Abstract
Vitamin D represents one of the major driving factors for the development of life on earth and for human evolution. While up to 10–20 % of the human organism’s requirements in vitamin D can be obtained by the diet (under most living conditions in the USA and Europe), approximately 90 % of all needed vitamin D has to be photosynthesized in the skin through the action of the sun (ultraviolet-B (UV-B)). The skin represents a key organ of the human body’s vitamin D endocrine system (VDES), being both the site of vitamin D synthesis and a target tissue for biologically active vitamin D metabolites. It was shown that human keratinocytes possess the enzymatic machinery (CYP27B1) for the synthesis of the biologically most active natural vitamin D metabolite 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), representing an autonomous vitamin D3 pathway. Cutaneous production of 1,25(OH)2D3 may exert intracrine, autocrine, and paracrine effects on keratinocytes and on neighboring cells. Many skin cells (including keratinocytes, sebocytes, fibroblasts, melanocytes, and skin immune cells) express the vitamin D receptor (VDR), an absolute pre-requisite for the mediation of genomic effects of 1,25(OH)2D3 and analogs. VDR belongs to the superfamily of trans-acting transcriptional regulatory factors, which includes the steroid and thyroid hormone receptors as well as the retinoid X receptors (RXR) and retinoic acid receptors (RAR). Numerous studies, including cDNA microarray analyses of messenger RNAs (mRNAs), indicate that as many as 500–1000 genes may be regulated by VDR ligands that control various cellular functions including growth, differentiation, and apoptosis. The observation that 1,25(OH)2D3 is extremely effective in inducing the terminal differentiation and in inhibiting the proliferation of cultured human keratinocytes has resulted in the use of vitamin D analogs for the treatment of psoriasis. This review gives an historical view and summarizes our present knowledge about the relevance of the VDES for the management of inflammatory and malignant skin diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vitamin D represents one of the major driving factors for the development of life on earth and for human evolution. There is convincing evidence that for more than 500 million years, phytoplankton and zooplankton have been producing vitamin D (rev. in 1–3). While the physiologic function of vitamin D in lower non-vertebrate organisms is, at present, still not well understood, it is well known that most vertebrates need an adequate source of vitamin D, in order to develop and maintain a healthy mineralized skeleton (rev. in 1–3). While up to 10–20 % of the human organism’s requirements in vitamin D can be obtained by the diet (under most living conditions in the USA and Europe), approximately 90 % of all needed vitamin D has to be photosynthesized in the skin through the action of the sun (ultraviolet-B (UV-B)) (rev. in 1–3). Thus, the skin represents a key organ of the human body’s vitamin D endocrine system (VDES), being both the site of vitamin D synthesis and a target tissue for biologically active vitamin D metabolites (rev. in 1–3).
2 The skin’s VDES
Vitamin D is photochemically synthesized from 7-dehydrocholesterol (7-DHC, pro-vitamin D) by UV-B (280–315 nm) radiation (maximum of spectral effectiveness: ≈ 297 nm) in the skin, which itself is a target tissue for the seco-steroid hormone 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3, calcitriol), the biologically active vitamin D metabolite (rev. in 1–3) (Fig. 1). Dependent on temperature and time, it has been estimated that <15 % of 7-DHC can be converted into pre-vitamin D, which thereafter isomerizes to vitamin D (rev. in 1–3). It was estimated that the effect of a single full-body exposure with 1.0 minimal erythemal dose (MEM) UV-B on vitamin D status corresponds to the oral intake of 10,000–25,000 IU vitamin D [1–3]. After binding to carrier proteins in the blood (particularly vitamin D binding protein (DBP, GC)), vitamin D3 is transported to the liver where it is hydroxylated by CYP2R1 and CYP27A1 at the C25 position, generating 25-hydroxyvitamin D3 [25(OH)D3] (rev. in 1–3). 25(OH)D represents the major circulating form of vitamin D in the human body, and analysis of its blood concentration is the best laboratory parameter to assess the vitamin D status of an individual person (rev. in 1–3). Factors that influence for a person’s vitamin D status include skin type, body mass index (BMI), age, and sun exposure (Fig. 2); limited data exists on genetic determinants of serum 25(OH)D concentrations [4, 5]. We have recently shown that variants (single-nucleotide polymorphisms (SNPs)) of several genes (including EXOC2, TYR, TYRP1, and DCT) involved in skin pigmentation are predictive of serum 25(OH)D levels [4, 5]. 25(OH)D3, bound to DBP, is transported to the kidney and to other tissues, where it is finally hydroxylated by CYP27B1 at C1α position to hormonally active 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (rev. in 1–3). 1,25(OH)2D3 not only acts in the kidney but also is transported by DBP to vitamin D receptor (VDR)-positive target tissues (mainly bone, intestine, and parathyroid gland) to exert genomic or non-genomic effects (rev. in 1–3). Intracellular catabolism of 1,25(OH)2D3 is induced by enzymatic hydroxylation (CYP24A1) at C24 position followed by further oxidation forming the water-soluble calcitroic acid that is excreted in the bile (Fig. 1).
Schematic representation of the synthesis and metabolism of vitamin D for skeletal and non-skeletal function. 1-OHase 25-hydroxyvitamin D-1a-hydroxylase, 24-OHase 25-hydroxyvitamin D-24-hydroxylase, 25(OH)D 25-hydroxyvitamin D, 1,25(OH) 2 D 1,25-dihydroxyvitamin D, CaBP calcium-binding protein, DBP vitamin D-binding protein, ECaC epithelial calcium channel, FGF-23 fibroblast growth factors, PTH parathyroid hormone, RANK receptor activator of the NF-κB, RANKL receptor activator of the NF-κB ligand, RXR retinoic acid receptor, TLR2/1 toll-like receptor 2/1, VDR vitamin D receptor, vitamin D vitamin D2 or vitamin D3. Published with kind permission of © Michael F. Holick 2013
Many skin cells (including keratinocytes, sebocytes, fibroblasts, and melanocytes) express the VDR, an absolute prerequisite for the mediation of genomic effects of 1,25(OH)2D3 and other biologically active vitamin D analogs (rev. in 1–3). However, experimental and clinical lines of investigation have convincingly demonstrated that serum concentration of 1,25(OH)2D3 is too low to induce VDR-mediated hormonal effects in the skin [6, 7]. It was found in vitro [8] and in vivo [9] that human keratinocytes express CYP27B1 and possess an autonomous vitamin D3 pathway. This path contains not only the well-known UVB-induced synthesis of vitamin D3 but also its further enzymatically regulated metabolism which results in substantial amounts of hormonally active 1,25(OH)2D3. Moreover, in vitro investigations have shown that dermal fibroblasts express the vitamin D-25-hydroxylase (CYP27A1) but not the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Therefore, fibroblasts might play an important role not only in providing 1,25(OH)2D3 precursors (vitamin D3 and 25(OH)D3) for keratinocytes but also for the circulation [10].
