Abstract
Tropical coral reef flats can be 3–4 °C warmer than surrounding deeper reef slopes, and some experience daily temperature fluctuations of up to 12 °C, which will be exacerbated as global temperatures continue to rise. Epaulette sharks (Hemiscyllium ocellatum), predominantly found on reef flats, may have evolved behavioural and/or physiological strategies to mitigate the effects of these dramatic temperature fluctuations. Here, juvenile sharks were acclimated, for at least 6 weeks, to average summer temperatures (28 °C) or predicted end-of-century summer temperatures (32 °C) to investigate the effects of elevated temperatures on growth, survival, and the use of movement to thermoregulate. In addition, sharks experience seasonal temperature changes; therefore, the upper critical thermal limits were determined for adult, wild sharks during both summer and winter months. We found that regardless of acclimation temperature, juveniles maintained the same food consumption rates (~ 5% body mass every other day), but for those living at 32 °C, this resulted in significantly decreased growth rates (body mass and total length). During winter months, maximum habitat temperatures (~ 24 °C) are far below adult sharks’ critical thermal limits (35.92 ± 0.21 °C). During summer months, maximum habitat temperatures (~ 35 °C) are closer to adult critical thermal limits (38.85 ± 0.31 °C). When estimating thermoregulatory behaviour of juvenile sharks maintained at 28 °C, those sharks examined in winter exhibited no thermoregulatory behaviour, while those examined in summer actively sought to control their thermal exposure, preferring 30.7 ± 1.04 °C (day) and 28.54 ± 0.75 °C (night). Furthermore, after acclimation to predicted end-of-century conditions, these same sharks behaviourally sought out 32.94 ± 0.46 °C (day) and 30.74 ± 0.68 °C (night); despite the cost of decreased growth and/or survival. Sharks maintained in control conditions had a mortality rate of 33% during the initial 90-day period of exposure, while mortality was 100% in those sharks exposed to elevated conditions. Ultimately, as ocean temperatures continue to rise, the distribution and abundance patterns for epaulette sharks and many other coral reef species are likely to change if trade-offs associated with acclimation outweigh the benefits of moving to more favourable habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical coral reef flats are among the warmest and most thermally fluctuating habitats within coral reef ecosystems (Harborne 2013). Conditions on these reef flats are ultimately driven by the daily tidal cycles that influence water depth and water quality (e.g. temperature, dissolved oxygen, pH) (Harborne 2013; Kline et al. 2015). Depending on the season, diurnal fluctuations, and duration of low tide, reef flats can fluctuate by as much as 12 °C over 24 h (Potts and Swart 1984) and are often 3–4 °C warmer than the conditions found on adjacent reef slopes (Harborne 2013). During the summer months along the Great Barrier Reef, Australia, coral reef flat temperatures can reach 33–34 °C, which contrasts with winter months when thermal conditions are much less severe and rarely exceed 30 °C (Chisholm et al. 1996; AIMS 2015a, b). As a result, resident reef flat species routinely experience conditions that could be thermally challenging (Harborne 2013).
Biochemical and physiological processes (e.g. growth, digestion, reproduction, swimming performance, etc.) directly depend on temperature (Fry and Hart 1948; Fry 1971; Pörtner and Peck 2010; Johansen and Jones 2011), and each process is thought to be optimized for a particular temperature or range of temperatures (optimum temperature, Topt) (Brett 1971; Pörtner and Farrell 2008). As temperatures increase or decrease away from the Topt, these processes may decline (Pörtner and Peck 2010). However, with prolonged exposure to altered temperatures some species can acclimate (e.g. shift their thermal range, performance indicators, or even behaviours) to these new conditions over days, months, or even over generations (Pörtner and Peck 2010). As the climate continues to warm, species that are not acclimating fast enough will increasingly experience conditions beyond their optimum, which may already be occurring in some equatorial populations (Munday et al. 2008; Tewksbury et al. 2008; Rummer et al. 2014, McLeod et al. 2015). Whether species have the capacity to eventually adapt to rising temperatures over long timescales (multiple generations) or if they will need to use other strategies including acclimation and behaviour to ameliorate sub-optimal conditions are of key importance in making long-term predictions about the effects that ocean warming will have at the species and ecosystem levels.
Organisms residing in variable thermal environments (e.g. reef flats, mangrove forests, intertidal zones) may regularly experience temperatures near those where performance starts to decline (Harborne 2013) and as temperatures increase, the energy required for an organism to maintain daily processes also increases (Schmidt-Nielson 1990). As a result, non-essential processes such as somatic growth and reproduction become depressed, as basic maintenance costs require a greater proportion of available energy (Parsons 1993). For example, bonnethead shark (Sphyrna tiburo) populations residing in warm waters display reduced growth rates, increased food consumption rates, and reach smaller body sizes than conspecifics living in cooler waters (Parsons 1993; Lombardi-Carlson et al. 2003; Bethea et al. 2007). A similar trend has been noted for large predatory coral trout (Plectropomus leopardus) on the Great Barrier Reef (Johansen et al. 2013, 2015). However, some tropical reef fish and shark species (Economakis and Lobel 1998; Harborne 2013) appear to thrive in fluctuating thermal conditions and consequently may either have adapted and/or acclimated to these conditions (i.e. shifting their thermal optima) and/or utilize strategies (such as movement) either of which may allow them to persist in future conditions.
Species residing in thermally fluctuating environments often rely on physiological/biochemical mechanisms, some of which may be protective (e.g. anaerobic metabolism, anti-oxidative defence mechanisms, stress activated protein responses, etc.) (Pörtner and Peck 2010), as well as behavioural responses to enable them to endure what could be deleterious conditions at least on a periodic basis. Species may also relocate to more favourable temperatures within their habitat (e.g. microhabitats, deeper depths) to avoid these extreme conditions (Coutant 1977; Armstrong et al. 2013; Furey et al. 2013; Furukawa et al. 2014; Nay et al. 2015; Habary et al. 2016). However, temporary relocation does not allow organisms to reside in challenging conditions indefinitely, as continued and prolonged exposure to elevated temperatures can incur high energetic costs eventually affecting processes such as reproduction and increasing predation risk, among others, which could lead to death (Parsons 1993; Munday et al. 2008; Rummer et al. 2014). As a result, coral reef flat residents, which endure daily, prolonged periods of elevated temperatures, may not only be well suited to tolerate thermal fluctuations but may also possess behavioural thermoregulatory strategies (e.g. movement, sheltering) to ease physiological constraints associated with increases in temperature (Harborne 2013, Nay et al. 2015, Habary et al. 2016); such species may be ideal models for investigating temperature mitigation strategies (e.g. behavioural thermoregulation and/or long-term acclimation) and whether these strategies will change as the oceans continue to warm.
