Abstract
Ocean warming can induce physiological and behavioural effects in marine predators that can cascade through ecosystems. A lack of understanding of the effects of elevated temperature on shark behaviour remains an impediment to forecasting ecosystem-wide impacts. Port Jackson shark eggs were incubated and reared at current and projected end-of-century temperatures (+ 3 °C). We tested juvenile’s learning ability with a quantity discrimination task. The mortality rate of sharks reared in warm water was 41.7% compared with no mortality in the present-day sharks. Contrary to expectations, our results suggest that surviving hatchlings from the elevated-temperature group took fewer days to reach learning criterion and had a higher proportion of correct choice compared with hatchlings reared under present conditions. Additionally, this is the first data suggesting that sharks can discriminate different quantities. Our results seem to indicate that learning and behaviour might play a role in allowing elasmobranchs to overcome some of the deleterious effects of climate warming, but further research is needed to fully comprehend these findings.
Significance statement
The world’s oceans are warming at an unprecedented rate, which will impair development and alter physiological and behavioural traits in marine predators. Learning may play a leading role in allowing apex and mesopredators to adapt to a rapidly changing environment; however, no studies have tested the impacts of ocean warming in their learning abilities. We incubated and reared Port Jackson shark eggs at current and projected end-of-century temperatures (+ 3 °C). Contrary to expectations, surviving juveniles from the elevated-temperature group showed better learning performance, potentially adding learning ability to a growing list of traits that incubation temperature can modify during early development in marine predators. Our results were not entirely negative; it is possible that increased learning performance might allow apex and mesopredators to increase foraging efficiency and match increased energetic demands caused by elevated temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is altering natural systems at an unprecedented rate. Global sea-surface temperature has increased by 1 °C over the past 120 years and is predicted to increase by a further 2.6–4.8 °C in the next century under ‘business-as-usual’ scenarios (Collins et al. 2013; Pörtner et al. 2014). Global warming has already affected multiple terrestrial and marine organisms around the globe, causing shifts in abundance, distribution, and phenotypic changes, among others (Parmesan 2006).
Ectothermic animals are especially vulnerable to global warming because their body temperature and basic physiological functions are regulated by environmental temperatures. Exposure to elevated temperatures impacts many morphological and physiological traits, with the most obvious influences on variables such as metabolic rate, growth, and locomotor performance (Cano and Nicieza 2006; Calosi et al. 2008; Munday et al. 2008). Thermal stress during embryonic development is particularly harmful and likely causes significant effects on brain development and cognitive performance (Jonson et al. 1976; Wang et al. 2007; Dayananda and Webb 2017). For example, velvet geckos (Amalosia lesueurii) incubated in warmer nest temperatures took longer to locate a shelter and made more mistakes compared with hatchlings from current-day temperatures (Dayananda and Webb 2017). Additionally, individuals with lower learning scores had lower post-release survival, suggesting that hampered cognitive performance influenced fitness and survival (Dayananda and Webb 2017). Teleost fish reared in future warming conditions also showed decreased growth, reproductive output, and antipredator and foraging behaviour (Munday et al. 2008; Nilsson et al. 2009; Donelson et al. 2010; Nowicki et al. 2012).
Changes in growth, metabolic demands, and foraging of predatory species are likely to have cascading effects through ecosystems (Estes et al. 2011). Apex and mesopredators shape ecosystem structure and function by inflicting mortality, inducing costly antipredator behaviour in their prey (Heithaus et al. 2008), and/or imposing a ‘landscape-of-fear’ (Laundré et al. 2014). Because of their important ecological influence, it is vital to understand how predatory species will be affected by global warming from a physiological and behavioural perspective. However, research in marine apex and mesopredators in the context of ocean warming is still very scarce.