As outlined above, epidermal keratinocytes are both the following: place of 1,25(OH)2D3 synthesis and target of this hormone. Cutaneous production of 1,25(OH)2D3 may exert intracrine, autocrine, and paracrine effects on keratinocytes and on neighboring cells. This potent seco-steroid hormone regulates growth, differentiation, apoptosis, and other biological processes via genomic and non-genomic effects. Non-genomic effects of 1,25(OH)2D3 and analogs are, in part, related to effects on intracellular calcium [11, 12]. It has been shown that in epidermal keratinocytes and in other cell types, 1,25(OH)2D3 rapidly increases free cytosolic calcium levels [11, 12]. Genomic effects of 1,25(OH)2D3 are mediated via binding to the VDR, a predominant nuclear receptor protein that is present in target tissues and binds 1,25(OH)2D3 with high affinity (KD 10−9–10−10 M) and low capacity [13–17]. VDR belongs to the superfamily of trans-acting transcriptional regulatory factors, which includes the steroid and thyroid hormone receptors as well as the retinoid X receptors (RXR) and retinoic acid receptors (RAR) [13, 14]. Evolutionarily, VDR is most closely related to the pregnane X receptor (PXR) that triggers xenobiotic detoxification and to the farnesoid X receptor (FXR) which governs bile acid metabolism [13, 14]. VDR binds its ligand 1,25(OH)2D3 with high affinity, resulting in heterodimerization with RXR and in zinc finger-mediated binding to vitamin D response elements (VDREs) in the regulatory region of target genes directly controlled by 1,25(OH)2D3 [13, 14]. As a consequence, vitamin D action in a particular cell strongly depends upon the metabolic production or delivery of sufficient concentrations of the 1,25(OH)2D3 ligand and expression of adequate VDR and RXR receptor proteins and of cell-specific programming of transcriptional responses to regulate selected genes that encode proteins that function in mediating the effects of vitamin D [13, 14] (Fig. 1). Numerous studies, including cDNA microarray analyses of messenger RNAs (mRNAs), indicate that as many as 500–1000 genes may be regulated by VDR ligands [13, 14]. Transcriptional regulation of cell cycle regulatory proteins including p21/WAF-1 (CDKN1A) and other proteins involved in cellular growth and differentiation, including ß3-integrin and fibronectin, by 1,25(OH)2D3 has been shown [13–17]. Both VDR and RXR-α are expressed in keratinocytes, fibroblasts, Langerhans cells, sebocytes (sebaceous gland cells), endothelial cells, and most cell types related to the skin immune system [18, 19]. There is a large number of genes in keratinocytes which are regulated by 1,25(OH)2D3, supporting the concept of a link between the therapeutic effect of UVB radiation in the treatment of psoriasis and the cutaneous vitamin D3 pathway [1–3].
3 Biological effects of 1,25(OH)2D3 in human skin
As outlined above, 1,25(OH)2D is not exclusively a calciotropic hormone. It regulates many cell types via endocrine, paracrine, and/or autocrine pathways and various cellular functions including cell growth and differentiation [1–3]. In vitro studies have revealed that 1,25(OH)2D3 is extremely effective in inducing the terminal differentiation and in inhibiting the proliferation of cultured human keratinocytes in a dose-dependent manner [20–22]. Additionally, 1,25(OH)2D3 exerts effects on many cell types involved in immunologic reactions, including lymphocytes, macrophages, and Langerhans cells [23, 24]. It has been shown that 1,25(OH)2D is a direct regulator of antimicrobial innate immune responses [25–27]. 1,25(OH)2D along with lipopolysaccharides (LPS) synergistically induces cathelicidin antimicrobial peptide (camp) expression in human keratinocytes, monocytes, and neutrophils [25–27]. Moreover, it has been reported that Toll-like receptor (TLR) activation of human macrophages upregulates expression of VDR and CYP27B1, leading to induction of cathelicidin and killing of intracellular Mycobacterium tuberculosis [25–27] (Fig. 1). Taken these data together, the effects of solar UV radiation on the innate and adapted immune system are not exclusively immunosuppressive, but also stimulate distinct immune response pathways.
Data published in the literature about the effects of 1,25(OH)2D3 on the melanin pigmentation system are still conflicting, but most studies do not support the concept that 1,25(OH)2D3 might directly regulate melanogenesis in human skin [28].
It was demonstrated that human sebocytes (sebum-producing cells that form the sebaceous glands) represent target cells for biologically active vitamin D metabolites, expressing VDR and the enzymatic machinery to synthesize and metabolize biologically active vitamin D analogs [29]. It was shown that incubation of SZ95 sebocytes with 1,25(OH)2D3 resulted in a cell culture condition-, time-, and dose-dependent modulation of cell proliferation, cell cycle regulation, lipid content, and interleukin-6/interleukin-8 secretion in vitro, while RNA expression of VDR and CYP24A1 was upregulated along with vitamin D analog treatment [29]. It was concluded that the VDES is of high importance for sebocyte function and physiology and that sebaceous glands represent potential targets for therapy with vitamin D analogs or for pharmacological modulation of 1,25(OH)2D3 synthesis and metabolism [29].
4 Biological effects of vitamin D compounds in psoriasis
The use of vitamin D compounds for the treatment of psoriasis resulted from two independent lines of investigation. It seemed reasonable that the antiproliferative effects of 1,25(OH)2D3 could be used for the treatment of this hyperproliferative skin disorder. However, before launching clinical trials in 1985, MacLaughlin and associates reported the observation that primary cultured fibroblasts from psoriatic skin are partially resistant to the antiproliferative effects of 1,25(OH)2D3 [30]. This observation prompted MacLaughlin and associates to speculate that 1,25(OH)2D3 may be effective in the treatment of the hyperproliferative skin disease psoriasis. The other line of investigation resulted from a clinical observation. In 1985, Morimoto and Kumahara reported that a patient, who was treated orally with 1α-(OH)D3 for osteoporosis, had a dramatic remission of psoriatic skin lesions [31]. Morimoto et al. reported a follow-up study, demonstrating that almost 80 % of 17 patients with psoriasis who were treated for up to 6 months orally with 1α-(OH)D3 at a dose of 1.0 μg/day showed clinically significant improvement [32].
Many studies have reported that 1,25(OH)2D3 and various analogs, including calcipotriol, tacalcitol, hexafluoro-1,25-dihydroxyvitamin D3 [33], and maxacalcitol, are effective and safe in the topical treatment of psoriasis [34–40] (Fig. 3). It was shown that topically applied 1,25(OH)2D3 and its analogs are very effective and safe for the long-term treatment of psoriasis [39–41]. Calcipotriol (MC 903) is a vitamin D analog with similar VDR binding properties compared to 1,25(OH)2D3, but low affinity for DBP. In vivo studies in rats showed that effects of calcipotriol on calcium metabolism are 100–200× lower as compared to 1,25(OH)2D3, while in vitro effects on proliferation and differentiation on human keratinocytes are comparable [42]. These differential effects are probably caused by the different pharmacokinetic profiles of calcipotriol and 1,25(OH)2D3 (different affinity for DBP). Serum half-life in rats of these vitamin D compounds was shown to be 4 min after treatment with calcipotriol in contrast to 15 min after treatment with 1,25(OH)2D3 [42]. The fast degradation of calcipotriol after systemic administration has limited its oral use but made it an ideal drug for topical use. Applied twice daily topically in amounts of up to 100 g of ointment (50 μg calcipotriol/g ointment) per week, calcipotriol was shown to be slightly more effective in the topical treatment of psoriasis than betamethasone 17-valerate ointment [41].