Here, we used the epaulette shark (Hemiscyllium ocellatum) as a model reef flat species to investigate the effects of elevated temperatures on growth and survival with predicted end-of-century conditions and the use of behaviour to regulate body temperature. Found almost exclusively on tropical reef flats from Papua New Guinea to Australia (Heupel and Bennett 1998; Payne and Rufo 2012), the epaulette shark is the most hypoxia-tolerant (Wise et al. 1998; Routley et al. 2002) and short-term anoxia-tolerant (more than 3 h) (Renshaw et al. 2002) elasmobranch studied to date. The effects of prolonged exposure to elevated temperatures are not completely understood, but we are starting to gather evidence suggesting that elevated temperatures can negatively affect early development in this species (e.g. colouration and patterns, see Gervais et al. 2016). Therefore, the overall aim of this study was to understand the influence of temperature variations on growth, activity, and preferred temperature in captive reared, juvenile epaulette sharks and understand the influence of seasonal conditions on the thermal tolerance of adult, wild sharks.
Methods
Collection and holding facilities
Epaulette shark eggs were collected from wild-caught, captive sharks (supplied by Cairns Marine) that had been maintained at either Sea World (SW), Gold Coast (n = 12) or Cairns Marine (CM) (n = 8), both located in Queensland, Australia. Eggs were collected as they became available during the breeding season (June–November) and shipped (via air), by 10 dpf (days post-fertilization), to rearing facilities at the Marine Aquaculture Research Facilities Unit (MARFU) at James Cook University, Townsville, Queensland, Australia (see Johnson et al. 2016). There were two hatching periods [Spring—September–mid-October (SW, n = 7; CM, n = 3) and Autumn—February–mid-March (SW, n = 5; CM, n = 5)]. Upon hatching, sharks (14.71 cm ± 2.23, 15.97 g ± 2.66; mean ± SD) were maintained in 200-L circular aquaria at 28 °C (summer average temperature on reef flats Heron Island, 23.442°S, 151.914°E) (AIMS 2015b) for a maximum of 30 days post-hatch (dph) until yolk resources were consumed (West and Carter 1990; Payne and Rufo 2012). Hatchlings were presented with food at 15 and 25 dph; however, it was not until 30 dph that hatchlings accepted food and, therefore, all sharks were fed regularly after 30 dph. At this point, sharks (now designated as juveniles, 17.07 cm ± 7.04, 13.96 g ± 2.92) were transferred to 50-L aquaria (each two to three sharks) and fed pilchard and squid every other day. Throughout the study, captive reared sharks were maintained under a 12:12 photoperiod. Prior to any experimentation, all sharks were fasted for 48 h to ensure a post-absorptive state (Heinrich et al. 2014).
Adult sharks (505.56 ± 80.24 g, 62.17 ± 4.67 cm) used to investigate the upper thermal limits for this species were collected by hand from reef flats on Heron Island, QLD (23.4423°S, 151.9148°E) during the summer (January, February 2015; n = 10) and winter (July 2015; n = 8) months (Marine Park Permit #G14/36697 and James Cook University accreditation). Sharks were maintained in the laboratory for between 60 and 72 h and kept on a natural photoperiod (11:13 during winter months and 13:11 during summer months). During this time, sharks were given at least 24 h to settle, followed by 12-h habituation to experimental tanks, and, following experimentation, allowed 24-h recovery before they were released back onto the reef flats.
All animal care and experimental protocols used in this study were approved by James Cook University Animal Ethics Committee regulations (permit: A2089) and conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and the Queensland Animal Care and Protection Act 2001.
Experimental protocols
Growth rates and food consumption rates
Growth and food consumption rates of juvenile sharks were recorded from 30 dph onward; therefore, only sharks that had consumed food at the start of data collection (i.e. by 30 dph) were included (N = 15 sharks; 28 °C, n = 9; 32 °C, n = 6). Body mass (g) and total length (mm) were measured prior to feeding, at least once per fortnight. Body mass was measured (nearest 0.01 g) and to determine total length, a photo was taken of each shark (with a ruler for scale) and analysed using FIJI software (Schindelin et al. 2012). To measure the change in food consumption over time, sharks were isolated in individual containers, and equal amounts of pilchard and squid were weighed (nearest 0.01 g) and offered to each shark, ad libitum. Food was kept in the individual feeding containers until sharks stopped consuming food (roughly 10 min) and all uneaten food was re-weighed and subtracted from the food offered. The food consumed was recorded (minimum of once per week) as the proportion of a shark’s total body mass.
Two time periods (pre-171 dph and post-171 dph) were analysed for each treatment group, as all sharks were maintained at 28 °C for the first 171 dph. Following 171 dph, a group of sharks (n = 6) were transitioned to 32 °C while, the control group was maintained at 28 °C throughout the entire duration of the study. This was to determine if age contributed to the changes recorded between sharks maintained at 28 °C and sharks transitioned to 32 °C.
Temperature preference
Equipment
A shuttle-box system (described in detail by Nay et al. 2015; Habary et al. 2016) was used to determine the Tpref of each juvenile shark. The temperature range around the Tpref and the number of movements that each animal made between chambers to maintain these preferred temperatures were also determined.
The system is a two-chamber aquarium made from PVC with a clear plexiglass floor. Both round aquaria (Ø 35 cm) are arranged side-by-side in a figure eight manner with a 50-mm wide passage between the two chambers that allows the shark to move freely between them. One chamber was randomly designated as a ‘warm chamber’ while the other was designated as a ‘cool chamber’. At all times, there was an average of 1 °C difference between the two chambers (following previous studies; see Petersen and Steffensen 2003; Killen 2014; Nay et al. 2015; Habary et al. 2016), which was maintained through counterclockwise water flow preventing mixing between the chambers.
During a trial, a custom software system monitored the temperature of the chambers, tracked the position of the shark, and activated/deactivated the corresponding water pumps depending on which chamber the shark was occupying. When a shark entered the ‘warm chamber’, the pump located in a warm water buffer tank would activate, and the temperature of the entire system would increase at a rate of 8 °C h−1. Conversely, when the shark entered the ‘cool chamber’, the warm pump would deactivate, and the cool pump would activate, thus decreasing the temperature at the same rate, while continuously maintaining the 1 °C differential between chambers. By moving between tanks, each shark was able to control their thermal environment and consequently their internal body temperature.
Each buffer tank supplied the corresponding chamber with seawater at a rate of 420 L h−1, which then passively returned to the buffer tank. To heat/cool the water in the buffer tanks, water moved via aquarium pumps through stainless steel coils located in either a cool or a warm reservoir. The temperature in the cool reservoir was maintained using two chillers (HC-130A, HC-1000A, Hailea, Guangdong, China and 1/3 hp Aqua One Arctic, Aqua One, Southampton, UK), and the warm reservoir was maintained using a 5000-W heater (Control Distributions, Carlton, Australia).
Temperature sensors (copper and tungsten thermocouple) constantly relayed temperatures to thermostats (N323, NOVUS Automation, Porto Alegre, Brazil) and were linked to the software system. The position of the shark was assessed using a video camera mounted on a wall 4.75 m away from the set up and aimed at an angled mirror (45° downward) above the shuttle-box system. A PC video frame-grabber (USB 2.0 DVD maker) transmitted the video signal from the camera to the computer software where a software program (LoliTrack, Loligo Systems, Tiele, Denmark) determined the shark’s position based on an X- and Y-coordinate system. From there, custom programs were written using Labtech Notebook Pro (Laboratories Technology Corp., Andover, MA, USA) to activate/deactivate the corresponding pumps. Infrared lights were installed beneath each chamber to ensure that the sharks could be detected at all hours.