A few studies have reported changes in physiology and behaviour of elasmobranchs reared under predicted end-of-century temperatures. Bamboo sharks (Chiloscyllium punctatum) incubated under elevated temperatures showed lower survival rates, higher metabolic and growth rate, and decreased body condition (Rosa et al. 2014, 2016). Epaulette sharks (Hemiscyllium ocellatum) had lower survival and abnormal coloration/patterns, and while their food consumption rate did not increase, juveniles incubated at higher temperatures had decreased growth rates (Gervais et al. 2016, 2018). In Port Jackson sharks (Heterodontus portusjacksoni), elevated temperature increased the rate of embryonic development, food consumption, and growth rate (Pistevos et al. 2015). In addition, little skates (Leucoraja erinacea) reared under simulated ocean warming had lower aerobic performance and scope and decreased escape responses (Di Santo and Bennett 2011; Di Santo 2016). With such consequences on development, physiology, and behaviour, it is likely that rapid ocean warming might also impact cognitive skills in elasmobranchs, particularly given the high energetic costs associated with maintaining a large brain. While learning and behaviour may play a leading role in allowing individuals to adapt to the rapidly changing environmental conditions (Brown 2012; Wong and Candolin 2015), there is a tremendous gap in empirical studies testing the impacts of ocean warming on the learning abilities of marine predators.
The capacity to make relative quantity judgements is one among the many abilities animals evolved to deal with the ecological and social challenges they face (Geary et al. 2014). Choosing to forage in a patch with the larger number of items or fewer competitors can improve foraging efficiency and joining a larger social group can reduce sexual harassment or predation risk (Hager and Helfman 1991; Boysen et al. 2001; Agrillo et al. 2007; Panteleeva et al. 2013). Numerical abilities have been observed in a wide range of taxa, from mammals (Boysen et al. 2001; Ward and Smuts 2007), birds (Hunt et al. 2008; Rugani et al. 2013), reptiles (Petrazzini et al. 2017), amphibians (Krusche et al. 2010), teleost fish (Agrillo et al. 2014), and some invertebrates (Chittka and Geiger 1995; Carazo et al. 2009), but remain to be tested in elasmobranchs. From an evolutionary perspective, it is likely that the selective pressures driving the evolution of numerical cognition are common to all vertebrates including elasmobranchs, considering they seem to share a basic cognitive toolbox (Schluessel 2015).
The Port Jackson shark is an oviparous species widely abundant in temperate Australian waters (Last and Stevens 2009). Port Jackson sharks are mesopredators and might play a role in regulating coastal reef environments (Burt et al. 2018). On the east coast of Australia, Port Jackson sharks undertake a long-distance migration every year from foraging areas to their breeding reef and show extremely high site fidelity to their breeding grounds within and between years (Bass et al. 2016). Females lay their eggs on shallow rocky crevices and, under ambient conditions, embryos have an incubation period of 10 to 11 months (Rodda and Seymour 2008). High site fidelity to traditional breeding locations might constrain their capacity to respond to rapidly changing conditions (Root et al. 2003; Calosi et al. 2008). Moreover, with such a long incubation period, Port Jackson shark embryos are exposed to prevailing environmental conditions and have little choice other than to adapt, acclimate, or die. These factors mean that Port Jackson shark populations may be susceptible to global warming and reduction in population size might cause ecosystem-wide trophic cascades. Since Port Jackson sharks are reasonably small and do well in captivity, they are an excellent species to test the effect of elevated temperature on behaviour and cognition. In this study, we tested the hypothesis that juvenile H. portusjacksoni can discriminate between two quantities and that rearing temperature influences their learning performance. We predicted that sharks incubated at elevated temperature would show impaired learning ability owing to thermal impacts during development.
Methods
Egg collection and incubation
We collected Port Jackson shark eggs from Jervis Bay, New South Wales (35.07′ S, 150.68′ E) in October–November 2016. All eggs had been laid within 6 weeks prior to collection (see Supporting Information S1). Eggs were held in 40 L tanks containing natural-filtered seawater and temperature was maintained using a custom-design environmental control mixing chamber. Following transport, eggs were left to rest for 7 days. We randomly divided eggs among two treatments: a control temperature treatment (‘C’; n = 12) incubated at 20.6 ± 0.5 °C, consistent with the annual average maximum temperature in Jervis Bay; and an elevated temperature treatment (‘ET’; n = 12) incubated at 23.6 ± 0.5 °C, representing an end of century projected sea-surface temperature increase under the representative concentration pathway (RCP) 8.5 climate model (Collins et al. 2013). This latter temperature was obtained by steady increases of 0.5 °C/day.
When the egg capsules’ mucous plug opened, approximately 4 months into development, the embryos were removed from the egg and placed in individual containers within the housing tank to directly monitor growth and development.