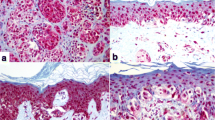
Twenty-six-year-old male with a 10-year history of psoriasis. Top panel: in a double-blinded manner, the psoriasis lesions on both forearms were treated either with placebo Vaseline (right forearm) or Vaseline containing 1,25-dihydroxyvitamin D3 [1,25(OH)2D3; 15 μg/Gm} (left forearm) for 2 months. The bottom panel shows photomicrographs of biopsies stained with hematoxylin and eosin and toluidine blue from the psoriasis lesions in the top panel. Published with kind permission of © Michael F. Holick 1996
Vitamin D analogs have been shown not to exhibit tachyphylaxis during treatment of psoriatic lesions, and topical treatment can be continued indefinitely. The results of four separate studies designed to evaluate specific local-safety parameters of various vitamin D analogs including cumulative irritancy, cutaneous contact sensitization, photoallergic contact sensitization, and phototoxicity were analyzed [43]. 1,25(OH)2D3 (3 μ/g) ointment was classified as non-irritant when compared to calcipotriol, tacalcitol, and white petrolatum (control). Petrolatum and tacalcitol were slightly irritant and calcipotriol moderately irritant. No sensitization was observed with 1,25(OH)2D3 (3 μg/g) ointment. With regard to phototoxic potential, sites treated with 1,25(OH)2D3 (3 μg/g) ointment or vehicle ointment were less irritated than those treated with white petrolatum or those that were untreated. Using standard photo-allergenicity testing methodology, there were no skin reactions of a photo-allergic nature to the study material [43].
Interestingly, a long-term study has convincingly demonstrated the efficacy and safety of oral 1,25(OH)2D3 as a potential treatment of psoriasis [44]. Of the 85 patients included in that study that received oral 1,25(OH)2D3 for 36 months, 88.0 % had some improvement in their disease, while 26.5, 26.3, and 25.3 % had complete, moderate, and slight improvement in their disease, respectively. Serum calcium concentrations and 24-h urinary calcium excretion increased by 3.9 and 148.2 %, respectively, but were not outside the normal range. Bone mineral density of these patients remained unchanged. A very important consideration for the use of orally administered 1,25(OH)2D3 is the dosing technique. To avoid its effects on enhancing dietary calcium absorption, it is very important to provide 1,25(OH)2D3 at night time. Perez et al. [44] showed that as a result of this dosing technique, doses of 2 to 4 μg/night are well tolerated by psoriatic patients.
Up to today, the precise mechanisms underlying the therapeutic effectiveness of vitamin D compounds in psoriasis are still not completely understood. Results from immunohistochemical and molecular biology studies performed several decades ago indicate that the antiproliferative effects of topically applied vitamin D compounds on epidermal keratinocytes are more pronounced as compared to effects on dermal inflammation. Modulation of various markers of epidermal proliferation (proliferating cell nuclear antigen (PCNA) and Ki-67 antigen) and differentiation (involucrin, transglutaminase K, filaggrin, cytokeratins 10, 16) in lesional psoriatic skin after topical application of vitamin D analogs was shown in situ [45]. Interestingly, effects of topical treatment with vitamin D analogs on dermal inflammation are less pronounced (CD antigens, cytokines, HLA-DR, etc.) as compared to effects on epidermal proliferation or differentiation. One reason for this observation may be that the bioavailability of this potent hormone in the dermal compartment may be markedly reduced as compared to the epidermal compartment [45].
Molecular biology studies have demonstrated that clinical improvement in psoriatic lesions treated topically with 1,25(OH)2D3 correlates with an elevation of VDR mRNA [46]. It is well known that some patients suffering from psoriasis are resistant to topical 1,25(OH)2D3 treatment. It was demonstrated that responders can be distinguished from non-responders at the molecular level since non-responders show no elevation of VDR mRNA in skin lesions along with the treatment and express relatively low levels of VDR. These findings indicate that the ability of 1,25(OH)2D3 to regulate keratinocyte growth is closely linked to the expression of VDR. The target genes of topical 1,25(OH)2D3 that are responsible for its therapeutic efficacy in psoriasis are still unknown. Major candidates for 1,25(OH)2D3 target genes that are responsible for the 1,25(OH)2D3-induced terminal differentiation in keratinocytes are distinct cell cycle-associated proteins (i.e., INK4 family), including p21/WAF-1 [47].
Data analyzing VDR expression and genotype in psoriasis are somewhat conflicting, some studies report a correlation between VDR expression or individual VDR genotypes (SNPs) and the skin eruptions of psoriasis, as well as with responsiveness to treatment with vitamin D analogs [46, 48, 49]. While no differences in VDR genotype between controls and psoriasis patients were reported at the BsmI site, some studies reported significant difference in terms of ApaI SNP [50] and FokI SNP [51]. Additionally, it was shown that VDR genotypes are not associated with clinical response to calcipotriol, at least in Korean psoriasis patients [52]. Kontula et al. [53] and Mee et al. [54] investigated the BsmI polymorphism and the response to calcipotriol treatment in psoriatic patients and found no association between them. According to Colin et al. [55], the FokI polymorphism was associated with the response to calcipotriol, and under conditions of vitamin D insufficiency, this finding might have clinical implications.
Data concerning serum levels of 1,25(OH)2D or 25(OH)D in psoriatic patients are conflicting. Some studies report reduced levels of 1,25(OH)2D in patients with manifest disease [56]. Additionally, the coincidence of pustular psoriasis with hypocalcemia [57] and the exacerbation of psoriasis under chloroquin therapy (thereby reducing 1,25(OH)2D levels via inhibition of 1α-(OH)ase) are well known [58].
5 Treatment of other skin disorders with vitamin D analogues
5.1 Vitamin D and ichthyosis
A double-blind, bilaterally paired, comparative study has demonstrated the effectiveness of topical treatment with calcipotriol ointment on congenital ichthyoses [59]. Reduction in scaling and roughness on the calcipotriol-treated side was seen in all patients with lamellar ichthyosis and bullous ichthyotic erythroderma of Brocq. The only patient treated with Comel-Netherton syndrome showed mild improvement, while the only patient suffering from ichthyosis bullosa of Siemens that was treated with calcipotriol did not show any change in severity on the calcipotriol-treated as compared to the vehicle-treated side. It has been reported that topical tacalcitol therapy was ineffective against ichthyoses that are characterized by retentive hyperkeratosis and a lack of epidermal hyperproliferation, including X-linked ichthyosis (XLI), ichthyosis vulgaris (IV), and acquired ichthyosis [60].
5.2 Vitamin D and scleroderma
Preliminary findings point to the efficacy of vitamin D analogs for the treatment of scleroderma. Humbert et al. [61] reported that oral administration of 1.0–2.5 μg/day 1,25(OH)2D3 improves skin involvement, probably via inhibition of fibroblast proliferation and dermal collagen deposition.
5.3 Vitamin D and acne
The therapeutic efficacy of vitamin D in acne has been investigated in the middle of the last century [62–64] with limited success. However, more recent laboratory investigations and animal studies indicate that vitamin D compounds may be effective in acne treatment [65, 66]. Acne vulgaris is the most common skin disorder affecting millions of people worldwide [65]. Inflammation that results from the immune response targeting Propionibacterium acnes (P. acnes) has an important role in its pathogenesis [65]. In a recent study, it was shown that P. acnes is a strong inducer of T helper 17 (Th17) and Th1, but not Th2 responses in human peripheral blood mononuclear cells (PBMCs). P. acnes stimulated expression of key Th17-related genes, including IL-17A, RORα, RORc, IL-17RA, and IL-17RC, and triggered IL-17 secretion from CD4(+), but not from CD8(+) T cells. Supernatants from P. acnes-stimulated PBMCs were sufficient to promote the differentiation of naive CD4(+)CD45RA T cells into Th17 cells. Furthermore, IL-17-expressing cells were present in skin biopsies from acne patients but not from normal donors. Interestingly, 125(OH)2D3 inhibited P. acnes-induced Th17 differentiation [65]. The authors concluded that P. acnes-induced IL-17 is present in acne lesions and that vitamin D analogs could be effective tools to modulate Th17-mediated diseases such as acne [65].
