Abstract
Spent coffee grounds (SCG), an important waste product of the coffee industry, contain approximately 15 wt% of coffee oil. The aim of this work was to investigate the utilization of oil extracted from SCG as a substrate for the production of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator H16. When compared to other waste/inexpensive oils, the utilization of coffee oil resulted in the highest biomass as well as PHB yields. Since the correlation of PHB yields and the acid value of oil indicated a positive effect of the presence of free fatty acids in oil on PHB production (correlation coefficient R 2 = 0.9058), superior properties of coffee oil can be probably attributed to the high content of free fatty acids which can be simply utilized by the bacteria culture. Employing the fed-batch mode of cultivation, the PHB yields, the PHB content in biomass, the volumetric productivity, and the Y P/S yield coefficient reached 49.4 g/l, 89.1 wt%, 1.33 g/(l h), and 0.82 g per g of oil, respectively. SCG are annually produced worldwide in extensive amounts and are disposed as solid waste. Hence, the utilization of coffee oil extracted from SCG is likely to improve significantly the economic aspects of PHB production. Moreover, since oil extraction decreased the calorific value of SCG by only about 9 % (from 19.61 to 17.86 MJ/kg), residual SCG after oil extraction can be used as fuel to at least partially cover heat and energy demands of fermentation, which should even improve the economic feasibility of the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coffee is one of the world’s most popular beverages and has been growing steadily in commercial importance during the last 150 years. Nowadays, coffee is, after petroleum, the second largest traded commodity in the world. In 2010, the worldwide annual production of coffee beans exceeded eight million tons (Murthy and Naidu 2012). Therefore, the coffee industry is responsible for the generation of large amounts of waste. Almost 50 % of worldwide coffee produced is processed for soluble coffee preparation (Ramalakshmi et al., 2009), and the solid residues after soluble coffee preparation are called spent coffee grounds (SCG) (Al-Hamamre et al. 2012). On an average, the manufacturing of one ton of green coffee generates about 650 kg of SCG (Murthy and Naidu 2012). Despite the fact that this waste stream of the coffee industry is considered as a raw substrate for the production of various compounds, such as polyphenols (Machado et al. 2012), bioethanol (Mussatto et al. 2012; Kwon et al. 2013), biodiesel (Al-Hamamre et al. 2012; Kwon et al. 2013), or mannooligosaccharides (Asano et al. 2004), most of this residue remains unutilized, being discharged to the environment or burned for elimination, which cannot be considered either environmentally friendly or economically feasible techniques (Machado et al., 2012).
SCG contain a significant portion of coffee oil—the oil content in SCG varies from 11 to 20 wt% (Al-Hamamre et al. 2012). Coffee oil has been identified as a suitable raw material for biodiesel production (Oliveira et al. 2007); however, the high content of free fatty acids in coffee oil might negatively influence the process of biodiesel production (Kwon et al. 2013).
Polyhydroxyalkanoates (PHA) are a group of hydroxyacid polyesters which are produced and accumulated in the form of intracellular granules by a wide variety of bacterial strains which use PHA as carbon, energy, and reducing power storage material (Kessler and Wilholt 1999). Out of the big family of PHA, a homopolymer of 3-hydroxybutyrate, poly(3-hydroxybutyrate) (PHB), is the most widespread in nature and the best characterized. Due to its mechanical properties similar to those of polypropylene or polyethylene, PHB is considered as an alternative to synthetic polymers. Moreover, PHB can be produced from renewable or even waste resources and it is completely biodegradable (Sudesh et al. 2000).
To compete with common plastics, the production costs of PHB have to be minimized. Because the costs of media component contribute most significantly (the carbon substrate up to 50 %) to the overall production costs of PHB, inexpensive substrates such as molasses, starch, surplus glycerol from biodiesel production and plant oils, and other cheap fatty substrates attract the attention of many researchers (Koller et al. 2005). Unlike other carbon sources, the theoretical yield coefficient of PHB production from oils is over 1 g PHB per g of plant oil, since oils comprise a higher number of carbon atoms per weight, and moreover, fatty acids are utilized via β-oxidation pathway directly yielding the precursor of PHB production—acetyl-CoA (Kahar et al. 2004).
