Abstract
Nonmelanoma skin cancers including basal and squamous cell carcinomas (SCC and BCC) represent a significant clinical problem due to their relatively high incidence, imposing an economic burden to healthcare systems around the world. It is accepted that ultraviolet radiation (UVR: λ = 290–400 nm) plays a crucial role in the initiation and promotion of BCC and SCC with UVB (λ = 290–320 nm) having a central role in this process. On the other hand, UVB is required for vitamin D3 (D3) production in the skin, which supplies >90% of the body’s requirement for this prohormone. Prolonged exposure to UVB can also generate tachysterol and lumisterol. Vitamin D3 itself and its canonical (1,25(OH)2D3) and noncanonical (CYP11A1-intitated) D3 hydroxyderivatives show photoprotective functions in the skin. These include regulation of keratinocyte proliferation and differentiation, induction of anti-oxidative responses, inhibition of DNA damage and induction of DNA repair mechanisms, and anti-inflammatory activities. Studies in animals have demonstrated that D3 hydroxyderivatives can attenuate UVB or chemically induced epidermal cancerogenesis and inhibit growth of SCC and BCC. Genomic and non-genomic mechanisms of action have been suggested. In addition, vitamin D3 itself inhibits hedgehog signaling pathways which have been implicated in many cancers. Silencing of the vitamin D receptor leads to increased propensity to develop UVB or chemically induced epidermal cancers. Other targets for vitamin D compounds include 1,25D3-MARRS, retinoic orphan receptors α and γ, aryl hydrocarbon receptor, and Wnt signaling. Most recently, photoprotective effects of lumisterol hydroxyderivatives have been identified. Clinical trials demonstrated a beneficial role of vitamin D compounds in the treatment of actinic keratosis. In summary, recent advances in vitamin D biology and pharmacology open new exciting opportunities in chemoprevention and treatment of skin cancers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction to the Ultraviolet Spectrum of Solar Radiation
Ultraviolet radiation (UVR : λ = 290–400 nm), depending on its wavelength (UVB: λ = 290–320 nm; UVA: λ = 320–400 nm), penetrates into different layers of the skin, with UVB being predominantly absorbed by the epidermis and reaching the upper portion of the papillary dermis, while UVA penetrates deep into the reticular dermis [69, 135, 164, 210, 238, 246]. UVR affects the integrity of DNA, RNA, and proteins and cell and tissue homeostasis, induces mutations, and changes the expression of a plethora of genes including oncogenes and tumor suppressor genes [29, 51, 132, 210, 241, 242]. It can also modify the expression and activity of growth factors, cytokines, neurohormones, neuropeptides, and their receptors and have local and systemic immunosuppressive [2, 30, 32, 62, 82, 105, 106, 126, 144, 156, 172, 174, 177,178,179,180,181, 193, 196, 210] as well as pro-pigmentary effects [148, 176, 182].
Excessive exposure to UVR results in skin aging, precancerous states such as solar/actinic keratosis (SA), and finally skin cancers including squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and melanoma (Fig. 13.1). Therefore, UVR (UVB and UVA) is defined as a major environmental stressor and full carcinogen responsible for the development and progression of BCC, SCC, and melanoma [11, 51, 100, 200].
Ultraviolet B as the double-edge sword in skin health
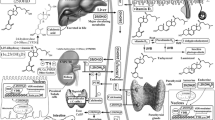
UVB not only induces skin cancers but also is necessary for phototransformation of 7DHC (7-dehydrocholesterol) to vitamin D3. BCC basal cell carcinoma, SCC invasive squamous cell carcinoma. (Reprinted from [208] with permission from Elsevier)
UVB, while representing only ~5% of UVR spectrum, exhibits a high efficiency for inducing biological effects in the skin through its interaction with cutaneous chromophores. It causes direct damage to DNA (a chromophore for UVB) by inducing covalent bond formation between adjacent pyrimidines, which leads to the production of mutagenic photoproducts such as cyclobutane pyrimidine dimers (CPD) and pyrimidine-pyrimidine adducts [29, 121, 241, 242]. To a lesser degree, its mechanism of action is linked to production of reactive oxygen species (ROS). UVB is an important etiological factor of BCC and SCC [121, 200, 241, 242]. UVB fingerprint mutations in p53 and CDKN2A genes have been identified in BCC and SCC [83]. UVB is more efficient in inducing SCC and BCC than UVA [52, 141] with some exceptions [151,152,153, 159]. The damaging effect of UVA, which is approximately 1,000 less efficient than UVB due to the limited number of target chromophores, is predominantly secondary to the action of ROS [24, 71, 245] or production of nitric oxide (NO) and nitroxyl (HNO) [1, 170, 210].
Vitamin D in the Skin
Vitamin D and Related Compounds in a Nutshell
UVB is also required for vitamin D3 formation in the skin which usually supplies >95% of the body’s requirement for this prohormone [18, 84, 85] (Fig. 13.1). The transformation of 7-dehydrocholesterol (7DHC) to vitamin D3 (D3) after absorption of UVB energy represents the most fundamental reaction in photobiology [84, 87]. The initial photoproduct, previtamin D3, undergoes thermal isomerization to vitamin D3 in the skin. With sustained UVB, previtamin D3 can undergo further photoisomerization to lumisterol (L3) and tachysterol (T3) [84]. These reactions are reversible and are dependent on the temperature and UVB dose.