In the rhino mouse model, a comedolytic effect of topically applied active vitamin D3 analog maxacalcitol on pseudocomedones was demonstrated [66]. The rhino (hr rh/hr rh) phenotype is due to an autosomal recessive mutation in the hairless (hr) gene [66]. In the rhino mouse, utriculi are derived from the infundibular zone of the initial follicular units and are histologically similar to comedones [66]. In that study [66], rhino mice were treated topically with tretinoin and maxacalcitol once daily for 2 and 4 weeks, respectively [66]. The dermal side of the epidermal sheet was observed to measure the size of the utricle [66]. Hematoxylin-and-eosin-stained vertical sections were used to determine utricle diameter and density and to evaluate histological changes. Maxacalcitol (25 μg/g) and tretinoin (0.1 %) significantly decreased the size and the diameter of the utricle after 1 week of treatment [66]. Histopathologically, maxacalcitol and tretinoin markedly induced epidermal hyperplasia associated with by a minor accumulation of inflammatory cells in the dermis, with and without hypercornification, respectively [66]. These promising results [66] indicate that maxacalcitol has an effect on comedolysis and that its mechanism of action may be different from that of retinoids. The clinical relevance of these observations remains to be elucidated in well-designed future investigations.
5.4 Vitamin D and rosacea
The pathogenesis of rosacea—a common, chronic inflammatory skin disease mainly affecting the central portions of the face—is, at present, still only partly understood [67]. In affected skin, the expression of cathelicidin is strongly increased [67]. In addition, the activity of cutaneous proteases is greatly increased leading to the generation of cathelicidin peptide fragments with pro-inflammatory activity [67]. UV irradiation and microbial factors contribute to this inflammatory cascade by increasing vitamin D metabolism and the activation of Toll-like receptors (TLRs) [67]. These insights might uncover the VDES as novel promising target for innovative therapy of this common, stigmatizing skin disease [67].
5.5 Vitamin D and cutaneous wound healing
Laboratory investigations and animal studies indicate that 1,25(OH)2D3 and its analogs may be effective agents to promote cutaneous wound healing [68]. The effect of 1,25(OH)2D3 or its analogs on wound healing is associated with an increased expression of the antimicrobial peptide cathelicidin [68]. However, the relevance of these promising results has to be further investigated in the future in well-designed clinical studies.
5.6 Vitamin D and other skin diseases
A number of case reports demonstrate positive effects of topical treatment with 1,25(OH)2D3 or its analogs in a variety of skin diseases such as transient acantholytic dermatosis (Grover’s disease), inflammatory linear verrucous epidermal naevus (ILVEN), disseminated superficial actinic porokeratosis, pityriasis rubra pilaris, epidermolytic palmoplantar keratoderma of Vorner, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), and Sjögren-Larsson syndrome [69, 70]. These promising observations will have to be further investigated in future clinical trials.
6 Relevance of the VDES for skin cancer prevention and therapy
6.1 Cross-talk between VDR and p53 signaling pathways
Increasing evidence indicates an important role of the VDES for skin carcinogenesis. It has been stated that the VDR, mostly due to its ligand-induced growth regulatory effects, acts as a tumor suppressor in skin [13, 71]. Both vitamin D and p53 signaling pathways have a significant impact on spontaneous or carcinogen-induced malignant transformation of cells, with VDR and p53 representing important tumor suppressors [13, 71]. VDR and the p53/p63/p73 proteins (the p53 family) all function typically as receptors/sensors that turn into transcriptional regulators upon stimulus, with the main difference being that the nuclear VDR is transcriptionally activated after binding its naturally occurring ligand 1,25(OH)2D3 with high affinity, while the p53 clan, mostly in the nucleoplasm, responds to a large number of alterations in cell homeostasis commonly referred to as stress [13]. Interestingly, an increasing body of evidence now convincingly demonstrates a cross talk between vitamin D and p53 signaling that occurs at different levels, that has genome-wide implications, and that should be of high importance for many malignancies, including non-melanoma skin cancer [13, 17]. One interaction involves the ability of p53 to regulate skin pigmentation [13]. It has been shown that p53 upregulates skin pigmentation via POMC derivatives including alpha-MSH and ACTH [13]. Increased pigmentation protects the skin against UV-induced DNA damage and skin carcinogenesis but, on the other hand, reduces cutaneous synthesis of vitamin D [13]. A second level of interaction may be through the ability of 1,25(OH)2D3 to increase the survival of skin cells after UV irradiation [13]. UV irradiation-surviving cells show significant reductions in thymine dimers in the presence of 1,25(OH)2D3 that are associated with increased nuclear p53 protein expression and significantly reduced NO products [13]. A third level of interaction is documented by the ability of vitamin D compounds to regulate the expression of the murine double minute (MDM2) gene in dependence of the presence of wild-type p53 [13, 17]. MDM2 has a well-established role as a key negative regulator of p53 activity [17]. The E3 ubiquitin ligase and transcriptional repressor MDM2 is a potent inhibitor of the p53 family of transcription factors and tumor suppressors [17]. It was reported that VDR is also bound and inhibited by MDM2 [17]. This interaction was not affected by vitamin D ligand [17]. VDR was ubiquitylated in the cell, and its steady-state level was controlled by the proteasome [17]. Strikingly, overproduced MDM2 reduced the level of VDR whereas knockdown of endogenous MDM2 increased the level of VDR [17]. In addition to ubiquitin-marking proteins for degradation, MDM2, once recruited to promoters by DNA-binding interaction partners, can inhibit the transactivation of genes [17]. Transient transfections with a VDR-responsive luciferase reporter revealed that low levels of MDM2 potently suppress VDR-mediated transactivation. Conversely, knockdown of MDM2 resulted in a significant increase of transcript from the CYP24A1 and p21 genes, noted cellular targets of transactivation by liganded VDR [17]. These findings [17] suggest that MDM2 negatively regulates VDR in some analogy to p53 [17]. Finally, p53 and its family members have been implicated in the direct regulation of the VDR [13].
6.2 VDES in non-melanoma skin cancer
Using immunohistochemical techniques and real-time PCR, strong expression of key components of the VDES (VDR, CYP24A1, CYP27A1, CYP27B1) has been demonstrated in cutaneous basal (BCC) and squamous (SCC) cell carcinomas previously [72–74]. Interestingly, expression of VDR, CYP24A1, and CYP27B1 is stronger in BCCs and SCC as compared to unaffected, normal skin [72–74]. These findings provide supportive evidence for the concept that endogenous synthesis and metabolism of vitamin D metabolites as well as VDR expression may regulate growth characteristics of BCCs and SCCs. It has been shown that mouse and human BCC and SCC cell lines respond well against the antiproliferative effects of biologically active vitamin D compounds [72, 75]. Additionally, it has been demonstrated that calcitriol inhibits proliferation and growth of BCCs of patched (Ptch) mutant mice in vitro and in vivo [75]. As assessed by reduced Gli1 transcription, it has recently shown that calcitriol inhibits canonical Hh signaling independently of VDR signaling and downstream of Ptch. An obvious molecular target of this VDR-independent effect of calcitriol is Smo, because Smo-deficient cells show no decreased Gli1 transcription in response to this substance. A similar observation has been made for the inactive form of calcitriol, vitamin D3 [76]. According to this work, Ptch might function as an efflux pump for vitamin D-related compounds with hedgehog (Hh)-inhibitory potential.