Protocol
Juveniles were used for temperature preference experiments as they were over ten times more likely to move than hatchlings were (Fig. S1). Temperature preference trials were performed twice for each individual to identify changes that may occur before and after prolonged exposure to elevated temperatures. The first trial for all juvenile sharks (171 ± 12.6 dph) was at 28 °C, following which juveniles were allocated to one of two temperature treatments (28 °C or 32 °C) representing current day summer averages and projected end-of-century temperatures (Collins et al. 2013, RCP 8.5). To reach 32 °C, the temperature of the holding system was increased at a rate of 0.3 °C per day. All sharks that were eventually brought to 32 °C (n = 4) were placed in the system during summer months (January–March) while the sharks maintained at 28 °C (n = 6) and hatched 6 months later were placed in the system during winter months (August–October). During the first trial, n = 15 sharks were tested at 28 °C; however, three sharks maintained at 28 °C (out of 9) and two sharks (out of six) that were maintained at elevated temperature perished before the second trial could commence. Sharks were maintained at target temperature for 9 weeks prior to any experiment to allow for acclimation of whole body processes (Guderley and Gawlicka 1992), then, after 100 days (66 days of which the sharks were maintained at treatment temperature; 28 °C, n = 6 or 32 °C, n = 4), the second trial for each shark was performed.
At 08:00 (hh:mm), an individual shark was placed into the system and allowed to habituate to the chambers for 2 h. At 10:00, the temperature limits of the system were set so that the shark could learn the heating and cooling pattern of the system. Then, for the following 7 h, the limits of the system would be expanded until the external reservoirs reached the maximum and minimum temperatures that the system could achieve if sharks maintained a position in one chamber indefinitely (50 °C and 8 °C, respectively). The system would continue monitoring the shark’s movements and temperatures throughout the night until 15:00 the following day. Sharks that maintained their position within a chamber were observed to make erratic and unusual movements as the temperatures in the system approached 35 °C or 17 °C (depending on which chamber they were residing). Continued and prolonged failure to change chambers resulted in temperatures increasing/decreasing continually. Around 37 °C and 15 °C, sharks started to become unresponsive, losing their righting response and/or exhibiting constant erratic movements. Therefore, sharks reaching 35 °C or 17 °C prior to 19:00 each evening were removed from the system, as they displayed a lack of movement necessary to avoid reaching lethal temperatures over the night portion of the trial.
Critical thermal limits
The upper critical thermal limits of adult epaulette sharks were investigated by following critical thermal methodology protocols (Becker and Genoway 1979; Paladino et al. 1980; Lutterschmidt and Hutchison 1997). The experimental tank contained an experimental chamber and a 2000-W heater (Omega 2000 W, Full Gauge TIC-17RGT thermostat), air stone, and aquarium pump (WH-500, Weipro®, Guangdong, China) to ensure that the temperature and oxygen concentration in the tank were homogenous. A buoyant basket (experimental chamber), which had holes to allow for generous water flow, was placed inside the experimental tank (100 L). A mesh net was attached to the top of the basket to prevent the sharks from escaping.
Prior to trials, sharks were moved indoors to individual 68-L holding aquaria for at least 12 h so they could habituate indoor conditions. Following this period, an individual was placed into the experimental chamber and allowed to settle (i.e. cease swimming movements) for at least 1 h. Then the temperature of the chamber was increased at a constant rate (0.26 ± 0.0026 °C min−1). As the temperature increased throughout the trial, ventilation rate (operculum beats min−1) was recorded every 10 min until ventilation rate initially increased, at which point ventilation rate was recorded every 5 min. Once the ventilation rate peaked, ventilation rate began to decrease, and the researcher would begin to check for the shark’s loss of righting reflex (LRR) by rotating the shark 180° along the longitudinal axis. The temperature at which the shark exhibited LRR was defined as the critical maximal temperature (CTMax). Immediately following LRR, the shark was removed from the experimental chamber and allowed to fully recover (at least 24 h) before being released back onto the reef flat.
Analysis
Growth rates and food consumption rates
Linear mixed effect models (LMM) were used to analyse growth (body mass and total length) and feeding (expressed as proportion of body mass) rates. Data were auto-correlated with time, and variation between sharks was controlled for by factoring each individual shark as they aged. The random effect was each individual shark. The fixed effects were body mass, total length, and food consumption rates over time, along with either the treatment temperature (28 °C or 32 °C) or the treatment period (pre-171 dph or post-171 dph). Four LMM comparisons for each variable (i.e. body mass, total length, and food consumption) were used to determine whether there were differences between 28 °C and 32 °C sharks over time and treatment. Specifically, the first LMM analysed the growth and food consumption rates in the would-be 32 °C sharks while they were still at 28 °C and then after they had been transitioned to 32 °C. The second LMM analysed growth and food consumption rates only in the 28 °C sharks up to 171 dph and then following 171 dph to determine if these rates were simply just changing with age. The third LMM analysed the differences between the 28 °C and would-be 32 °C sharks (while both groups were still maintained at 28 °C) up to 171 dph to determine if there were differences between the groups prior to the temperature treatment. The fourth LMM analysed growth and food consumption rates between the 28 °C and 32 °C group from 171 dph onward, after target temperatures were reached.
Fisher’s exact test was used to compare differences in mortality percentage between shark treatments [i.e. mortality between sharks maintained at 28 °C (n = 9) and sharks maintained at 32 °C (n = 6)] for 90 days of exposure to treatment temperatures.
Temperature preference
The average Tpref, the range of temperatures selected, and the number of movements required for each shark to maintain their Tpref were all calculated from the logged data. The Tpref was defined as the temperature at which the shark spent the most time, while the range of temperatures selected was determined as the difference between the minimum and maximum temperatures sharks reached during each trial. The total number of movements from one chamber to the next was recorded to calculate the number of movements each shark made to maintain their Tpref. Furthermore, as these sharks are nocturnally active (Heupel and Bennett 1998), the three metrics were not only compared between treatment groups but also compared between night and day time periods. For each shark, two 5-h data periods—one during the night and the other during the day—were used to make calculations (Nay et al. 2015). A LMM was used to compare the change in the average Tpref, range of temperatures selected, and number of movements required for each shark to maintain their Tpref. Data for each shark (random effect) were compared between temperature treatments (i.e. 28 °C and 32 °C) and time periods (i.e. day and night).
Critical thermal limits
One-way ANOVAs were used to detect the seasonal responses of the sharks to progressive temperature changes. Four variables were analysed during each season: (a) temperature at which the shark lost its righting reflex (i.e. its CTMax), (b) the ventilation rate at the loss of righting reflex temperature (final ventilation rate), (c) the maximum ventilation rate during acute warming, and (d) the temperature at the time of maximum ventilation rate during acute warming. No differences were detected between males and females, and all sharks were of similar size (505.56 g ± 80.24, 62.17 cm ± 4.67; mean ± SD); therefore, these factors were not included in the statistical analysis. Additionally, the time required to settle was not significantly different between treatments, sex, or size (1.722 h ± 0.771).