The bacterial strain Cupriavidus necator (formerly Alcaligenes eutrophus, Ralstonia eutropha, and Wautersia eutropha) is considered as the candidate for the industrial PHB production from fatty substrates due to its ability to reach high cell densities and to accumulate high amounts of PHB (Obruca et al. 2010; Budde et al. 2011; Pradella et al. 2012).
In this work, oil extracted from SCG was tested as raw material for PHB production employing C. necator H16. The suitability of coffee oil as a substrate was compared to other waste/inexpensive plant oils, and further, PHB production from coffee oil was tested in a laboratory fermentor in batch and fed-batch mode of cultivation. Finally, based on the results obtained from the laboratory bioreactor, the model industrial process of PHB production from coffee oil was evaluated.
Material and methods
Material and microorganism
Spent coffee grounds (SCG) were obtained from a coffee automatic machine at the Faculty of Chemistry, Brno University of Technology, Czech Republic. The waste material was firstly dried to a constant weight (80 °C for 24 h), and coffee oil extraction was performed using n-hexane in Soxhlet extractor apparatus as described by Al-Hamamre et al. (2012). Approximately 70 g of SCG were weighted into a thimble of Soxhlet extractor and fitted to a conical flask. Coffee oil was extracted with 250 ml of n-hexane, and the extraction was stopped when the solvent reflux was clear. After the extraction had been accomplished, the solvent was distilled off in a vacuum rotatory evaporator and recovered in order to be used in the next batch of extraction. The amount of oil in SCG was determined from the original SCG sample weight and the weight of the extraction cup before and after extraction, i.e., by directly weighing the extracted oil.
Where w 1 is the weight of the empty extraction cup, w 2 is the weight of the extraction cup with the extract, and w 3 stands for the weight of the spent coffee ground sample.
Waste frying rapeseed oil was obtained from a university canteen (Faculty of Chemistry, Brno University of Technology, Czech Republic); waste frying palm oil, originally used for doughnut production, was provided by Strelna bakery (Strelna, Czech Republic); waste frying sunflower oil was obtained from a restaurant in Valasske Mezirici (Czech Republic); and crude rapeseed oil was provided by Agrofert Co., Czech Republic. C. necator H16 (CCM 3726) was purchased from the Czech Collection of Microorganisms, Brno, Czech Republic.
Analysis of oil extracted from SCG
The fatty acid composition of the oil extracted from SCG was determined by gas chromatography after their conversion to fatty acid methyl esters (FAME) as described previously (Obruca et al. 2013). Briefly, oil was transesterified using methanol to form FAME and the analysis of FAME composition was performed using GC-FID (Trace GC Ultra, Thermo Scientific). The sample injected was separated in a SLB-IL100 (Supelco) column (60 m × 0.25 mm × 0.20 μm). The Supelco® 37 Component FAME Mix was used as a standard for the identification of individual FAME.
Further features of coffee oil are as follows: (i) the acid value expressed in milligrams of KOH per gram of sample, which is an indication of the free fatty acid content in the sample; (ii) its saponification value, expressed in milligrams of KOH per gram of sample, which is the amount of alkali necessary to saponify a certain quantity of the sample; and (iii) its iodine value, expressed as the number of grams of iodine absorbed per 100 g of sample, which is a measurement of the unsaturations of the sample determined as described by Kartika et al. (2013).
