site. Aquat Biol 24:175–184. https://doi.org/10.3354/ab00648
Tricas TC, Michael SW, Sisneros JA (1995) Electrosensory optimization to conspecific phasic signals for mating. Neurosci Lett 202:129–132. https://doi.org/10.1016/0304-3940(95)12230-3
Trujillo JE, Pardo LM, Vargas-Chacoff LV, Valdivia N (2019) Sharks in the forest: relationships between kelp physical-complexity attributes and egg deposition sites of the red-spotted catshark. Mar Ecol Prog Ser 610:125–135. https://doi.org/10.3354/meps12818
Vila Pouca C, Gervais C, Reed J, Michard J, Culum B (2019) Quantity discrimination in Port Jackson sharks incubated under elevated temperatures. Behav Ecol Sociobiol 73:93. https://doi.org/10.1007/s00265-019-2706-8
Wallman HL, Bennett WA (2006) Effects of parturition and feeding on thermal preference of Atlantic stingray, Dasyatis sabina (Lesueur). Environ Biol Fishes 75:259–267. https://doi.org/10.1007/s10641-006-0025-1
Ward-Paige CA, Keith DM, Worm B, Lotze HK (2012) Recovery potential and conservation options for elasmobranchs. J Fish Biol 80:1844–1869. https://doi.org/10.1111/j.1095-8649.2012.03246.x
Weijs L, Briels N, Adams DH, Lepoint G, Das K, Blust R, Covaci A (2015a) Bioaccumulation of organohalogenated compounds in sharks and rays from the southeastern USA. Environ Res 137:199–207. https://doi.org/10.1016/j.envres.2014.12.022
Weijs L, Briels N, Adams DH, Lepoint G, Das K, Blust R, Covaci A (2015b) Maternal transfer of organohalogenated compounds in sharks and stingrays. Mar Pollut Bull 92:59–68. https://doi.org/10.1016/j.marpolbul.2014.12.056
White WT, Blaber SJM, Craig JF (2012) The current status of elasmobranchs: biology, fisheries and conservation. J Fish Biol 80:897–900. https://doi.org/10.1111/j.1095-8649.2012.03268.x
Wosnick N, Awruch CA, Adams KR, Gutierre SMM, Bornatowski H, Prado AC, Freire CA (2018) Impacts of fisheries on elasmobranch reproduction: high rates of abortion and subsequent maternal mortality in the shortnose guitarfish. Anim Conserv 22:198–206. https://doi.org/10.1111/acv.12458
Acknowledgements
CRW is supported by a research assistantship jointly through the Anderson Cabot Center for Ocean Life at New England Aquarium and the University of Massachusetts Boston as well as a graduate fellowship from the American Australian Association. RR is supported by Fundação para a Ciência e Tecnologia (FCT, Portugal) through the MARE strategic project UID/MAR/04292/2019 and a project grant PTDC/AAG-GLO/1926/2014. JLR is supported by an Australian Research Council (ARC) Early Career Discovery Fellowship (2015–2018), a L’Oréal-UNESCO Women in Science Foundation Fellowship (2015–2016), and research allocation from the ARC Centre of Excellence for Coral Reef Studies at James Cook University. We thank the three anonymous reviewers for their insightful feedback and suggestions on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wheeler, C.R., Gervais, C.R., Johnson, M.S. et al. Anthropogenic stressors influence reproduction and development in elasmobranch fishes. Rev Fish Biol Fisheries 30, 373–386 (2020). https://doi.org/10.1007/s11160-020-09604-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-020-09604-0