Data were log transformed where needed to meet assumptions for normality and equal variance. Linear mixed models were generated using S-PLUS for Windows Version 8.0 (Insightful Corp.). Fishers’ exact test and one-way ANOVAs were conducted using SigmaPlot for Windows Version 12.1.0.15 (Systat Software, Inc., Chicago, IL, USA). A FDR correction (Benjamini and Hochberg 1995) was used post hoc (when necessary) to account for running multiple tests on the same individuals within experiments.
Results
Growth rates and food consumption rates
There was a significant reduction in growth rates (body mass and total length) in juvenile sharks maintained at 32 °C when compared to juvenile sharks maintained under control conditions (body mass, F1,581 = 6.409, p = 0.0116; total length, F1,493 = 30.494, p < 0.0001). While maintained under control conditions, sharks grew at an average rate of 0.45 g and 1.69 mm per week; however, growth rates slowed to 0.01 g and 0.27 mm per week after sharks were transferred to 32 °C (Fig. 1a, b). This was not a product of age/development, as the growth rates of sharks maintained under control conditions for the entire duration of the study did not significantly change (body mass 0.42–0.67 g and total length 1.52–1.76 mm per week; F1,805 = 3.577, p = 0.059, and F1,450 = 0.640, p = 0.424, respectively) from before and after the 171 dph time point (Fig. 1a, b). Further evidence suggesting that slowed growth was not a result of age (or hatching period) comes from a comparison to the control group. Prior to transfer to 32 °C, sharks grew at a similar rate as their control group counterparts at the same age (body mass, F1,1021 = 0.505, p = 0.478; total length, F1,781 = 0.312, p = 0.577). However, after transfer to 32 °C (171 dph), these sharks grew significantly slower than their similarly aged control counterparts (body mass, F1,353 = 38.248, p < 0.0001; total length, F1,151 = 22.047, p < 0.0001).
Growth mass (a) and total length (b) and change in food consumption rates (c) for juvenile sharks transferred from control conditions (28 °C) to 32 °C after 171 days post-hatch (n = 6, right side, dark colour) or those maintained under control conditions for the entire duration of the study (n = 9, left side, light colour). Dashed lines indicate 95% confidence intervals. Analyses are from LMM output and significant differences within a group or between groups are denoted by an asterisk (*)
Regardless of treatment and/or time period (prior to 171 dph or after 171 dph), all sharks consumed, on average, 5.7 ± 0.09% of their body mass per feeding for the duration of the study. While under control conditions, sharks that were later transitioned to 32 °C did not display a change in food consumption rates (food mass relative to body mass) in comparison to after transfer to 32 °C (Fig. 1c, LMM, F1,524 = 0.446, p = 0.504). Control sharks consistently consumed similar amounts of food (per feeding) over the entire study (Fig. 1c, F1,784 = 0.189, p = 0.664). Food consumption rates in sharks prior to being transitioned to 32 °C were not significantly different from the food consumption rates of similarly aged sharks maintained at 28 °C during the same pre-exposure period (Fig. 1c, F1,960 = 2.873, p = 0.090). Furthermore, after exposure to elevated temperatures, 32 °C sharks consumed food at a similar rate and amount to that of control sharks maintained at 28 °C after 171 dph (F1,339 = 0.161, p = 0.689).
Mortality
Following transition to treatment temperature, sharks transferred to and maintained at 32 °C experienced a significantly higher mortality than sharks maintained at 28 °C (Fig. 2, Fisher’s exact test, p < 0.05). All sharks maintained at 32 °C died by 80 days at target temperature, while only three sharks (33.3%) that were maintained at 28 °C had died by 80 dph.
Temperature preference
There were significant differences in the average Tpref for each shark (n = 4), which were eventually transitioned to and maintained at 32 °C, depending on the time of day (LMM, F1,9 = 14.298, p < 0.005) and their treatment temperature (LMM, F1,9 = 14.928, p < 0.005). But there were no significant differences between the range of selected temperatures (LMM diel period, F1,9 = 0.1.445, p = 0.260; treatment temperature F1,9 = 0.978, p = 0.349) and the number of movements required for each shark to maintain their Tpref (LMM diel period, F1,7 = 3.218, p = 0.116; temperature treatment, F1,7 = 0.048, p = 0.8323) between sharks before (28 °C) and after they were transitioned to 32 °C (Table 1).
During the day, sharks, regardless of treatment temperature, preferred temperatures roughly 2 °C warmer than during the night [28 °C (day) 30.7 ± 1.04 °C vs. (night) 28.54 ± 0.75 °C; 32 °C (day) 32.94 ± 0.46 °C vs. 30.74 ± 0.68 °C (night); Table 1], and similarly, after acclimation to 32 °C, sharks preferred warmer temperatures than while at 28 °C, regardless of time of day [day (28 °C) 30.7 ± 1.04 °C vs. (32 °C) 32.94 ± 0.46 °C; night (28 °C) 28.54 ± 0.75 °C vs. (32 °C) 30.74 ± 0.68 °C; Table 1]. There were no differences in either the range of temperatures each shark selected, or the number of movements required for each shark to maintain their Tpref between acclimation temperatures or diurnal periods; however, in general, sharks increased the number of movements made at night (Table 1).
In contrast, all sharks (n = 6) that were tested under control conditions (28 °C) during the winter and then maintained under control conditions for a further 100 days into winter failed to use movement to control their body temperature both during the initial trial (171 ± 12.6 dph) or 100 days later for the second trial (i.e. they did not move within the experimental setup). A total of n = 6 sharks were run in the system during the summer months at 28 °C, all successfully using movement to regulate their external environment; however, during acclimation but prior to the second trial at 32 °C two sharks died.
Critical thermal limits
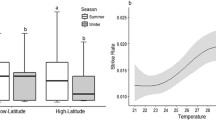
Adult, wild sharks exhibited a significantly higher upper critical thermal temperature (CTMax) and maximum ventilation rates during the warmer summer months (25–32 °C, summer temperature range on Heron Island reef flat), than during the cooler winter months (14–26 °C, winter temperature range on Heron Island reef flat) (AIMS 2015b); however, neither the ventilation rate at the upper critical thermal limit (final ventilation rate) nor the temperature at which the shark exhibited maximum ventilation rates was different between seasons. During the summer months, sharks lost their righting reflex at significantly higher temperatures (n = 10; 38.85 ± 0.3 °C) than sharks during the winter months (n = 8; 35.92 ± 0.2 °C) (Fig. 3a, one-way ANOVA, F1,17 = 45.744, p < 0.001). Furthermore, sharks during the summer months doubled their ventilation rates from 39.22 ± 5.09 min−1 at rest to maximal rates of 78.10 ± 4.2 min−1, a significantly higher maximal rate than what was observed in sharks during winter months, where resting rates were 19.00 ± 2.46 min−1 and maximal rates were 52.00 ± 4.2 min−1 (Fig. 3b, one-way ANOVA, F1,17 = 26.487, p < 0.001). However, these maximum ventilation rates occurred at the same temperature regardless of season (35.04 ± 0.5 °C; one-way ANOVA, F1,17 = 2.116, p = 0.165). Furthermore, there were no significant differences in the ventilation rates (57.78 ± 3.3 min−1) at the sharks’ CTMax regardless of season (one-way ANOVA, F1,17 = 3.959, p = 0.064).