Cultivation in Erlenmeyer flasks
The nutrient broth (10 g/l peptone, 10 g/l yeast extract, 5 g/l NaCl) (NB) medium was used for inoculum development and culture preservation (agar plates). The mineral salt (MS) medium was used in all experiments: 3 g/l (NH4)2SO4, 1 g/l KH2PO4, 11.1 g/l Na2HPO4⋅12H2O, 0.2 g/l MgSO4⋅7H2O, 1 ml microelement solution, and 1 l distilled water. The microelement solution consisted of 9.7 g/l FeCl3, 7.8 g/l CaCl2, 0.156 g/l CuSO4⋅5H2O, 0.119 g/l CoCl2, 0.118 g/l NiCl2, 0.062 g/l CrCl2, and 0.1 mol/l HCl. Oil, salt solutions, and microelement solutions were autoclaved separately (121 °C, 25 min) and then aseptically reconstituted at room temperature prior to the inoculation. The concentration of individual waste/inexpensive oils was set to 20 g/l. The pH was adjusted at 7.0 using 1 mol/l NaOH/H2SO4. The cultivations were performed in Erlenmeyer flasks (volume 250 ml) containing 50 ml of MS medium. The temperature was set at 30 °C and the agitation at 180 rpm. After 72 h of cultivation, the cells were harvested (centrifugation, 8,000 × g, 5 min), and the biomass as well as the PHB yields were determined as described below. All the cultivations were performed in triplicate.
Cultivations in the fermentor
The fermentor vessel (2 l, Biostat B plus, Sartorius Stedim Biotech, Germany) containing 1.35 l of MS medium and coffee oil (final concentration 30 g/l) was inoculated with 150 ml of a 24-h culture grown in Erlenmeyer flasks (flask volume 500 ml) on MS medium (20 g/1 of coffee oil). The temperature was set at 30 °C, and pH was maintained at 7 by 2 mol/l H2SO4 and 30 % (w/v) NaOH. The dissolved oxygen (DO) concentration was monitored by an O2 electrode, and the DO value was maintained at the level of 30 % to air saturation by varying the agitation speed automatically, and the aeration was set at 1.0 l of air per minute. In the fed-batch cultivation, an additional dose of coffee oil (corresponding to 20 g/l of coffee oil) and (NH4)2SO4 (3 g/l) was introduced at 14 h of cultivation. The last dose of coffee oil corresponding to its concentration of 10 g/l was introduced at 20 h of cultivation. The samples were withdrawn at regular intervals and analyzed for biomass concentration and PHB content. All the cultivations were performed in duplicate.
Analytical methods
To determine the biomass concentration and the PHA content in cells, the samples (10 ml) were centrifuged and the cells were washed with 5 % (vol/vol) Triton X (10 ml) and distilled water, respectively. The biomass concentration expressed as cell dry weight (CDW) was analyzed as reported previously (Obruca et al. 2010). The PHB content of dried cells was analyzed by gas chromatography (Trace GC Ultra, Thermo Scientific, USA) as reported by Brandl et al. (1988). Commercially available PHB (Sigma Aldrich, Germany) was used as a standard; benzoic acid (LachNer, Czech Republic) was used as an internal standard. To determine the molecular weight of PHB, the polyesters were extracted from the dried cells and analyzed by gel permeation chromatography [Agilent 1100 Series; column PLgel Mixed B (300 9 7.5 mm; 10 lm)] as described by Kusaka et al. (1997).
The calorific value of SCG samples before and after the coffee oil extraction was measured by an automatic oxygen bomb calorimeter (Parr 1281, Parr Instruments, USA). Measurements were performed in duplicate on a weighed amount of about 1 g.
Evaluation of fermentation strategies
The evaluation of PHB production was performed as described by Koller et al. (2005). The basis for the calculations was the number of production cycles per year (n c). This value includes typically 8,640 annual working hours. The cycle time (t c) was calculated by the addition of the laboratory-scale fermentation time plus 12 h for the preparation and follow-up treatment of the bioreactor. The following equations were used for all calculations:
where (1) = the production cycles per year, (2) = the production hours per year, (3) = duration of production cycle plus 12 h (typical value for the preparation and follow-up treatment of the bioreactor),
(4) = annual production of PHB in the 100-m3 bioreactor, (5) = bioreactor scale, (6) = final concentration of PHB, (7) = factor for the fermentation broth ~ total volume of the bioreactor, and (8) = factor for the calculation of tons (Koller et al. 2005).
Results
Oil extraction and characterization
At first, the extraction of coffee oil from the SCG was performed in order to estimate the amount of coffee oil in the waste material and also to obtain the substrate for further experiments. The coffee oil content of SCG was determined to be 15.1 ± 0.2 wt%.