Husbandry and rearing
Approximately 1 month after ‘hatching’ (external yolk completely exhausted, internal yolk virtually depleted, and disappearance of slime coat; stage 15, Rodda and Seymour 2008), individuals were moved to the Sydney Institute of Marine Science (SIMS). Sharks were housed in groups of six animals in 1000 L tanks maintained at incubation temperatures using submersible heaters (one 2000 W titanium stick heater or four 300 W AquaOne glass heaters). For additional details see Supporting Information S2.
Five sharks from the ‘elevated temperature’ treatment did not survive the second month after ‘hatching’ (three deaths and two euthanized because they were not feeding). We therefore started the procedure with seven ‘ET’ (4 females, 3 males) and twelve ‘C’ (5 females, 7 males) sharks, 58.3 and 100% of our initial sample size for each group, respectively.
Experimental apparatus
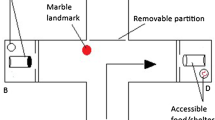
The experimental tank (180 × 100 × 40 cm, Fig. 1a) was maintained at incubation temperatures using four to six 300 W AquaOne glass heaters. The tank contained an opaque, enclosed start box (40 × 20 × 40 cm) at one end, with a sliding door facing the middle of the tank, and a black wall at the opposite end. Water inflow was provided from both sides of the tank and water outflow was located on the left side of the start box area. Stimuli were printed within a 9 × 9 cm white square and were displayed on either side of the black wall, adjacent to the bottom of the tank (since Port Jackson sharks usually swim close to the ground; Fig. 1b). The daily food intake per individual was equivalent to 2% of its wet body weight in squid (Loligo opalescens) pieces.
Procedure
Familiarisation
Familiarisation was set up to allow the shark to overcome any stress associated with moving tanks and become familiar with the learning procedure. During this phase, the shark could swim freely in the experimental tank for a 30-min period. We presented them with two ‘sham stimuli’—a black geometric shape in white background, randomly selected from a set of four (square, triangle, cross, or x mark; Fig. 1c). After 30 min elapsed, the shark was fed with long aquarium tongs within a 20-cm radius of either stimulus (‘decision zone’; Fig. 1a). The familiarisation phase occurred over 4 days. On day five, each shark ran a behavioural laterality assay for another study (Vila Pouca et al. 2018).
Quantity discrimination (3 vs. 6)
Training sessions started the day following the laterality test and were conducted once a day, always at the same time. All sharks were trained with the same numerical contrast: 3 vs. 6. For half of the individuals in each treatment group, the smaller numerosity (3) was chosen as the positive stimulus, and for the other half, the larger numerosity (6) was chosen as the positive stimulus. To avoid or minimise correct identification of the positive stimulus based on pattern recognition, different stimulus pairs with different spatial arrangement and/or sized dots were shown on each trial, pseudo-randomly chosen from a set of three options per numerosity (Fig. 1c). To decrease the difficulty of the task, we did not control for ‘continuous quantities’ (non-numerical cues such as cumulative surface area, sum of perimeter of the figures, overall space occupied by the array, or luminance); therefore, the sharks could solve the task by using numerical and/or quantity information. The position of the stimuli (left–right) was counterbalanced over trials, with each numerosity never shown more than twice consecutively on the same side. Each session consisted of 5 min of acclimation in the experimental tank, followed by six training trials (see Supporting Information S3 for details). For each trial, we recorded the sharks’ first choice and latency to push against the stimulus, and latency to eat the food reward.
Data analysis
Trials were video recorded and trial statistics were collected by two observers using BORIS v. 2.62 (Friard and Gamba 2016). It was not possible to record data blind because our individuals have uniquely identifying markings. Statistical analyses were conducted in R v. 3.4.3 (R Core Team 2017).
Quantity discrimination (3 vs. 6)
Lack of motivation was apparent in some trials, in which the sharks did not press their nose against one of the stimuli (hereafter referred to as null trials). Separate Mann–Whitney U tests were used to compare the overall proportion of null trials of ‘C’ and ‘ET’ sharks and of sharks that learnt or did not learn the task.
We considered that a shark was successful during training if it made a correct choice in 9 out of 12 consecutive trials. If a shark did not reach learning criterion after 35 days, it was excluded from the analysis. Due to our small sample size, we used separate Mann–Whitney U tests to compare the learning performance of the two treatments. Our response variables included (1) number of days to learn the task; (2) proportion of correct choice; (3) proportion of correct choice excluding null trials; (4) latency of choice; (5) latency of correct choice; and (6) latency to retrieve food reward.