Boxplots showing the upper critical thermal limits (a) and maximum ventilation rates during acute arming (b) for wild sharks during summer (n = 10) and winter months (n = 8). Significant differences are denoted by an asterisk (*). The boxes represent first and third quartiles, and the whiskers (errors) represent the minimum, and maximum values within each box mean (solid line) values are included. Summer values are in orange boxplots. Winter values are in dark blue boxplots
Discussion
Exposure to elevated temperatures significantly depressed growth rates (body mass and total length) of juvenile epaulette sharks, with overall growth rates approaching zero after prolonged exposure to 32 °C. Movement can be a strategy used by many reef-associated species to avoid potentially deleterious conditions (e.g. elevated temperatures and other changes in water quality and physical habitat associated with low tide, see Harborne 2013), and to select conditions that may be more energetically and/or ecologically advantageous (Nay et al. 2015; Habary et al. 2016). In this study, we revealed potential seasonal differences in the strategies used by epaulette sharks—and perhaps other similar reef flat residents—to cope with fluctuating temperatures. Juvenile sharks displayed the ability to regulate their thermal environment through movement; however, the time of year may influence the extent to which this species utilizes this behaviour. Specifically, during summer months, sharks that were initially maintained at 28 °C and later transitioned to 32 °C preferred temperatures at, or slightly higher than, their acclimation temperatures. During the winter months, none of the juveniles (all maintained at 28 °C) used behaviour to regulate their thermal environment and thus their body temperature. Juveniles may be able to use behavioural strategies to mitigate the negative effects of elevated temperatures (e.g. depressed growth and increased energetic costs) by moving to areas where temperatures are more favourable.
With exposure to elevated temperatures, many species require a greater amount of energy as basic living costs increase. Some species such as bonnethead sharks (Bethea et al. 2007) are shown to compensate for the increased energetic demands associated with living at elevated temperatures by increasing food consumption. Epaulette sharks acclimated to 32 °C were seemingly unable to meet their increased energetic demand by increasing the amount of food they ate. It is likely that the animals were morphologically restricted and could not eat more or at a faster rate than the 28 °C sharks could within their feeding time. Digestive rates or gut passage time may change at higher temperatures, necessitating more frequent meals or more energy-rich food items in the wild to compensate for increased energetic demands (Bethea et al. 2007). If sharks maintained at higher temperatures are able to find sufficient food over a longer timescale, it is possible that the depressed growth rates observed in this study may not occur in nature. However, the finding that acclimation to 32 °C resulted in higher mortality and depressed growth rate despite similar food consumption when compared with 28 °C sharks does suggest apparent trade-offs at higher temperatures. Species unable to meet these increased energetic demands directly, will need to either shift their diet to include prey items with greater energy (Bethea et al. 2007) or else move to cooler temperatures to slow down digestion, increase nutrient uptake, and/or mitigate the effects of elevated temperatures (Neer et al. 2007). Such strategies could allow species to maintain physiological performance under these challenging conditions (Bethea et al. 2007; Neer et al. 2007; Pistevos et al. 2015; Johansen et al. 2013). With these elevated energetic costs, those species most affected will need to be able to use strategies such as behavioural thermoregulation, and if the energetic costs still cannot be met, biological fitness could be compromised at the species or population levels.
Epaulette sharks displayed the capacity to acclimate both their upper critical thermal limits as adults and their preferred temperatures as juveniles, despite the apparent cost that preferring a higher temperature may have to traits such as growth and even survival. In past studies, some species that were maintained at a range of temperatures shifted their critical thermal limits (upper and lower) and/or their preferred temperatures (Brett 1952; Cherry et al. 1977; Noyola et al. 2013; Habary et al. 2016). These traits, among others such as aerobic scope and swimming performance, are thought to be relatively plastic and may benefit from acclimation (Brett 1952; Cherry et al. 1975, 1977; Bulger and Tremaine 1985; Clark and Green 1991; Beitinger et al. 2000; Healy and Schulte 2012; Cocherell et al. 2014; Zhang and Kieffer 2014; Habary et al. 2016). While the small sample size and lack of sharks tested under elevated temperature in winter months should be considered, juvenile sharks showed similar trends as a group and preferred higher temperatures after prolonged exposure to elevated temperatures compared to when they were maintained under control temperatures. This is particularly interesting as they preferred temperatures similar to or just above their acclimation temperature despite the fact that during the day they preferred temperatures above which growth was depressed. While only temperature preference and growth were directly measured, other performance traits and physiological processes could benefit from higher temperatures, at least in the short term. In nature, epaulette sharks are exposed to variable conditions during which sharks may choose to conserve energy and minimize other deleterious effects during the day (i.e. increased visibility by aerial predators, UV exposure, etc.) and thus appear to prefer warmer temperatures because they only move when absolutely necessary. Juvenile sharks that failed to use movement to regulate their external thermal environment may have been trying to “wait out” the unfavourable conditions they were experiencing to conserve energy. While in nature this strategy may be effective, the shuttle-box system used here relies on active movement and the failure to move elicits exposure to continually increasing/decreasing conditions. Those that failed may miss the chance to effectively thermoregulate by “waiting out”, and as the sharks that failed to move approached their thermal extremes, they would become erratic near prior to becoming unresponsive. Those sharks that did move, while not significant, moved more at night both at 28 °C and at 32° than during daytime hours. This is reasonable, as this shark species is naturally most active at night (Heupel and Bennett 1998) and may be more willing to move at night than during the day when conditions are harshest. Moving between temperatures on a diel schedule could be beneficial, as sharks conserve energy during the day—only moving when absolutely necessary—while actively moving around at night, during which time the risks of moving are reduced (e.g. less predation and/or reduced exposure to extreme conditions).
Species that experience regular, environmental changes on a seasonal scale may naturally acclimate as conditions change. Here, the upper thermal limits in adult epaulette sharks changed in accordance with environmental conditions, with sharks being able to survive temperatures around 3 °C warmer in the summer months compared to the winter months. Furthermore, at elevated temperatures during summer months, increased ventilation rates may compensate for increased oxygen demand and lower dissolved oxygen concentrations in the water (Gehrke and Fielder 1988; Routley et al. 2002). Despite the fact that juvenile sharks were held under constant conditions throughout the study, the only sharks that were successful in using movement to select their thermal environment were those that were tested during the summer months. While this result is intriguing, we suggest that, as parent sharks were all wild-caught adults and thus would have experienced both summer and winter conditions, transgenerational acclimation could have occurred. In teleost fishes, the environmental conditions during spawning have been shown to influence the performance of offspring (Munday 2014, Murray et al. 2014); however, further studies are required to investigate, as to date, transgenerational acclimation has not been studied in sharks.