Considering the importance of the VDES for carcinogenesis of BCCs and SCCs that is outlined in this review, it is no surprise that low 25(OH)D serum concentrations and genetic variants of the VDES have recently drawn attention as potential risk factors for occurrence and prognosis of non-melanoma skin cancer. Expression and function of the VDR protein can be affected by SNPs in the VDR gene [77, 78]. Associations indicate that the Apa1 and Taq1 genotypes of VDR may be of importance for carcinogenesis of BCCs, but not for SCCs [79]. Associations of the BSM1 polymorphism with BCC [80] and SCC [81] have also been reported. In conclusion, an increasing body of evidence now indicates that the VDES is of relevance for carcinogenesis and progression of non-melanoma skin cancer and that vitamin D compounds may hold promise as effective agents for the prevention and treatment of these malignancies.
6.3 VDES in melanoma
The relevance of the VDES for tumorigenesis and prognosis of malignant melanoma has been realized for several decades [82]. The presence of the VDES (VDR, CYP27A1, CYP27B1, CYP24A1) in normal melanocytes and in malignant melanoma has been characterized in vitro and in situ [83], indicating that endogenous synthesis and metabolism of vitamin D metabolites as well as VDR expression may modulate growth both of normal melanocytes and of melanoma cells in vitro and in vivo [83].
When the effects of 1,25(OH)2D3, its analog seocalcitol (EB 1089), and 25(OH)D3, on the proliferation of seven melanoma cell lines were analyzed in vitro [83], three cell lines (MeWo, SK-Mel-28, SM) responded to antiproliferative effects of active vitamin D analogs, while the remaining (SK-Mel-5, SK-Mel-25, IGR, MelJuso) was resistant. A strong increase (up to 7000-fold) of CYP24A1 mRNA was observed in responsive cell lines after stimulation with 1,25(OH)2D3, indicating functional integrity of VDR-mediated transcription. In contrast, induction of CYP24A1 was much lower in resistant melanoma cells (70-fold). VDR mRNA was induced up to 3-fold both in responsive and in resistant cell lines after stimulation with 1,25(OH)2D3. In that study, RNA for vitamin D-activating enzymes CYP27A1 and CYP27B1 was detected in all melanoma cell lines analyzed; additionally, splicing variants of CYP27B1 were shown in SK-Mel-28 cells. Expression of CYP27A1 and CYP27B1 was marginally modulated along with treatment. Growth of melanoma cells was not inhibited by treatment with 25(OH)D3, indicating no induction of endogenous production of 1,25(OH)2D3. In conclusion, the VDES has been characterized in melanoma cells, and it was demonstrated that the majority of melanoma cell lines analyzed are resistant to antiproliferative effects of 1,25(OH)2D3. The authors concluded that only a minority of cases with metastasizing melanoma may represent a promising target for palliative treatment with new vitamin D analogs that exert little calcemic side effects or for pharmacological modulation of endogenous 1,25(OH)2D3 synthesis/metabolism. Remarkably, it was previously that 1,25(OH)2D3 sensitivity of melanoma cells can, at least partially, be restored by co-stimulation with the histone deacetylase inhibitor (HDACI) trichostatin A (TSA) or with the DNA methyltransferase inhibitor (DNMTI), 5-azacytidine (5-Aza) [84]. It was shown that stimulation with 1,25(OH)2D3 and/or epigenetic drugs (5-Aza, TSA) modulated the VDR mRNA expression in 1,25(OH)2D3-responsive and -resistant melanoma cell lines and in cultured normal human melanocytes (NHM). Treatment with 5-Aza, but not with TSA, reduced the expression of a VDR regulating microRNA (miR-125b) in 1,25(OH)2D3-responsive and 1,25(OH)2D3-resistant melanoma cell lines and in NHM. Treatment with 1,25(OH)2D3 and/or epigenetic drugs (5-Aza, TSA) reduced the expression of another VDR-regulating microRNA (miR 27b) in three out of four melanoma cell lines. It was concluded that responsiveness to 1,25(OH)2D3 corresponds to the expression level of VDR mRNA which, in turn, might be regulated by VDR microRNAs (miR-27b, miR-125b) or by epigenetic modulation [84].
Considering the importance of the VDES for cancer, it is no surprise that low 25(OH)D serum concentrations and genetic variants of the VDES have drawn attention as potential risk factors for occurrence and prognosis of melanoma. In 2000, an association of Fok 1 restriction fragment length polymorphisms (RFLP) of the VDR gene with occurrence and outcome of malignant melanoma, as predicted by Breslow thickness, was reported [85]. The same laboratory demonstrated thereafter that a SNP in the promotor region of VDR (A-1012G, adenine-guanine substitution −1012 bp relative to the exon 1a transcription start site) is associated in melanoma patients with greater Breslow thickness and with the development of metastatic disease [86]. The authors concluded that polymorphisms of the VDR gene, which can be expected to result in impaired function of biologically active vitamin D metabolites, are associated with susceptibility and prognosis in malignant melanoma. In recent years, many studies have convincingly reported an association of VDR SNPs with occurrence and outcome of malignant melanoma, although it has to be noted that a few investigations showed negative results. The interaction between VDR polymorphisms and sun exposure was investigated in a population-based multinational study comparing 1138 patients with a multiple (second or subsequent) primary melanoma (cases) to 2151 patients with a first primary melanoma (controls) [87]. This was essentially a case-control study of melanoma in a population of melanoma survivors. Sun exposure was assessed using a questionnaire and interview and was shown to be associated with multiple primary melanoma. VDR was genotyped at the FokI and BsmI loci, and the main effects of variants at these loci and their interactions with sun exposure were investigated. The authors reported that only the BsmI variant was associated with multiple primary melanoma (odds ratio [OR] = 1.27, 95 % confidence interval [CI], 0.99–1.62 for the homozygous variant genotype) and concluded that these findings indicate a higher risk of multiple primary melanomas in people who have the BsmI variant of VDR.
The association of VDR polymorphisms and the risk of cutaneous melanoma was analyzed in a meta-analysis [88]. Six studies (cases, 2152; controls, 2410) that investigated the association between five VDR polymorphisms (TaqI, FokI, BsmI, EcoRV, and Cdx2) and the risk of melanoma were retrieved and analyzed. The model-free approach was applied to meta-analyze these molecular association studies. Available data suggested a significant association between the BsmI VDR polymorphism and melanoma risk (pooled OR, 1.30; 95 % CI, 1.11–1.53; P = .002; heterogeneity Cochran Q test, P > .1), and the population-attributable risk was 9.2 %. In contrast, the FokI polymorphism did not appear to be associated with such risk (OR, 1.09; 95 % CI, 0.99–1.21; P = .07; heterogeneity Cochran Q test, P > .1). For the TaqI and the EcoRV polymorphisms, significant between-study heterogeneity did not support genotype data pooling. Only one study investigated the Cdx2 variant, and the findings were negative. Current evidence is in favor of an association between one VDR gene polymorphism (BsmI) and the risk of developing melanoma. These findings prompt further investigation on this subject and indirectly support the hypothesis that sun exposure may have an antimelanoma effect through activation of the VDES.