To investigate if individuals that failed to reach learning criterion developed a side bias and if side choice and outcome in one trial would influence side choice on the following trial, we estimated discrete-time Markov chain (DTMC) transition probability matrices between trials (t—1) and t for each individual shark (package markovchain, Spedicato et al. 2016). Transition matrices were computed excluding days 1–6 (when incorrect choices were not scored). Confidence intervals of individual transition matrices should be considered cautiously due to low raw counts of transition steps.
Data availability
The data collected and analysed during the current study is available from the corresponding author upon request.
Results
Five sharks were excluded from the quantity discrimination task: two ‘ET’ individuals did not acclimatise and were not eating in the experimental setup and three ‘C’ individuals did not participate in the experiment. Nine ‘C’ sharks and five ‘ET’ sharks remained in the experiment. Of these, ‘ET’ sharks participated in the task more often than ‘C’ sharks (Supporting Information, Fig. S1; W = 41, P = 0.0162). We found no differences in the proportion of null trials between sharks that learnt or did not learn the task (W = 22, P = 0.846).
Learning outcome
Three out of nine (33.3%) ‘C’ sharks and three out of five (60%) ‘ET’ sharks reached learning criterion. All three ‘C’ sharks were trained to select the larger numerosity (6) and all three ‘ET’ sharks were trained to select the smaller numerosity (3). For this reason, incubation treatment and positive stimulus were confounded; however, positive stimulus was not linked to the proportion of successful sharks or the number of days to reach learning criterion and thus seems to have been less influential than incubation treatment. We therefore used incubation treatment alone when analysing performance of successful sharks but are aware that we cannot fully disentangle the effects of each variable individually. Of the six ‘C’ sharks that failed learning criterion, 50% had been trained to select ‘3’ and 50% had been trained to select ‘6’; the two ‘ET’ sharks that failed learning criterion had been trained to ‘6’.
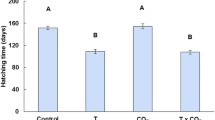
In contrast to our prediction, the three ‘ET’ sharks took fewer days to reach learning criterion (mean ± SD = 12.7 ± 2.3 days) compared with the three ‘C’ sharks (mean ± SD = 31.0 ± 4.6 days; Fig. 2). Individual variation within the groups was low, which suggests the difference in number of days to learn the task might not be due to chance alone. However, since our sample size is low, we do not have statistical power to compare the two groups.
Correct choice performance
‘ET’ sharks had a higher proportion of correct choice over all trials compared with ‘C’ sharks (Fig. 3a; W = 338.5, P < 0.001), even when excluding null trials (Fig. 3b; W = 416.5, P = 0.0427). In the latter, the difference seems to be stronger in the initial days, driven by the low participation of ‘C’ in the task (Supporting Information Fig. S1). Unlike classic learning curves, sharks did not show a positive trend in the proportion of correct choices (excluding null trials) over training days. Visual inspection of individual data indicates that most animals performed close to random most of the training days, followed by a steep increase in performance over 2/3 days prior to reaching learning criterion.
Proportion of correct choices a over all trials and b excluding null trials, by incubation treatment. The thick black lines represent the medians, the boxes encompass the interquartile ranges, the whiskers extend to the most extreme data points within 1.5 × the interquartile range outside the box, and the circles show data points beyond the whiskers. *P < 0.05, ***P < 0.001
Choice and reward latency
We found no differences between treatments in choice latency (Fig. 4a; W = 506, P = 0.312) or in latency of correct choice (Fig. 4b; W = 487, P = 0.517). The average choice latency over all trials was 30.82 ± 9.96 s (mean ± SD) in ‘ET’ sharks and 29.75 ± 18.88 s (mean ± SD) in ‘C’ sharks. The latency to retrieve the food reward also did not differ between treatments (Fig. 4c; W = 484, P = 0.494). In all three response variables (choice latency, correct choice latency, and reward latency), individual variation seemed to be lower in the ‘ET’ group compared with ‘C’ sharks.