Although H. ocellatum and many other reef flat species may be well adapted to fluctuating and extreme environmental conditions for short, seasonal periods (Renshaw et al. 2002; Nilsson and Ostlund-Nilsson 2004; Heinrich et al. 2015), not all of these reef flat species may be capable of withstanding exposure to elevated temperatures for longer periods of time. It is expected that ocean warming will increase the overall temperature of reef flats, and if conditions fail to improve, some species will be pushed past their thermal limits, and biological fitness will decline. Already some species and populations may be nearing the upper bounds of their temperature ranges for performance and may be unable to endure prolonged exposure to predicted end-of-century conditions (Gardiner et al. 2010; Harborne 2013; Rummer et al. 2014). In the current study, all 32 °C-acclimated sharks perished by 251 dph (80 days at 32 °C). In contrast, there was a 33% mortality in sharks maintained under control conditions at the end of 251 dph (80 days at 28 °C), which is about average when compared against rates reported in other husbandry studies (Payne and Rufo 2012; 19.5% mortality; West and Carter 1990). While our study was conducted in a laboratory setting, natural mortality of neonate and juvenile sharks in the wild can be relatively high (Gruber et al. 2001; Heupel and Simpfendorfer 2002). In fact, mortality of neonate blacktip reef sharks (Carcharhinus limbatus), within the first 15 days after birth, can be between 62 and 92% (Heupel and Simpfendorfer 2002). While this study showed that there are detrimental effects to this species when they are maintained at a constant + 4 °C above current summer temperatures, coral reef flats do experience wide ranges in temperatures, which can depend on tidal and daily fluctuations. Given that the average temperatures on these reefs are expected to increase, the characteristic daily fluctuations may offer some relief, as nocturnal periods contrast with the even more extreme daytime periods that these reef flat specialists must endure.
Ocean warming will continue to have substantial effects on many reef fishes, causing declines in growth, reproduction, and survival. Elevated temperatures, especially during early life stages of fishes, can have a profound impact on population structure and ecosystem health, influencing recruitment rates and patterns via impacts on growth, behaviours such as predator avoidance, and overall survivorship (Marine and Cech 2004; Munday et al. 2008; Martins et al. 2012). Without inherent plasticity or adaptations to avoid or compensate for challenging thermal habitats, knock-on effects could include limited reproductive output, changes in population demographics, or even local extinctions (Munday et al. 2008, Vergés et al. 2014). Even species such as the epaulette shark, which is understood to be well adapted to particularly challenging environmental conditions (e.g. hypoxia, anoxia, elevated CO2; Wise et al. 1998; Renshaw et al. 2002; Routley et al. 2002; Heinrich et al. 2014, 2015; Johnson et al. 2016), are not immune to rising temperatures. However, this species does seem to utilize strategies, such as movement and/or acclimation which may be able to offset some of the negative effects of prolonged exposure to elevated temperatures. Yet, the depressed growth and high mortality rate under elevated temperatures observed in this study may suggest that prolonged exposure to these elevated temperatures is of concern. Utilizing behavioural thermoregulatory strategies, such as movement, gives species that are strongly affected by temperature a way to alleviate and/or escape areas unfit for survival under future ocean conditions. As conditions in coral reefs and other tropical habitats decline, the use of these behaviours may become more frequent and could ultimately lead to entire populations redistributing as species move to deeper waters or towards the poles.
References
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Armstrong JB, Schindler DE, Ruff CP, Brooks GT, Bentley KE, Torgersen CE (2013) Diel horizontal migration in streams: juvenile fish exploit spatial heterogeneity in thermal and trophic resources. Ecology 94:2066–2075. https://doi.org/10.1890/12-1200.1
Australian Institute of Marine Science (AIMS) (2015a) Sea water temperature logger data at Lizard Island, Great Barrier Reef from 27 Oct 1995 to 06 Dec 2014, http://data.aims.gov.au/metadataviewer/faces/view.xhtml?uuid=b8fc8578-fef9-43fa-9483-222ade016c2b. Accessed 31 Oct 2015
Australian Institute of Marine Science (AIMS) (2015b) Sea water temperature logger data at Heron Island, Great Barrier Reef from 24 Nov 1995 to 30 Mar 2015. http://data.aims.gov.au/metadataviewer/faces/view.xhtml?uuid=446a0e73-7c30-4712ddb-ba1fc29b8b9a. Accessed 25 Nov 2015
Becker CD, Genoway RG (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of fresh water fish. Environ Biol Fish 4:245–256
Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish 58:237–275. https://doi.org/10.1023/a:1007676325825
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 57:289–300
Bethea D, Hale L, Carlson J, Cortés E, Manire C, Gelsleichter J (2007) Geographic and ontogenetic variation in the diet and daily ration of the bonnethead shark, Sphyrna tiburo, from the eastern Gulf of Mexico. Mar Biol 152:1009–1020. https://doi.org/10.1007/s00227-007-0728-7
Brett JR (1952) Temperature tolerance in young Pacific salmon, genus Oncorhynchus. J Fish Res Bd Can 9:265–323. https://doi.org/10.1139/f52-016
Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon Oncorhynchus nerka. Am Zoo 11:99–113
Bulger AJ, Tremaine SC (1985) Magnitude of seasonal effects on heat tolerance in Fundulus heteroclitus. Physiol Zoo 58:197–204
Chave EHN, Randall HA (1971) Feeding behavior of the moray eel, Gymnothorax pictus. Copeia 1971:570–574. https://doi.org/10.2307/1442464
Cherry DS, Dickson KL, Cairns J Jr (1975) Temperatures selected and avoided by fish at various acclimation temperatures. J Fish Res Board Can 32:485–491. https://doi.org/10.1139/f75-059
Cherry DS, Dickson KL, Cairns J Jr, Stauffer JR (1977) Preferred, avoided, and lethal temperatures of fish during rising temperature conditions. J Fish Res Board Can 34:239–246. https://doi.org/10.1139/f77-035
Chisholm JJ, Barnes DD, Devereux MM (1996) Measurement and analysis of reef flat community metabolism at Lizard Island, Great Barrier Reef, Australia in March 1996, p 150
Clark DS, Green JM (1991) Seasonal variation in temperature preference of juvenile Atlantic cod (Gadus morhua), with evidence supporting an energetic basis for their diel vertical migration. Can J Zoo 69:1302–1307. https://doi.org/10.1139/z91-183