Several studies reported a strong inverse correlation between serum 25(OH)D concentrations and Breslow thickness [89–92]. Among the patients with malignant melanoma, significantly reduced serum 25(OH)D levels were found in the stage IV patients as compared to stage I patients, and those with low 25(OH)D serum levels (<10 ng/ml) may develop earlier distant metastatic disease compared to those with higher 25(OH)D serum levels (>20 ng/ml) [90]. In a follow-up study [91], serum 25(OH)D concentrations were retrospectively analyzed in a cohort of melanoma patients (n = 324) and healthy controls (n = 141) to test the hypothesis that serum 25(OH)D concentrations are predictive of melanoma risk, thickness of primary melanomas, and overall survival (OS). Median serum 25(OH)D concentrations were significantly lower (P = 0.004) in melanoma patients (median = 13.6 ng/ml) as compared to controls (median = 15.6 ng/ml) [91]. Primary tumors of patients with low serum 25(OH)D concentrations (<10 ng/ml) had significantly (P = 0.006) greater Breslow thickness (median 1.9 mm) as compared to patients with higher levels (>20 ng/ml; median 1.00 mm). Patients with 25(OH)D serum concentrations in the lowest quartile had inferior OS (median 80 months) comparing with the highest quartile (median 195 months; P = 0.049).
Our results are in agreement with a recent study that analyzed plasma samples from 1042 prospectively observed patients with melanoma for 25(OH)D serum concentration and C-reactive protein (CRP) [92]. The associations of demographics and CRP with 25(OH)D serum concentration were determined, followed by a determination of the association between 25(OH)D serum concentration and stage and outcome measures from the date of blood draw [92]. The median follow-up time was 7.1 years [92]. In that study, a lower 25(OH)D serum concentration was associated with the blood draw during fall/winter months (P < .001), older age (P = .001), increased CRP (P < .001), increased tumor thickness (P < .001), ulcerated tumor (P = .0105), and advanced melanoma stage (P = .0024) [92]. On univariate analysis, lower 25(OH)D serum concentration was associated with poorer overall survival (OS; P < .001), melanoma-specific survival (MSS; P = .0025), and disease-free survival (DFS; P = .0466) [92]. The effect of 25(OH)D serum concentration on these outcome measures persisted after adjustment for CRP and other covariates [92]. Multivariable hazards ratios per unit decrease of 25(OH)D serum concentration were 1.02 for OS (95 % CI, 1.01 to 1.04; P = .0051), 1.02 for MSS (95 % CI, 1.00 to 1.04; P = .048), and 1.02 for DFS (95 % CI, 1.00 to 1.04; P = .0427) [92]. Although lower 25(OH)D serum concentration was strongly associated with higher CRP, the associations of lower 25(OH)D serum concentration with poorer OS, MSS, and DFS were independent of this association [92]. The authors concluded that lower 25(OH)D serum concentrations in melanoma patients were associated with poorer outcomes and that analysis of mechanisms responsible for these associations may be of value to patients with melanoma [92].
In summary, these findings support the concept that serum 25(OH)D concentrations are associated with risk and prognosis of melanoma. However, it has to be noted that most of these investigations are association studies that do not allow a conclusion of a causal relationship and that randomized controlled trials are still lacking. Whether normalizing serum 25(OH)D concentrations in these patients improves outcomes will require testing in future clinical trials.
In light of inverse relationships reported in observational studies of vitamin D intake and serum 25(OH)D concentrations with risk of non-melanoma skin cancer (NMSC) and melanoma, the effects of vitamin D (400 IU daily) combined with calcium supplementation (1000 mg daily) on skin cancer were recently evaluated in a randomized placebo-controlled trial analyzing postmenopausal women age 50 to 79 years (N = 36,282) enrolled onto the Women’s Health Initiative (WHI) calcium/vitamin D clinical trial (mean follow-up period of 7.0 years) [93]. Neither incident NMSC nor melanoma rates differed between treatment hazard ratio [HR], 1.02; 95 % CI, 0.95 to 1.07) and placebo groups (HR, 0.86; 95 % CI, 0.64 to 1.16) [93]. In subgroup analyses, women with a history of NMSC assigned to CaD had a reduced risk of melanoma versus those receiving placebo (HR, 0.43; 95 % CI, 0.21 to 0.90; P(interaction) = .038), which was not observed in women without a history of NMSC [93]. The authors concluded that vitamin D supplementation at a relatively low-dose plus calcium did not reduce the overall incidence of NMSC or melanoma [93]. However, in women with a history of NMSC, CaD supplementation reduced melanoma risk, suggesting a potential role for calcium and vitamin D supplements in this high-risk group [93]. The authors concluded that results from this post hoc subgroup analysis should be interpreted with caution but warrant additional investigation [93]. It can be speculated whether vitamin D supplementation at a more appropriate, higher dose (e.g., 1000–2000 IU) would have reduced the overall incidence of NMSC or melanoma in that study.
References
Mason RS, Reichrath J. Sunlight vitamin D and skin cancer. Anti Cancer Agents Med Chem. 2013;13:83–97. doi:10.2174/187152013804487272.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi:10.1056/NEJMra070553.
Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–25.
Saternus R, Pilz S, Gräber S, Kleber M, März W, Vogt T, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39–47.
Rossberg W, Saternus R, Wagenpfeil S, Kleber M, März W, Vogt Th, et al. Skin pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016.
Matsumoto K, Azuma Y, Kiyoki M, Okumura H, Hashimoto K, Yoshikawa K. Involvement of endogenously produced 1,25-dihydroxyvitamin D-3 in the growth and differentiation of human keratinocytes. Biochim Biophys Acta. 1991;1092:311–8.
Prystowsky JH, Muzio PJ, Sevran S, Clemens TL. Effect of UVB phototherapy and oral calcitriol (1,25-dihydroxyvitamin D3) on vitamin D photosynthesis in patients with psoriasis. J Am Acad Dermatol. 1996;35:690–5.
Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1α,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–85.
Lehmann B, Sauter W, Knuschke P, Dreßler S, Meurer M. Demonstration of UVB-induced synthesis of 1α,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res. 2003;295:24–8.
Vatieghem K, Dehaes P, Bouillon R, Segaert S. Cultured fibroblasts produce non-active vitamin D metabolites that can be activated by cultured keratinocytes. In: Abstracts Twelfth Workshop on Vitamin D, July 6-10, 2003, Maastricht, The Netherlands, page 27.
Bittiner B, Bleehen SS, Mac NS. 1α-25-(OH)2 vitamin D3 increases intracellular calcium in human keratinocytes. Br J Dermatol. 1991;124:12230–5.
MacLaughlin JA, Cantley LC, Holick MF. 1,25(OH)2D3 increases calcium and phosphatidylinositol metabolism in differentiating cultured human keratinocytes. J Nutr Biochem. 1990;1:81–7.
Reichrath J, Reichrath S, Heyne K, Vogt T, Roemer K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front Physiol. 2014;5:166. doi:10.3389/fphys.2014.00166.
Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92(2):77–98. doi:10.1007/s00223-012-9619-0.
Baker AR, Mc Donnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988;85:3294–8.