Latency of a all choices and b correct choices, by incubation treatment. The thick black lines represent the medians, the boxes encompass the interquartile ranges, the whiskers extend to the most extreme data points within 1.5 × the interquartile range outside the box, and the circles show data points beyond the whiskers
Side bias
Choice/outcome DTMC transition probabilities for seven sharks that failed to learn the task show an overall bias to choose the stimulus on the right (Fig. 5; shark C407 was not included since it made very few consecutive choices). Individual variation in choice strategy was also apparent: ET433 and C430 both had a higher probability of choosing right after a right-side choice and of choosing left after a left-side choice; ET455 and C460 had an overall bias to choose right, but C460 chose left more often after gaining a reward on the left and ET455 after missing a reward on the left; and C456 tended to alternate to the right after choosing left and choose both sides randomly after a right-side choice.
Discussion
Our results suggest that Port Jackson shark juveniles incubated under predicted end-of-century temperatures performed better in a visual learning task compared with sharks incubated under current-day conditions. In addition, this study provides the first evidence of quantitative abilities in an elasmobranch species.
Incubation at elevated temperature is known to hamper survival, brain development, and learning ability in some invertebrate and vertebrate species (Jonson et al. 1976; Jones et al. 2005; Rosa et al. 2014; Dayananda and Webb 2017). However, despite higher mortality in our ‘ET’ group (41.7% did not survive the second month cf. 0% mortality in the control group), the surviving ‘ET’ hatchlings showed faster learning and higher proportion of correct choices over the course of the experiment, largely driven by their increased participation in the task. It is unclear if the results observed in the present study were due to plastic responses during development or selective mortality of sharks that would be ‘below average’ learners. Nonetheless, it is apparent that climate change will impact elasmobranchs in many ways and our study potentially adds learning ability to a growing list of traits that incubation temperature can influence during vulnerable early developmental stages. These results seem to be in accordance with similar studies in reptiles. Three-lined skinks (Bassiana duperreyi) incubated at higher temperatures also outperformed ‘cold’-incubated individuals in multiple learning tasks (Amiel and Shine 2012; Clark et al. 2014), though both thermal regimes tested were typical natural nest conditions from low (hot) or high (cold) elevations. Elevated temperature during incubation induces significant metabolic and ventilatory costs, as well as an increase in food consumption rates, in a range of ectotherms, including reptiles, teleosts, and elasmobranchs (Cano and Nicieza 2006; Nilsson et al. 2009; Di Santo and Bennett 2011; Rosa et al. 2014; Pistevos et al. 2015). It is possible that in this study, ‘ET’ hatchlings also had increased metabolic requirements and might have valued rewards at a higher level compared with sharks from ambient conditions. In addition, thermal regimes during incubation might cause a change in endocrine pathways linked with brain development. For example, changes in temperature can affect endocrine homeostasis responsible for gonadal differentiation in reptiles and fish (Van Der Kraak and Pankhurst 1997; Amiel and Shine 2012). Thus, thermal effects on hormone levels or receptors may also induce structural variation of brain regions. Indeed, incubation treatment has been shown to cause differences in size and volume of specific brain regions, and in neuron size, number, and density in the few species examined, yet the mechanisms underlying these changes are still largely unknown (Jonson et al. 1976; Jones et al. 2005; Wang et al. 2007; Amiel et al. 2017). It is important to note, however, that our sample size was low and therefore we lack statistical power to make strong comparisons between the treatments.
This study provides the first data on quantitative abilities in elasmobranchs, the only vertebrate group not investigated to date. All fourteen sharks included in the experiment showed a decrease in latency to choose one of the stimuli and in latency to retrieve the reward, and six individuals reached learning criterion within 35 training days. While the result shows that the species is likely equipped with the neuro-cognitive systems required to discriminate two quantities, about half of the individuals failed to acquire the discrimination. Two main hypotheses could be advanced. First, it is possible that those individuals required further training to reach learning criterion. This hypothesis is supported by the high individual-level variability we observed in the number of days to reach criterion. Alternatively, learning and memorising attributes such as quantities (that in the wild might translate to patch quality, for example) is costly given the fact that it relies on underlying brain tissues (Fagan et al. 2013). It is therefore possible that, for some individuals, attribute memory incurs extensive costs based on interindividual differences (e.g. physiological or internal states) that strongly influence the net fitness benefits of memory (Fagan et al. 2013). Additionally, the forced-choice training procedure with 2D stimuli presented in a card could also present an impediment, as learning an association between the numerosity of arbitrary stimuli and a reward is unlikely to occur in nature. Spontaneous choice tests with groups of conspecifics or food are the typical alternative to operant training procedures in numerical competency tasks (Agrillo and Bisazza 2014), but we are not convinced they would be a better alternative for this species. Juvenile Port Jackson sharks do not actively associate with conspecifics (Vila Pouca and Brown 2019), and the aquatic environment presents difficulties in controlling for olfactory cues, added to satiation effects, if pieces of food were used as stimuli.