Oil extracted from SCG was characterized in terms of fatty acid composition and basic parameters such as acid, iodine, ester, and saponification values. The results of the coffee oil characterization are summarized in Table 1. Common fatty acids such as linoleic acid (43.7 %), palmitic acid (35.7 %), and oleic acid (9.4 %) were identified as the predominant fatty acids in coffee oil. Further, in agreement with the other authors (Al-Hamamre et al. 2012; Kwon et al. 2013), we determined the relatively high acid value (7.1 mg KOH per g of coffee oil), which indicates the presence of a significant amount (approx. 3.6 %) of free fatty acids (FFA).
Comparison of several waste/inexpensive oils
The suitability of coffee oil as a substrate for PHB production by C. necator H16 was compared to other waste/inexpensive plant oils in cultivations performed in Erlenmeyer flasks, and the results of the experiment are presented in Table 2. Among the oils tested, the utilization of coffee oil resulted in the highest biomass as well as PHB yields, which indicates that this waste stream can be considered as a promising substrate for PHB production. Interestingly, it seems that the presence of free fatty acids in oil is an important parameter significantly influencing PHB production by C. necator, because the higher the acid value of oil, the higher the PHB yields obtained (correlation coefficient between acid value of oil and PHB yields: R 2 = 0.9058).
In the following experiment, coffee oil was mixed with waste frying rapeseed oil in various ratios to evaluate the influence of mixed substrate application (Table 3). The biomass growth as well as the PHB yields increased with the increased portion of coffee oil in the oil mixture. This experiment proved that coffee oil can be utilized not only as a sole carbon source, but it can be also fed in combination with other waste plant oils and that the introduction of coffee oil may improve the production parameters of the process of PHB production.
PHB production in the laboratory bioreactor
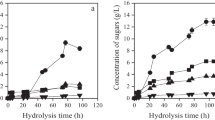
The production of PHB from coffee oil was tested also in the 2-l laboratory bioreactor. At first, the cultivation was performed in batch mode using 30 g/l of coffee oil as a sole carbon substrate. The cultivation lasted for 40 h and yielded 29.4 g/l of biomass which contained 90.1 wt% of PHB resulting in the PHB yield of 26.5 g/l. The time development of the biomass and PHB yields during the batch cultivation are shown in Fig. 1a.
Furthermore, the fed-batch pulsed feeding cultivation mode was employed in order to increase the productivity of the process. The coffee oil concentration applied at the beginning of the cultivation was 30 g/l, and the second dose of coffee oil corresponding to 20 g/l along with a supplementary dose of (NH4)2SO4 as a nitrogen source (corresponding to final concentration of 3 g/l) was applied at 14 h of cultivation. The last dose of coffee oil corresponding to its concentration of 10 g/l was introduced at 20 h of cultivation. Therefore, the total concentration of coffee oil used was 60 g/l. This feeding strategy gained the biomass yield, the PHB content of cells, and the PHB yields of 55.0 g/l, 89.1 %, and 49.4 g/l, respectively, in cultivation lasting 37 h. The time course of the fed-batch cultivation is provided in Fig. 1b.
Both cultivation modes are compared in Table 4. The utilization of the batch mode of cultivation resulted in higher biomass and product yield coefficients taking into account either coffee oil (0.98 and 0.88, respectively) or SCG (0.15 and 0.13, respectively) than those gained in fed-batch cultivation (biomass yield coefficient 0.92 and product yield coefficient 0.82 for coffee oil and 0.14 and 0.12 considering SCG as a substrate). On the contrary, the application of the fed-batch cultivation mode provides significantly higher PHB yield (26.5 g/l batch and 49.4 g/l fed-batch) and, therefore, also significantly higher volumetric productivity (0.66 g/(l h) batch and 1.33 g/(l h) fed batch) of the process. Since volumetric productivity is a crucial parameter for the biotechnological processes, the fed-batch cultivation mode should be considered as the mode of choice for the industrial production of PHB from coffee oil. Furthermore, the molecular mass of the polymer produced in fed-batch mode (M w = 4.74 · 105) was slightly higher than that of the polymers produced in batch cultivation (M w = 4.27 · 105). Thus, the fed-batch mode of cultivation seems to be superior to batch cultivation for PHB production using coffee oil as substrate.