Yu VC, Deisert C, Andersen B, Holloway JM, Devary OV, Näär AM, et al. RXRβ: a coregulator that enhances binding of retinoic acid, thyroid hormone and vitamin D receptors to their cognate response elements. Cell. 1991;67:1251–66.
Heyne K, Heil TC, Bette B, Reichrath J, Roemer K. MDM2 binds and inhibits vitamin D receptor. Cell Cycle. 2015;14(13):2003–10. doi:10.1080/15384101.2015.1044176.
Milde P, Hauser U, Simon R, Mall G, Ernst V, Haussler MR, et al. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol. 1991;97:230–9.
Reichrath J, Münssinger T, Kerber A, Rochette-Egly C, Chambon P, Bahmer FA, et al. In situ detection of retinoid-X receptor expression in normal and psoriatic human skin. Br J Dermatol. 1995;133:168–75.
Smith EL, Walworth NC, Holick MF. Effect of 1α-25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown under serum-free conditions. J Invest Dermatol. 1986;86:709–14.
Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1-alpha 25-dihydroxy-vitamin D3. Endocrinol. 1983;113:1950–7.
Gniadecki R, Serup J. Stimulation of epidermal proliferation in mice with 1 alpha, 25-dihydroxyvitamin D3 and receptor-active 20-EPI analogues of 1 alpha, 25-dihydroxyvitamin D3. Biochem Pharmacol. 1995;49:621–4.
Rigby WFC. The immunobiology of vitamin D. Immunol Today. 1988;9:54–8.
Texereau M, Viac J. Vitamin D, immune system and skin. Eur J Dermatol. 1992;2:258–64.
Gombard HF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–77.
Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12.
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3.
Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1α,25-dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593–8.
Krämer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol. 2009;113(1-2):9–16.
MacLaughlin JA, Gange W, Taylor D, Smith E, Holick MF. Cultured psoriatic fibroblasts from involved and uninvolved sites have partial but not absolute resistance to the proliferation-inhibition activity of 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1985;82:5409–12.
Morimoto S, Kumahara Y. A patient with psoriasis cured by 1α-hydroxyvitamin D3. Med J Osaka Univ. 1985;35:3–4. 51-4.
Morimoto S, Yochikawa K, Kozuka T, Kitano Y, Imawaka S, Fukuo K, et al. An open study of vitamin D3 treatment in psoriasis vulgaris. Br J Dermatol. 1986;115:421–9.
Holick MF, Chen ML, Kong XF, Sanan DK. Clinical uses for calciotropic hormones 1,25-dihydroxyvitamin D3 and parathyroid hormone related peptide in dermatology: a new perspective. J Invest Dermatol (Symp Proc). 1996;1:1–9.
Perez A, Chen TC, Turner A, Raab R, Bhawan J, Poche P, et al. Efficacy and safety of topical calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol. 1996;134:238–46.
Kragballe K, Beck HI, Sogaard H. Improvement of psoriasis by topical vitamin D3 analogue (MC 903) in a double-blind study. Br J Dermatol. 1988;119:223–30.
van de Kerkhof PCM, van Bokhoven M, Zultak M, Czarnetzki BM. A double-blind study of topical 1α-25-dihydroxyvitamin D3 in psoriasis. Br J Dermatol. 1989;120:661–4.
Barker JN, Ashton RE, Marks R, Harris RI, Berth-Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo-controlled, double-blind, dose-finding study with active comparator. Br J Dermatol. 1999;141(2):274–8.
Durakovic C, Malabanan A, Holick MF. Rationale for use and clinical responsiveness of hexafluoro-1,25-dihydroxyvitamin D3 for the treatment of plaque psoriasis: a pilot study. Br J Dermatol. 2001;144(3):500–6.
Miyachi Y, Ohkawara A, Ohkido M, Harada S, Tamaki K, Nakagawa H, et al. Long-term safety and efficacy of high-concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. Eur J Dermatol. 2002;12(5):463–8.
van de Kerkhof PC, Berth-Jones J, Griffiths CE, Harrison PV, Honigsmann H, Marks R, et al. Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br J Dermatol. 2002;146(3):414–22.
Kragballe K, Gjertsen BT, de Hoop D, Karlsmark T, van de Kerhof PCM, Larko O, et al. Double-blind right/left comparison of calcipotriol and betametasone valerate in treatment of psoriasis vulgaris. Lancet. 1991;337:193–6.
Binderup L, Latini S, Binderup E, Bretting C, Calverley M, Hansen K. 20-epi-vitamin D3 analogues: a novel class of potent regulators of cell growth and immune response. Biochem Pharmacol. 1991;42:1569–75.
Queille-Roussel C, Duteil L, Parneix-Spake A, Arsonnaud S, Rizova E. The safety of calcitriol 3 microg/g ointment. Evaluation of cutaneous contact sensitization, cumulative irritancy, photoallergic contact sensitization and phototoxicity. Eur J Dermatol. 2001;11(3):219–24.
Perez A, Raab R, Chen TC, Turner A, Holick MF. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol. 1996;134:1070–8.
Reichrath J, Müller SM, Kerber A, Baum HP, Bahmer FA. Biologic effects of topical calcipotriol (MC 903) treatment in psoriatic skin. J Am Acad Dermatol. 1997;36:19–28.
Chen ML, Perez A, Sanan DK, Heinrich G, Chen TC, Holick MF. Induction of vitamin D receptor mRNA expression in psoriatic plaques correlates with clinical response to 1,25-dihydroxyvitamin D3. J Invest Dermatol. 1996;106:637–41.
Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, et al. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associaated p300 protein in terminal differentiation. Proc Natl Acad Sci U S A. 1995;92:5451–5.
Dayangac-Erden D, Karaduman A, Erdem-Yurter H. Polymorphisms of vitamin D receptor gene in Turkish familial psoriasis patients. Arch Dermatol Res. 2007;299(10):487–91.
Okita H, Ohtsuka T, Yamakage A, Yamazaki S. Polymorphism of the vitamin D(3) receptor in patients with psoriasis. Arch Dermatol Res. 2002;294(4):159–62.
Park BS, Park JS, Lee DY, Youn JI, Kim IG. Vitamin D receptor polymorphism is associated with psoriasis. J Investig Dermatol. 1999;112(1):113–6.
Saeki H, Asano N, Tsunemi Y, Takekoshi T, Kishimoto M, Mitsui H, et al. Polymorphisms of vitamin D receptor gene in Japanese patients with psoriasis vulgaris. J Dermatol Sci. 2002;30(2):167–71.
Lee DY, Park BS, Choi KH, Jeon JH, Cho KH, Song KY, et al. Vitamin D receptor genotypes are not associated with clinical response to calcipotriol in Korean psoriasis patients. Arch Dermatol Res. 2002;294(1-2):1–5.
Kontula K, Välimäki S, Kainulainen K, Viitanen AM, Keski-Oja J. Vitamin D receptor polymorphism and treatment of psoriasis with calcipotriol. Br J Dermatol. 1997;136:147–8.
Mee JB, Cork MJ. Vitamin D receptor polymorphism and calcipotriol response in patients with psoriasis. J Invest Dermatol. 1998;110:301–2.
Colin EM, Weel AEAM, Uitterlinden AG, Buurman CJ, Birkenhäger JC, Pols HAP, et al. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin Endocrinol. 2000;52:211–6.