Within each treatment group, the sharks that acquired the discrimination were trained towards the same numerosity (‘ET’ sharks trained to ‘3’ as positive stimulus, and ‘C’ sharks trained to ‘6’ as positive stimulus). In relative quantity judgements, animals can use both numerical and non-numerical information that covaries with number (‘continuous quantities’), and most studies suggest that individuals will spontaneously use continuous quantities if they are available (Geary et al. 2014). Animals also tend to use relative numerosity rules over absolute contrasts, even though they can learn with either criteria (Agrillo et al. 2011). In this study, we did not control for all non-numerical information; therefore, the sharks could solve the task by using numerical and/or quantity information. Since the same number of animals succeeded in the task with ‘3’ or ‘6’ as positive stimulus (three individuals in both cases), and considering our low sample size, we are unable to determine whether one quantity would be easier to learn compared with the other.
It is interesting to note that, in this study, sharks that failed to learn the quantity discrimination developed a side bias towards the stimuli shown on the right side of the wall. We observed a similar bias to choose the option on right in individuals that failed to discriminate between two auditory stimuli (Vila Pouca and Brown 2018), and similar side preferences have been reported in other species under a learning context (e.g. eastern water skink (Eulamprus quoyii), Szabo et al. 2019). This side bias could be an expression of brain lateralisation (Vallortigara and Rogers 2005; Vila Pouca et al. 2018) but might also arise from a decision-making context, where having a default option in a two-choice situation yields a consistent payoff (the animal always receives 50% of the rewards) compared with a variable payoff rate obtained from random choice (Monteiro et al. 2013).
In conclusion, our results show that juvenile sharks are capable of quantity discrimination and suggest that elevated temperature during embryonic development might alter behavioural and cognitive abilities. This study provides an indication that elasmobranchs may be affected by future ocean warming, though our results on the surviving juveniles were not entirely negative. It seems that behavioural and cognitive mechanisms might allow surviving individuals to compensate for some of the challenges imposed by climate change. Further studies with greater sample sizes and on other shark species are required before we can fully understand the effects of climate warming on shark cognition and behaviour.
References
Agrillo C, Bisazza A (2014) Spontaneous versus trained numerical abilities. A comparison between the two main tools to study numerical competence in non-human animals. J Neurosci Methods 234:82–91
Agrillo C, Dadda M, Bisazza A (2007) Quantity discrimination in female mosquitofish. Anim Cogn 10:63–70
Agrillo C, Piffer L, Bisazza A (2011) Number versus continuous quantity in numerosity judgments by fish. Cognition 119:281–287
Agrillo C, Petrazzini MEM, Bisazza A (2014) At the root of math: numerical abilities in fish. In: Geary DC, Berch DB, Koepke KM (eds) Evolutionary origins and early development of number processing. Academic Press, London, pp 3–34
Amiel JJ, Shine R (2012) Hotter nests produce smarter young lizards. Biol Lett 8:372–374
Amiel JJ, Bao S, Shine R (2017) The effects of incubation temperature on the development of the cortical forebrain in a lizard. Anim Cogn 20:117–125
Bass NC, Mourier J, Knott NA, Day J, Guttridge T, Brown C (2016) Long-term migration patterns and bisexual philopatry in a benthic shark species. Mar Freshw Res 68:1414–1142. 1411
Boysen ST, Berntson GG, Mukobi KL (2001) Size matters: impact of item size and quantity on array choice by chimpanzees (Pan troglodytes). J Comp Psychol 115:106–110
Brown C (2012) Experience and learning in changing environments. In: Candolin U, Wong BB (eds) Behavioural responses to a changing world: mechanisms and consequences. Oxford University Press, Oxford, pp 46–62
Burt JM, Tinker MT, Okamoto DK, Demes KW, Holmes K, Salomon AK (2018) Sudden collapse of a mesopredator reveals its complementary role in mediating rocky reef regime shifts. Proc R Soc B 285(1883):20180553