Determination of calorific value of SCG
To estimate whether residual SCG after the extraction of coffee oil can be used as a fuel to at least partially cover the energy demands of the biotechnological process, the calorific value of SCG before and after the oil extraction was estimated. The calorific value of SCG before the coffee oil extraction was 19.61 MJ/kg. Once oil was extracted from the SCG, the calorific value of residual solids decreased in about 9 % to 17.86 MJ/kg.
Discussion
The methods of coffee waste management are outlined to create awareness of the opportunities and constraints associated with the maximization of coffee by-product utilization and the reduction of environmental pollution (Murthy and Naidu 2012). The utilization of oil extracted from SCG as a feedstock for PHA production presents several advantages. The coffee industry is steadily growing; the annual worldwide production of green coffee beans exceeded eight million tons (Murthy and Naidu 2012). Therefore, coffee by-products and especially spent coffee grounds (SCG) are available in millions of tons especially in coffee-producing countries (Pfluger 1975). On the other hand, Europe is also among the regions with a strong coffee industry and high per capita. In 2012, approx. 3.12 million tons of green coffee beans were imported into EU to be processed and manufactured. For instance, 355,777 tons of soluble coffee was produced in 2012 in EU (European Coffee 2012/2013). Because manufacturing of 1 kg of soluble coffee generates approximately 0.91–1.2 kg of SCG (Silva et al. 1998), the amount of SCG available only from coffee manufactories in EU countries represents more than 330,000 tons. Furthermore, the important point is that SCG can be easily collected not only from coffee-processing factories but also from fast food chains, cafeterias, restaurants, etc. Thus, Zuorro and Lavecchia (2012) estimated that almost 10,000 tons of SCG can be collected per year only in the province of Rome.
There is also another good reason why SCG should be utilized for various purposes. SCG are highly pollutant agents due to the presence of organic material that requires a great quantity of oxygen in order to degrade. Being discharged to the environment, they can cause contamination and environmental pollution problems due to their toxic nature (presence of caffeine, tannins, and polyphenols). Furthermore, simply piled up, they can ferment and produce spontaneous combustion (Silva et al. 1998; Mussatto et al. 2012). Therefore, the valorization of SCG does not only represent a theoretically economically feasible process (due to low cost of entering substrate), but also an ecologically friendly approach to the elimination of potentially problematic waste.
Nowadays, plant oils and similar fatty materials were identified as substrates which may enable the economically feasible production of polyhydroxyalkanoates (Du et al. 2012). Therefore, PHA production was accomplished on palm oil (Riedel et al. 2012), sunflower oil (Kimura et al. 1999), soybean oil (Taniguchi et al. 2003), olive oil, or corn oil (Chaudhry et al. 2011). Apart from noble plant oils, there are also attempts to utilize waste oils such as waste frying oils (Obruca et al. 2010; Rao et al. 2010; Obruca et al. 2013) for the production of PHA. These potential sources provide numerous advantages over noble oils—they do not compete with human food chain, their utilization does not place demands on agricultural land, and moreover, potentially problematic waste is transformed into a high value product. Coffee oil can be considered among these promising waste substrates since it meets all these requirements.
Oil extracted from SCG was recently identified as a potential raw material for the production of biodiesel (Oliveira et al. 2007; Al-Hamamre et al. 2012; Kwon et al. 2013). The fundamental problem of this technology is that a high level of FFA in coffee oil negatively influences the transesterification process and, thus, the biodiesel production. A high content of FFA in oil tends to form large amounts of undesirable soap by-products during the alkali-catalyzed transesterification process. This increases the viscosity of the reactant mixture, creates serious problems at the biodiesel separation from glycerol, and ultimately reduces the yield substantially (Sharma et al. 2008; Al-Hamamre et al. 2012). This may be partially solved by the application of acid-catalyzed transesterification; however, the high content of FFA affects also the physical properties of oil because it increases the kinematic viscosity of oil which results in high power consumption when mixing the reactant in the esterification process (Meher et al. 2006).