Cocherell DE, Fangue NA, Klimley PA, Cech JJ (2014) Temperature preferences of hardhead Mylopharodon conocephalus and rainbow trout Oncorhynchus mykiss in an annular chamber. Environ Biol Fish 97:865–873. https://doi.org/10.1007/s10641-013-0185-8
Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski W, Johns T, Krinner G (2013) Long-term climate change: projections, commitments and irreversibility. Cambridge University Press, Cambridge
Coutant CC (1977) Compilation of temperature preference Ddata. J Fish Res Bd Can 34:739–745. https://doi.org/10.1139/f77-115
Depczynski M, Bellwood DR (2003) The role of cryptobenthic reef fishes in coral reef trophodynamics. Mar Ecol Prog Ser 256:183–191
Economakis AE, Lobel PS (1998) Aggregation behavior of the grey reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, central Pacific Ocean. Environ Biol Fish 51:129–139
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic, New York, pp 1–98
Fry FEJ, Hart JS (1948) Cruising speed of goldfish in relation to water temperature. J Fish Board Can 7:169–175
Furey NB, Dance MA, Rooker JR (2013) Fine-scale movements and habitat use of juvenile southern flounder Paralichthys lethostigma in an estuarine seascape. J Fish Biol 82:1469–1483. https://doi.org/10.1111/jfb.12074
Furukawa S, Tsuda Y, Nishihara GN, Fujioka K, Ohshimo S, Tomoe S, Nakatsuka N, Kimura H, Aoshima T, Kanehara H, Kitagawa T, Chiang W-C, Nakata H, Kawabe R (2014) Vertical movements of Pacific bluefin tuna (Thunnus orientalis) and dolphinfish (Coryphaena hippurus) relative to the thermocline in the northern East China Sea. Fish Res 149:86–91. https://doi.org/10.1016/j.fishres.2013.09.004
Gardiner NM, Munday PL, Nilsson GE (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS One 5:e13299. https://doi.org/10.1371/journal.pone.0013299
Gehrke PC, Fielder DR (1988) Effects of temperature and dissolved oxygen on heart rate, ventilation rate and oxygen consumption of spangled perch, Leiopotherapon unicolor (Günther 1859), (Percoidei, Teraponidae). J Comp Phys B 157:771–782. https://doi.org/10.1007/bf00691008
Gervais C, Mourier J, Rummer J (2016) Developing in warm water: irregular colouration and patterns of a neonate elasmobranch. Mar Biodivers 4:1–2
Gruber SH, De Marignac JR, Hoenig JM (2001) Survival of juvenile lemon sharks at Bimini, Bahamas, estimated by mark–depletion experiments. Trans Am Fish Soc 130:376–384
Guderley H, Gawlicka A (1992) Qualitative modification of muscle metabolic organization with thermal acclimation of rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 10:123–132
Habary A, Johansen JL, Nay TJ, Steffensen JF, Rummer JL (2016) Adapt, move or die—how will tropical coral reef fishes cope with ocean warming? Glob Change Biol. https://doi.org/10.1111/gcb.13488
Harborne AR (2013) The ecology, behaviour and physiology of fishes on coral reef flats, and the potential impacts of climate change. J Fish Biol 83:417–447. https://doi.org/10.1111/jfb.12203
Healy TM, Schulte PM (2012) Factors affecting plasticity in whole-organism thermal tolerance in common killifish (Fundulus heteroclitus). J Comp Physiol B 182:49–62. https://doi.org/10.1007/s00360-011-0595-x
Heinrich DDU, Rummer JL, Morash AJ, Watson S-A, Simpfendorfer CA, Heupel MR, Munday PL (2014) A product of its environment: the epaulette shark (Hemiscyllium ocellatum) exhibits physiological tolerance to elevated environmental CO2. Conserv Physiol. https://doi.org/10.1093/conphys/cou047
Heinrich DD, Watson S-A, Rummer JL, Brandl SJ, Simpfendorfer CA, Heupel MR, Munday PL (2015) Foraging behaviour of the epaulette shark Hemiscyllium ocellatum is not affected by elevated CO2. ICES J Mar Sci 7:633–640. https://doi.org/10.1093/icesjms/fsv085
Heupel MR, Bennett MB (1998) Observations on the diet and feeding habits of the epaulette shark, Hemiscyllium ocellatum (Bonnaterre), on Heron Island Reef, Great Barrier Reef, Australia. Mar Freshw Res 49:753–756. https://doi.org/10.1071/MF97026
Heupel MR, Simpfendorfer CA (2002) Estimation of mortality of juvenile blacktip sharks, Carcharhinus limbatus, within a nursery area using telemetry data. Can J Fish Aquat Sci 59:624–632. https://doi.org/10.1139/f02-036
Johansen JL, Jones GP (2011) Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob Change Biol 17:2971–2979. https://doi.org/10.1111/j.1365-2486.2011.02436.x
Johansen JL, Messmer V, Coker DJ, Hoey AS, Pratchett MS (2013) Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob Change Biol. https://doi.org/10.1111/gcb.12452
Johansen JL, Pratchett MS, Messmer V, Coker DJ, Tobin AJ, Hoey AS (2015) Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci Rep 5(1):13830. https://doi.org/10.1038/srep13830
Johnson MS, Kraver DW, Renshaw GMC, Rummer JL (2016) Will ocean acidification affect the early ontogeny of a tropical oviparous elasmobranch (Hemiscyllium ocellatum)? Conserv Physiol. https://doi.org/10.1093/conphys/cow003
Killen SS (2014) Growth trajectory influences temperature preference in fish through an effect on metabolic rate 83:1513–1522. https://doi.org/10.1111/1365-2656.12244
Kline DI, Teneva L, Hauri C, Schneider K, Miard T, Chai A, Marker M, Dunbar R, Caldeira K, Lazar B, Rivlin T, Mitchell BG, Dove S, Hoegh-Guldberg O (2015) Six month in situ high-resolution carbonate chemistry and temperature study on a coral reef flat reveals asynchronous pH and temperature anomalies. PLoS One 10:e0127648. https://doi.org/10.1371/journal.pone.0127648
Lombardi-Carlson LA, Cortes E, Parsons GR, Manire CA (2003) Latitudinal variation in life-history traits of bonnethead sharks, Sphyrna tiburo, (Carcharhiniformes : Sphyrnidae) from the eastern Gulf of Mexico. Mar Freshw Res 54:875–883. https://doi.org/10.1071/MF03023
Lough JM (1999) Sea surface temperatures on the Great Barrier Reef: a contribution to the study of coral bleaching. Great Barrier Reef Marine Park Authority, Townsville
Lutterschmidt WI, Hutchison VH (1997) The critical thermal maximum: history and critique. Can J Zoo 75:1561–1574. https://doi.org/10.1139/z97-783
Marine KR, Cech JJ (2004) Effects of high water temperature on growth, smoltification, and predator avoidance in juvenile Sacramento River Chinook salmon. N Am J Fish Manage 24:198–210. https://doi.org/10.1577/M02-142
Martins EG, Hinch SG, Cooke SJ, Patterson DA (2012) Climate effects on growth, phenology, and survival of sockeye salmon (Oncorhynchus nerka): a synthesis of the current state of knowledge and future research directions. Rev Fish Biol Fisher 22:887–914. https://doi.org/10.1007/s11160-012-9271-9
McLeod IM, McCormick MI, Munday PL, Clark TD, Wenger AS, Brooker RM, Takahashi M, Jones GP (2015) Latitudinal variation in larval development of coral reef fishes: implications of a warming ocean. Mar Ecol Prog Ser 521:129–141
Meekan M, Fuiman L, Davis R, Berger Y, Thums M (2015) Swimming strategy and body plan of the world’s largest fish: implications for foraging efficiency and thermoregulation. Front Mar Sci. https://doi.org/10.3389/fmars.2015.00064