Staberg B, Oxholm A, Klemp P, Christiansen C. Abnormal vitamin D metabolism in patients with psoriasis. Acta Derm Venereol. 1987;67(1):65–8.
Stewart AF, Battaglini-Sabetta J, Millstone L. Hypocalcemia-induced pustular psoriasis of von Zumbusch. New experience with an old syndrome. Ann Intern Med. 1984;100(5):677–80.
Stone OJ. Chloroquine, ground substance, aggravation of psoriasis. Int J Dermatol. 1985;24(8):539.
Lucker GP, van de Kerkhof PC, van Dijk MR, Steijlen PM. Effect of topical calcipotriol on congenital ichthyosis. Br J Dermatol. 1994;131:546–50.
Okano M. Assessment of the clinical effect of topical tacalcitol on ichthyoses with retentive hyperkeratosis. Dermatology. 2001;202(2):116–8.
Humbert P, Dupond JL, Agache P, Laurent R, Rochefort A, Drobacheff C, et al. Treatment of scleroderma with oral 1,25-dihydroxyvitamin D3: evaluation of skin involvement using non-invasive techniques. Results of an open prospective trial. Acta Derm Venereol (Stockh). 1993;73:449–51.
Garnier G. Vitamin D2 in treatment of acne conglobata. Arch Belg Dermatol Syphiligr. 1964;20:105–8.
Cerri B. Attempted therapy for lupus vulgaris and juvenile acne with provitamins of the D group. Minerva Dermatol. 1952;27(3):53–7.
Maynard MT. Vitamin D in acne: a comparison with X-ray treatment. Cal West Med. 1938;49(2):127–32.
Agak GW, Qin M, Nobe J, Kim MH, Krutzik SR, Tristan GR, et al. Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J Invest Dermatol. 2014;134(2):366–73. doi:10.1038/jid.2013.334.
Hayashi N, Watanabe H, Yasukawa H, Uratsuji H, Kanazawa H, Ishimaru M, et al. Comedolytic effect of topically applied active vitamin D3 analogue on pseudocomedones in the rhino mouse. Br J Dermatol. 2006;155(5):895–901.
Schauber J. Antimicrobial peptides, vitamin D3 and more. How rosacea may develop. Hautarzt. 2011;62(11):815–9. doi:10.1007/s00105-011-2142-9.
Heilborn JD, Weber G, Grönberg A, Dieterich. Topical treatment with the vitamin D analogue calcipotriol enhances the upregulation of the antimicrobial protein hCAP18/LL-37 during wounding in human skin in vivo. Exp Dermatol. 2009.
Reichrath J, Holick MF. Clinical utility of 1,25-dihydroxyvitamin D3 and its analogs for the treatment of psoriasis and other skin diseases. In: Holick MF, editor. Vitamin D. Physiology, molecular biology and clinical applications. New Jersey: Humana Press Totowa; 1999. p. 357–74.
Carrozzo AM, Gatti S, Ferranti G, Primavera G, Vidolin AP, Nini G. Calcipotriol treatment of confluent and reticulated papillomatosis (Gougerot-Carteau syndrome). JEADV. 2000;14:131–3.
Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11(56):7–17.
Reichrath J, Kamradt J, Zhu XH. Kong Xf, Tilgen W, Holick MF: Analysis of 1,25-dihydroxyvitamin D3 receptors in basal cell carcinomas. Am J Pathol. 1999;155(2):583–9.
Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gärtner BC, et al. Analysis of the vitamin D system in cutaneous squamous cell carcinomas (SCC). J Cutan Pathol. 2004;31(3):224–31.
Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gärtner BC, et al. Analysis of the vitamin D system in basal cell carcinomas (BCCs). Lab Investig. 2004;84(6):693–702.
Uhmann A, Niemann H, Lammering B, Henkel C, Heß I, Nitzki F, et al. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of Hedgehog (Hh) signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther. 2011;10(11):2179–88.
Biijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched dependent (pro)vitamin D3 secretion. PLoS Biol. 2006;4:e232.
Köstner K, Denzer N, Müller CSL, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a meta-analysis of the literature. Anticancer Res. 2009;29:3511–36.
Denzer N, Vogt T, Reichrath J. Vitamin D receptor (VDR) polymorphisms and skin cancer: a systematic review. Dermatoendocrinol. 2011;3(3):205–10.
Köstner K, Denzer N, Koreng M, Reichrath S, Gräber S, Klein R, et al. Association of genetic variants of the vitamin D receptor (VDR) with cutaneous squamous cell carcinomas (SCC) and basal cell carcinomas (BCC): a pilot study in a German population. Anticancer Res. 2012;32(1):327–33.
Ramachandran S, Fryer AA, Lovatt TJ, Smith AG, Lear JT, Jones PW, et al. Combined effects of gender, skin type and polymorphic genes on clinical phenotype: use of rate of increase in numbers of basal cell carcinomas as a model system. Cancer Lett. 2003;189(2):175–81.
Han J, Colditz GA, Hunter DJ. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. 2007;28(2):390–7.
Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147(2):197–213.
Reichrath J, Rech M, Moeini M, Meese E, Tilgen W, Seifert M. In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells. Cancer Biol Ther. 2007;6(1):48–55.
Essa S, Reichrath S, Mahlknecht U, Montenarh M, Vogt T, Reichrath J. Signature of VDR miRNAs and epigenetic modulation of vitamin D signaling in melanoma cell lines. Anticancer Res. 2012;32(1):383–9.
Hutchinson PE, Osborne JE, Lear JT, Smith AG, Bowers PW, Morris PN, et al. Vitamin D receptor polymorphisms are associated with altered prognosis in patients. 2000.
Halsall JA, Osborne JE, Potter L, Pringle JH, Hutchinson PE. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibility and prognosis in malignant melanoma. Br J Cancer. 2004;91(4):765–70.
Mandelcorn-Monson R, Marrett L, Kricker A, Armstrong BK, Orlow I, Goumas C, et al. Sun exposure, vitamin D receptor polymorphisms FokI and BsmI and risk of multiple primary melanoma. Cancer Epidemiol. 2011;35(6):e105–10.
Mocellin S, Nitti D. Vitamin D receptor polymorphisms and the risk of cutaneous melanoma: a systematic review and meta-analysis. Cancer. 2008;113(9):2398–407.
Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F, et al. Serum 25-hydroxyvitamin D3 levels are associated with Breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27(32):5439–44.
Nürnberg B, Gräber S, Gärtner B, Geisel J, Pföhler C, Schadendorf D, et al. Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res. 2009;29(9):3669–74.
Bade B, Zdebik A, Wagenpfeil S, Gräber S, Geisel J, Vogt T, et al. Low serum 25-hydroxyvitamin d concentrations are associated with increased risk for melanoma and unfavourable prognosis. PLoS One. 2014;9(12):e112863. doi:10.1371/journal.pone.0112863.
Fang S, Sui D, Wang Y, Liu H, Chiang YJ, Ross MI, et al. Association of vitamin D levels with outcome in patients with melanoma after adjustment for C-reactive protein. J Clin Oncol. 2016.
Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women’s health initiative randomized controlled trial. J Clin Oncol. 2011;29(22):3078–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare (for all authors).
Rights and permissions
About this article
Cite this article
Reichrath, J., Zouboulis, C.C., Vogt, T. et al. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev Endocr Metab Disord 17, 405–417 (2016). https://doi.org/10.1007/s11154-016-9353-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-016-9353-4