On the contrary, it seems that a high content of FFA is a positive factor supporting the cell growth and PHB production by C. necator when using plant oils as a substrate. It is due to the good correlation between the acid value of oils and the PHB yields (R 2 = 0.9058). The inducible enzymatic system of C. necator responsible for the utilization of triacylglycerols (TAG) was recently described by Lu et al. (2013). It involves extracellular lipase (LipA) and its lipase-specific foldase (LipB). LipA was determined to be a nonspecific extracellular lipase, because it is able to completely hydrolyze TAG into fatty acids and glycerol (Lu et al. 2013). Oppositely, FFA are simple substrates which may be easily absorbed and utilized by bacteria cells without previous enzymatic treatment, which may be important during the initial stage of cultivation when lipolytic enzymes are about to be excreted. Furthermore, FFA act as natural surfactants and, therefore, enable the emulsification of oil and make TAG susceptible to the action of lipases (Budde et al. 2011). Moreover, FFA are likely to serve as inducers of LipA and LipB expression (Lu et al. 2013). Therefore, it seems logical that a higher content of free fatty acids in oil increases the growth and the metabolic activity of C. necator resulting in enhanced PHB yields. From this point of view, coffee oil possessing a high content of FFA can be considered as an excellent substrate for PHB production.
Moreover, the bacteria culture growth and the PHA accumulation on coffee oil can also be supported by other substances which are co-extracted along with coffee oil. Such materials could be proteins, carbohydrates, lipid-soluble vitamins, and other nonpolar substances (Mercier et al. 1980; Al-Hamamre et al. 2012). These molecules may be either utilized by bacteria culture and, thus, provide additional source of nutrients or they might contribute to oil emulsification and, thus, its availability for the bacteria culture (Budde et al. 2011).
The important fact is that, unlike other plant oils, coffee oil itself causes foaming when used as a sole carbon substrate for PHB production in a bioreactor. However, this undesirable property of coffee oil can be relatively simply solved by the application of antifoaming agents. Since coffee oil can be used in mixture with other plant oils (with the higher portion of coffee oil in a mixture, a higher PHB yield was achieved, Table 3), other plant oils such as waste frying oils can serve as antifoaming agents.
The utilization of coffee oil as a substrate for PHB production resulted in a high productivity coefficient Y P/S 0.88 and 0.82 g of PHB per g of oil for batch and fed-batch cultivation, respectively. These values are more than comparable to those reported in literature for other oils employing C. necator: 0.76 (Kahar et al. 2004) and 0.85 (Pradella et al. 2012) for soybean oil, 0.6 for palm oil (Riedel et al. 2012), or 0.83 for waste rapeseed oil (Obruca et al. 2010). These results prove the suitability of coffee oil as a substrate for PHB production. Moreover, the fact that PHB production on coffee oil was accompanied by high PHB content within the biomass (90.1 and 89.1 wt% batch and fed-batch, respectively), it has a positive impact not only on the productivity of the system, but also in the reduction of the cost of PHB isolation, because the PHB content in cells is an important factor influencing the economy of PHB downstream processing (Jacquel et al. 2008).
Apart from coffee oil, which represents approx. 15 wt% of SCG, this waste stream from the coffee industry is also rich in carbohydrates, especially hemicelluloses (37 wt%) and cellulose (9 wt%), proteins (13 wt%), and lignin (29 wt%) (Kwon et al. 2013). Once oil is extracted, the residual material (representing approx. 85 % of initial mass of SCG) consists of these substances. To completely utilize SCG, the residual material after the oil extraction can be hydrolyzed to form fermentable sugars which can be biotechnologically converted into other valuable products such as ethanol (Mussatto et al. 2012; Kwon et al. 2013).
Another option of solid residues management is its pelleting and combustion to produce heat and energy to cover the energy demands of the biotechnological process. Due to its high calorific value, which can be compared to the value of coal, SCG has already been identified as a potential fuel for industrial boilers to produce heat and energy (Silva et al. 1998). Oil extraction decreased the calorific value of SCG only in about 9 % to 17.86 MJ/kg. This value is still comparable to the calorific value of other agricultural wastes and wood materials such as wheat straw (17.51 MJ/kg), rice husk (15.29 MJ/kg), rice straw (16.78 MJ/kg), or birch wood (18.40 MJ/kg) (Zuorro and Lavecchia 2012) which proves the high energy potential of SCG even after the oil extraction.