Calosi P, Bilton DT, Spicer JI (2008) Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol Lett 4:99–102
Cano J, Nicieza A (2006) Temperature, metabolic rate, and constraints on locomotor performance in ectotherm vertebrates. Funct Ecol 20:464–470
Carazo P, Font E, Forteza-Behrendt E, Desfilis E (2009) Quantity discrimination in Tenebrio molitor: evidence of numerosity discrimination in an invertebrate? Anim Cogn 12:463–470
Chittka L, Geiger K (1995) Can honey bees count landmarks? Anim Behav 49:159–164
Clark BF, Amiel JJ, Shine R, Noble DWA, Whiting MJ (2014) Colour discrimination and associative learning in hatchling lizards incubated at ‘hot’ and ‘cold’ temperatures. Behav Ecol Sociobiol 68:239–247
Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G (2013) Long-term climate change: projections, commitments and irreversibility. In: IPCC (ed) Climate change 2013 - the phsyical science basis. Cambridge University Press, Cambridge, pp 1029–1136
Dayananda B, Webb JK (2017) Incubation under climate warming affects learning ability and survival in hatchling lizards. Biol Lett 13:20170002
Di Santo V (2016) Intraspecific variation in physiological performance of a benthic elasmobranch challenged by ocean acidification and warming. J Exp Biol 219:1725–1733
Di Santo V, Bennett WA (2011) Effect of rapid temperature change on resting routine metabolic rates of two benthic elasmobranchs. Fish Physiol Biochem 37:929–934
Donelson JM, Munday PL, McCormick MI, Pankhurst NW, Pankhurst PM (2010) Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar Ecol Prog Ser 401:233–243
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet earth. Science 333:301–306
Fagan WF, Lewis MA, Auger-Méthé M, Avgar T, Benhamou S, Breed G, LaDage L, Schlägel UE, Tang WW, Papastamatiou YP, Forester J, Mueller T (2013) Spatial memory and animal movement. Ecol Lett 16:1316–1329
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330
Geary DC, Berch DB, Koepke KM (2014) Evolutionary origins and early development of number processing. Academic Press, London
Gervais C, Mourier J, Rummer J (2016) Developing in warm water: irregular colouration and patterns of a neonate elasmobranch. Mar Biodivers 46(4):743–744
Gervais C, Nay TJ, Renshaw G, Johansen JL, Steffensen JF, Rummer J (2018) Too hot to handle? Using movement to alleviate effects of elevated temperatures in a benthic elasmobranch, Hemiscyllium ocellatum. J Mar Biol 165:162
Hager MC, Helfman GS (1991) Safety in numbers: shoal size choice by minnows under predatory threat. Behav Ecol Sociobiol 29:271–276
Heithaus MR, Frid A, Wirsing AJ, Worm B (2008) Predicting ecological consequences of marine top predator declines. Trends Ecol Evol 23:202–210
Hunt S, Low J, Burns K (2008) Adaptive numerical competency in a food-hoarding songbird. Proc R Soc Lond B 275:2373–2379
Jones JC, Helliwell P, Beekman M, Maleszka R, Oldroyd BP (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A 191:1121–1129
Jonson KM, Lyle JG, Edwards MJ, Penny RHC (1976) Effect of prenatal heat stress on brain growth and serial discrimination reversal learning in the guinea pig. Brain Res Bull 1:133–150
Krusche P, Uller C, Dicke U (2010) Quantity discrimination in salamanders. J Exp Biol 213:1822–1828
Last PR, Stevens JD (2009) Sharks and rays of Australia. CSIRO Publishing, Melbourne
Laundré JW, Hernández L, Medina PL, Campanella A, López-Portillo J, González-Romero A, Grajales-Tam KM, Burke AM, Gronemeyer P, Browning DM (2014) The landscape of fear: the missing link to understand top-down and bottom-up controls of prey abundance? Ecology 95:1141–1152
Monteiro T, Vasconcelos M, Kacelnik A (2013) Starlings uphold principles of economic rationality for delay and probability of reward. Proc R Soc B 280:20122386
Munday PL, Kingsford MJ, O’Callaghan M, Donelson JM (2008) Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs 27:927–931
Nilsson GE, Crawley N, Lunde IG, Munday PL (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Chang Biol 15:1405–1412
Nowicki JP, Miller GM, Munday PL (2012) Interactive effects of elevated temperature and CO2 on foraging behavior of juvenile coral reef fish. J Exp Mar Biol Ecol 412:46–51
Panteleeva S, Reznikova Z, Vygonyailova O (2013) Quantity judgments in the context of risk/reward decision making in striped field mice: first “count,” then hunt. Front Psychol 4:53