Munday PL (2014) Transgenerational acclimation of fishes to climate change and ocean acidification. F1000prime reports 6
Munday P, Kingsford M, O’Callaghan M, Donelson J (2008) Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs 27:927–931. https://doi.org/10.1007/s00338-008-0393-4
Murray CS, Malvezzi A, Gobler CJ, Baumann H (2014) Offspring sensitivity to ocean acidification changes seasonally in a coastal marine fish. Mar Ecol Prog Ser 504:1–11
Nay T, Johansen J, Habary A, Steffensen J, Rummer J (2015) Behavioural thermoregulation in a temperature-sensitive coral reef fish, the five-lined cardinalfish (Cheilodipterus quinquelineatus). Coral Reefs. https://doi.org/10.1007/s00338-015-1353-4
Neer JA, Rose KA, Cortes E (2007) Simulating the effects of temperature on individual and population growth of Rhinoptera bonasus: a coupled bioenergetics and matrix modeling approach. Mar Ecol Prog Ser 329:211–223
Nilsson GE, Ostlund-Nilsson S (2004) Hypoxia in paradise: widespread hypoxia tolerance in coral reef fishes. Proc R Soc B 271:S30–S33. https://doi.org/10.1098/rsbl.2003.0087
Noyola J, Caamal-Monsreal C, Díaz F, Re D, Sánchez A, Rosas C (2013) Thermopreference, tolerance and metabolic rate of early stages juvenile Octopus maya acclimated to different temperatures. J Therm Biol 38:14–19. https://doi.org/10.1016/j.jtherbio.2012.09.001
Paladino FV, Spotila JR, Schubauer JP, Kowalski KT (1980) The critical thermal maximum a technique used to elucidate physiological stress and adaptation in fishes. Rev Can Biol 39:115–122
Parsons G (1993) Geographic variation in reproduction between two populations of the bonnethead shark, Sphyrna tiburo. Environ Biol Fish 38:25–35. https://doi.org/10.1007/BF00842901
Payne EJ, Rufo KS (2012) Husbandry and growth rates of neonate epaulette sharks, Hemiscyllium ocellatum in captivity. Zoo Biol 31:718–724. https://doi.org/10.1002/zoo.20426
Petersen MF, Steffensen JF (2003) Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes at normoxia and moderate hypoxia. J Exp Biol 206:359–364. https://doi.org/10.1242/jeb.00111
Pistevos JCA, Nagelkerken I, Rossi T, Olmos M, Connell SD (2015) Ocean acidification and global warming impair shark hunting behaviour and growth. Sci Rep 5:16293. https://doi.org/10.1038/srep16293
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Pörtner HO, Peck MA (2010) Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J Fish Biol 77:1745–1779. https://doi.org/10.1111/j.1095-8649.2010.02783.x
Potts D, Swart P (1984) Water temperature as an indicator of environmental variability on a coral reef. Limnol Oceanogr 29:504–516
Reavis RH (1997) The natural history of a monogamous coral-reef fish, Valenciennea strigata (Gobiidae): 1. Abundance, growth, survival and predation. Exp Biol Fish 49:239–246
Renshaw GMC, Kerrisk CB, Nilsson GE (2002) The role of adenosine in the anoxic survival of the epaulette shark, Hemiscyllium ocellatum. Comp Biochem Physiol A 131:133–141
Routley MH, Nilsson GE, Renshaw GMC (2002) Exposure to hypoxia primes the respiratory and metabolic responses of the epaulette shark to progressive hypoxia. Comp Biochem Physiol A Mol Integr Physiol 131:313–321
Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL (2014) Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob Change Biol 20:1055–1066. https://doi.org/10.1111/gcb.12455
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Schmidt-Nielson K (1990) Animal physiology: adaptation and environment. Cambridge University Press, Cambridge
Sims DW, Wearmouth VJ, Genner MJ, Southward AJ, Hawkins SJ (2004) Low-temperature-driven early spawning migration of a temperate marine fish. J Anim Ecol 73:333–341. https://doi.org/10.1111/j.0021-8790.2004.00810.x
Tewksbury JJ, Huey RB, Deutsch CA (2008) Putting the heat on tropical animals. Science 320:1296–1297. https://doi.org/10.1126/science.1159328
Vergés A, Steinberg PD, Hay ME, Poore AGB, Campbell AH, Ballesteros E, Heck KL, Booth DJ, Coleman MA, Feary DA, Figueira W, Langlois T, Marzinelli EM, Mizerek T, Mumby PJ, Nakamura Y, Roughan M, van Sebille E, Gupta AS, Smale DA, Tomas F, Wernberg T, Wilson SK (2014) The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc R Soc B. https://doi.org/10.1098/rspb.2014.0846
West J, Carter S (1990) Observations on the development and growth of the epaulette shark Hemiscyllium ocellatum (Bonnaterre) in captivity. J Aquaric Aquatic Sci 5:111–117
Wise G, Mulvey JM, Renshaw GMC (1998) Hypoxia tolerance in the epaulette shark (Hemiscyllium ocellatum). J Exp Zoo 281:1–5. https://doi.org/10.1002/(SICI)1097-010X(19980501)281:1%3c1:AID-JEZ1%3e3.0.CO;2-S
Zhang Y, Kieffer JD (2014) Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Can J Zoo 92:215–221. https://doi.org/10.1139/cjz-2013-0223
Acknowledgements
We want to thank SeaWorld, Gold Coast for donating sharks and D. Kraver and M. Johnson for rearing embryos. Additionally, thanks are due to the staff of the Marine and Aquatic Research Facilities Unit (MARFU) at James Cook University for help with infrastructure and logistical support, as well as the Heron Island staff for their support in the field. This work was supported by an Australian Research Council (ARC) Super Science Fellowship, ARC Early Career Discovery Award, and ARC Centre of Excellence for Coral Reef Studies research allocation to J. L. R. In addition, this work was funded in part by a Griffith University (Gold Coast) Climate Change Response Group research grant to G. R. and J. L. R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Collection permit under the James Cook University accreditation and Marine Park permit, #G14/36697, from the Great Barrier Reef Marine Park Authority, was obtained.
Ethical approval
All animal care and experimental protocols used in this study were approved by James Cook University Animal Ethics Committee regulations (permit: A2089) and conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and the Queensland Animal Care and Protection Act 2001. This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
No conflict interests exist. C. Gervais declares that he has no conflict of interest. T. Nay declares that she has no conflict of interest. G. Renshaw declares that she has no conflict of interest. J. Johansen declares that he has no conflict of interest. J. Steffensen declares that he has no conflict of interest. J. Rummer declares that she has no conflict of interest.
Additional information
Responsible Editor: A. Todgham.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gervais, C.R., Nay, T.J., Renshaw, G. et al. Too hot to handle? Using movement to alleviate effects of elevated temperatures in a benthic elasmobranch, Hemiscyllium ocellatum. Mar Biol 165, 162 (2018). https://doi.org/10.1007/s00227-018-3427-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3427-7