The entire intended process starts with oil extraction from SCG, and the organic solvent from the extraction can be recovered and used repeatedly for further oil extractions, while the coffee oil is directly used to produce PHB. The solid residues after the oil extraction can be used for the generation of heat and energy in industrial boilers, which should at least partially cover the energy demands of the oil extraction, bioreactor sterilization, fermentation, and also polymer isolation. Therefore, the whole SCG mass has a great potential to be utilized for PHB production. Considering the data obtained from the laboratory bioreactor in the fed-batch mode of cultivation scaled up to a common industrial bioreactor of the volume of 100 m3, the annual production of PHB is estimated to make 668 tons utilizing 5,573 tons of SCG (Table 4). Since the coffee oil content in SCG is about 15 %, 4,737 tons of SCG after the oil extraction can be combusted generating theoretically approx. 85 TJ (2.3 ⋅ 107 kWh) of energy, and the efficiency of industrial biomass boilers presents the value between 65 and 85 % (Silva et al. 1998). The generation of such a high amount of energy would significantly improve the economic aspects of the process.
To sum up, coffee oil extracted from SCG represents an inexpensive and highly suitable substrate for PHB production by C. necator. Unlike in biodiesel production where FFA represent the technological problem, the high content of FFA in coffee oil is a positive factor supporting PHB accumulation by C. necator H16. The combustion of residual material after the oil extraction generates a substantial amount of energy. Therefore, the combination of utilization of oil extracted from SCG—inexpensive substrate which is present in sufficient amounts—for PHB production and the co-generation of energy might significantly reduce the producing costs of PHB.
References
Al-Hamamre Z, Foerster S, Hartmann F, Kroger M, Kaltschmitt M (2012) Oil extracted from spent coffee ground as a renewable source for fatty acid methyl ester manufacturing. Fuel 96:70–76
Asano I, Umemura M, Fujii S, Hoshino H, Iino H (2004) Effects of mannooligosaccharides from coffee mannan on fecal microflora and defecation in healthy volunteers. Food Sci Technol Res 10:93–97
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential application as a biodegradable polyester. Appl Environ Microbiol 54:1977–1982
Budde CF, Riedel SL, Hubner F, Risch S, Popovic MK, Rha C, Sinskey AJ (2011) Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl Microbiol Biotechnol 89:1611–1619
Chaudhry W, Jamil N, Ali I, Ayaz M, Hasnain S (2011) Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann Microbiol 61:623–629
Du C, Sabirova J, Soetaert W, Lin SKC (2012) Polyhydroxyalkanoates production from low-cost sustainable raw materials. Curr Chem Biol 6:14–25
European Coffee Report 2012/2013, European Coffee Federation, available on-line at http://www.ecf-coffee.org/publications
Jacquel N, Lo CW, Wei YH, Wu HS, Wang SS (2008) Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem Eng J 39:15–27
Kahar P, Tsuge T, Taguchi K, Doi Y (2004) High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym Degrad Stabil 83:79–86
Kartika IA, Yani M, Ariono D, Evon P, Rigal L (2013) Biodiesel production from jatropha seeds: solvent extraction and in situ transesterification in a single step. Fuel 106:111–117
Kessler B, Wilholt B (1999) Poly(3-hydroxyalkanoates). In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology—fermentation, biocatalysis and bioseparation. John Wiley, New York, pp 2024–2040
Kimura H, Takahashi T, Hiraka H, Iwama M, Takeishi M (1999) Effective biosynthesis of poly(3-hydroxybutyrate) from plant oils by Chromobacterium sp. Polym J 31:210–212
Koller M, Bona R, Hermann C, Horvat P, Martinz J, Neto J, Pereira L, Varila P, Braunegg G (2005) Biotechnological production of poly(3-hydroxybutyrate) with Wautersia eutropha by application of green grass juice and silage juice as additional complex substrates. Biocatal Biotransform 23:329–337