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Petrazzini MEM, Fraccaroli I, Gariboldi F, Agrillo C, Bisazza A, Bertolucci C, Foà A (2017) Quantitative abilities in a reptile (Podarcis sicula). Biol Lett 13:20160899
Pistevos JC, Nagelkerken I, Rossi T, Olmos M, Connell SD (2015) Ocean acidification and global warming impair shark hunting behaviour and growth. Sci Rep 5:16293
Pörtner H-O, Karl DM, Boyd PW et al (2014) Ocean systems. In: climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. In: Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 411–484
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org
Rodda K, Seymour R (2008) Functional morphology of embryonic development in the Port Jackson shark Heterodontus portusjacksoni (Meyer). J Fish Biol 72:961–984
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Rosa R, Baptista M, Lopes VM, Pegado MR, Ricardo-Paula J, Trübenbach K, Leal MC, Calado R, Repolho T (2014) Early-life exposure to climate change impairs tropical shark survival. Proc R Soc B 281:20141738
Rosa R, Pimentel M, Galan JG, Baptista M, Lopes VM, Couto A, Guerreiro M, Sampaio E, Castro J, Santos C, Calado R, Repolho T (2016) Deficit in digestive capabilities of bamboo shark early stages under climate change. Mar Biol 163:60
Rugani R, Cavazzana A, Vallortigara G, Regolin L (2013) One, two, three, four, or is there something more? Numerical discrimination in day-old domestic chicks. Anim Cogn 16:557–564
Schluessel V (2015) Who would have thought that ‘jaws’ also has brains? Cognitive functions in elasmobranchs. Anim Cogn 18:19–37
Spedicato GA, Kang TS, Yalamanchi SB, Yadav D (2016) The markovchain package: a package for easily handling Discrete Markov Chains in R. https://cran.r-project.org/web/packages/markovchain/markovchain.pdf
Szabo B, Noble DWA, Whiting MJ (2019) Context-specific response inhibition and differential impact of a learning bias in a lizard. Anim Cogn 22:317–329
Vallortigara G, Rogers LJ (2005) Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28:575–633
Van Der Kraak G, Pankhurst NW (1997) Temperature effects on the reproductive performance of fish. In: Wood CM, McDonald DG (eds) Global warming: implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 159–176
Vila Pouca C, Brown C (2018) Food approach conditioning and discrimination learning using sound cues in benthic sharks. Anim Cogn 21:481–492
Vila Pouca C, Brown C (2019) Lack of social preference between unfamiliar and familiar juvenile Port Jackson sharks Heterodontus portusjacksoni. J Fish Biol:1–7
Vila Pouca C, Gervais C, Reed J, Brown C (2018) Incubation under climate warming affects behavioral lateralisation in Port Jackson sharks. Symmetry 10:184
Wang X, Green DS, Roberts SP, de Belle JS (2007) Thermal disruption of mushroom body development and odor learning in Drosophila. PLoS One 2:e1125
Ward C, Smuts BB (2007) Quantity-based judgments in the domestic dog (Canis lupus familiaris). Anim Cogn 10:71–80
Wong B, Candolin U (2015) Behavioral responses to changing environments. Behav Ecol 26:665–673
Acknowledgments
We thank the members and interns of The Fish Lab and staff at SIMS, in particular, Andrew Niccum, for husbandry and aquarium maintenance assistance. We also thank the two anonymous reviewers and editors whose suggestions helped improve and clarify this manuscript.
Funding
This research was funded by the Department of Biological Sciences at Macquarie University, and CVP was supported by an Endeavour Postgraduate (PhD) Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Egg collection occurred under NSW Fisheries permit P08/0010-4.2. The experiments were approved by the Macquarie University Animal Ethics Committee (ARA 2016-027). All animals were euthanised at the end of the experiment with a lethal dose of MS-222 (tricaine methane-sulfonate; 1.5 g/L seawater) for brain anatomy studies.
Additional information
Communicated by S. D. Twiss
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vila Pouca, C., Gervais, C., Reed, J. et al. Quantity discrimination in Port Jackson sharks incubated under elevated temperatures. Behav Ecol Sociobiol 73, 93 (2019). https://doi.org/10.1007/s00265-019-2706-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2706-8