Kusaka S, Abe H, Lee SY, Doi Y (1997) Molecular mass of poly[(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Appl Microbiol Biotechnol 47:140–143
Kwon EE, Yi H, Jeon YJ (2013) Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour Technol 136:475–480
Lu J, Brigham CJ, Rha C, Sinskey AJ (2013) Characterization of an extracellular lipase and its chaperon from Ralstonia eutropha H16. Appl Microbiol Biotechnol 97:2443–2454
Machado EMS, Rodriguez-Jasso RM, Teixeira JA, Mussatto SI (2012) Growth of fungal strains on coffee industry residues with removal of polyphenolic compounds. Biochem Eng J 60:87–90
Meher LC, Dharmagadda Vidya SS, Naik SN (2006) Optimization of alkali-catalyzed transesterification of Pongamia pinnata oil for production of biodiesel. Bioresour Technol 97:1392–1397
Mercier C, Charbonniere R, Grebaut D, De La Gueriviere JF (1980) Formation of amylose–lipid complexes by twin-screw extrusion cooking of manioc starch. Cereal Chem 57:4–9
Murthy PS, Naidu MM (2012) Sustainable management of coffee industry by-products and value addition—a review. Resour Conserv Recycl 66:45–58
Mussatto SI, Machado EMS, Carneiro LM, Teixeira JA (2012) Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl Energy 92:763–768
Obruca S, Marova I, Snajdar O, Mravcova L, Svoboda Z (2010) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol Lett 32:1925–1932
Obruca S, Snajdar O, Svoboda Z, Marova I (2013) Application of random mutagenesis to enhance the production of polyhydroxyalkanoates by Cupriavidus necator H16 on waste frying oil. World J Microbiol Biotechnol 29:2417–2428
Oliveira LS, Franca AS, Camargos RRS, Ferraz VP (2007) Coffee oil as a potential feedstock for biodiesel production. Bioresour Technol 99:3244–3250
Pfluger RA (1975) Soluble coffee processing. In: Mantel CL (ed) Solid wastes: origin, collection, processing and disposal. Wiley, New York, pp 365–376
Pradella JGD, Ienczak JL, Delgado CR, Taciro MK (2012) Carbon source pulsed feeding to attain high yield and high productivity in poly(3-hydroxybutyrate) (PHB) production from soybean oil using Cupriavidus necator. Biotechnol Lett 34:1003–1007
Ramalakshmi K, Rao LJM, Takano-Ishikawa Y, Goto M (2009) Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem 115:79–85
Rao U, Sridhar R, Sehgal PK (2010) Biosynthesis and biocompatibility of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil. Biochem Eng J 49:13–20
Riedel SL, Bader J, Brigham CJ, Budde CF, Yusof ZAM, Rha C, Sinskey AJ (2012) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentation. Biotechnol Bioeng 109:74–83
Sharma YC, Singh B, Upadhyay SN (2008) Advancements in development and characterization of biodiesel: a review. Fuel 87:2355–2373
Silva MA, Nebra SA, Machado Silva MJ, Sanchez CG (1998) The use of biomass residues in the Brazilian soluble coffee industry. Biomass Bioenergy 14:457–467
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Taniguchi I, Kagotani K, Kimura Y (2003) Microbial production of poly(hydroxyalkanoates)s from waste edible oils. Green Chem 5:545–548
Zuorro A, Lavecchia R (2012) Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J Clean Prod 34:49–56
Acknowledgments
This work was supported by the project “Centre for Materials Research at FCH BUT” No. CZ.1.05/2.1.00/01.0012 from the European Regional Development Fund (ERFD) and by the project “Excellent young researcher at BUT” No. CZ.1.07./2.3.00/30.0039. The authors kindly thank Dr. Tomas Opravil (Brno University of Technology, Czech Republic) for collection of spent coffee grounds and also for the analysis of calorific value.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obruca, S., Petrik, S., Benesova, P. et al. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl Microbiol Biotechnol 98, 5883–5890 (2014). https://doi.org/10.1007/s00253-014-5653-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5653-3