Vitamin D3 is a prohormone that is activated by sequential hydroxylations in positions C25 and C1α, both at the systemic (liver and kidney) and local (skin) levels, to produce 1,25(OH)2D3 [13, 84, 85]. The first reaction is catalyzed by CYP2R1 or CYP27A1, while the C1α hydroxylation is catalyzed by CYP27B1 [15, 16, 84, 85]. Dietary vitamin D2 is activated to 1,25(OH)2D2 by CYP2R1 and CYP27B1, and inactivated by CYP24A1, by similar pathways [15, 16, 228].
Vitamin D can also be activated by CYP11A1, the first enzyme in the steroid biosynthesis pathway [78, 184, 185]. The major products of CYP11A1 action on vitamin D3 are 20(OH)D3 and 20,23(OH)2D3 [192, 224]. Other products of CYP11A1 action on vitamin D3 are 22(OH)D3, 20,22(OH)2D3, 17,20(OH)2D3, and 17,20,23(OH)3D3 [184, 224, 225]. The CYP11A1-derived metabolites can be further hydroxylated by CYP27A1, CYP27B1, CYP2R1, and/or CYP3A4 producing many more metabolites including 1,20(OH)2D3, 1,20,23(OH)3D3, 20,24(OH)2D3, 20,25(OH)2D3, and 20,26(OH)2D3 [213, 215, 217, 218, 223, 228]. Most of these metabolites have been detected in the human skin and/or serum indicating that the pathways occur in vivo (Fig. 13.2), and most have been tested in cultured cells and found to display biological activity, including inhibition of skin cell proliferation [192, 202,203,204, 228]. CYP11A1 can also act on vitamin D2 producing 20(OH)D2, which displays activities similar to 20(OH)D3, plus a number of other metabolites, including 17,20(OH)2D2 [140, 185, 188, 198, 228]. 20(OH)D2 can also be metabolized further by CYP27B1.
Detection of CYP11A1-derived 7DHC and D3 hdroxyderivatives in the human epidermis and serum
LC-MS spectra were measured on fractions with retention times corresponding to either 22(OH)7DHC or 20,22(OH)27DHC or 20(OH)D3, 22(OH)D3, or 25(OH)D3 that were pre-purified on a Waters C18 column (250 × 4.6 mm, 5 μm particle size) with a gradient of acetonitrile in water as described in [202]. Arrows indicate the retention times of the corresponding standards. Inserts show the mass spectra corresponding to the retention time of detected compound. In the outer panel, extracted ion chromatograms are shown for human epidermis (a and d), serum (b and e), and the pig adrenal (c and f). The work is reprinted from [202] under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) with small modifications
Lumisterol (L3), the major 7DHC photoproduct found in the skin following prolonged UVB radiation [86], can be metabolized by both CYP11A1 and CYP27A1 [206, 226, 227]. CYP11A1 produces primarily 22(OH)L3, 24(OH)L3, and 20,22(OH)2L3, with only minor production of pregnalumisterol which contains a cleaved side chain [226]. Lumisterol and its hydroxyderivatives have been detected in the skin and serum, illustrating that this pathway occurs in vivo (Fig. 13.3). The presence of relatively high concentrations of L3 in the serum indicates that it can leave the site of its production in the skin and potentially be delivered to tissues, such as the adrenal cortex, which expresses a high level of CYP11A1, for further metabolism [206]. The major products of CYP11A1 action on L3 are biologically active, with some, but not all activities, being similar to those of 1,25(OH)2D3 (see below) [41, 206]. More recently, we reported that lumisterol is an excellent substrate for CYP27A1, which converts it to 25(OH)L3 and both C25 epimers of 27(OH)L3, which in initial testing are able to inhibit melanoma cell proliferation [227].
Detection of novel lumisterol hdroxyderivatives in the human epidermis and serum
LC-MS spectra were measured on fractions with retention times corresponding to either of the hydroxyderivatives listed that were pre-purified on a Waters C18 column as described in [202]. Arrows indicate the retention times of the corresponding standards. Inserts show the mass spectra corresponding to the retention time of the detected compound. The work is reprinted from [202] under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) with small modifications
Finally, tissues expressing CYP11A1 are able to transform 7DHC to 22(OH)7DHC, 20,22(OH)27DHC, and finally to 7-dehydropregnenolone (7DHP) [183, 186, 191]. The latter can be further hydroxylated or converted to dehydroprogesterone by steroidogenic enzymes [191]. 20(OH)7DHC has been identified in human epidermis [206], while 22(OH)7DHC, 20,22(OH)27DHC, and 7DHP were detected in human epidermis and serum (Fig. 13.2) [202]. 7DHP and its metabolites can be transformed by UVB to the corresponding secosteroids, as predicted [183] and as has been experimentally substantiated [251,252,253] (Fig. 13.4). In addition, 20(OH)7DHC, 22(OH)7DHC, and 20,22(OH)27DHC can be converted to the corresponding vitamin D3, lumisterol and tachysterol hydroxyderivatives, after absorption of UVB energy by the B-ring (Fig. 13.4).
UVB-induced phototransformation of 7DHC, its hydroxyderivatives, and 7DHP to the corresponding secosteroidal, lumisterol, and tachysterol compounds
Shown is the metabolism of 7DHC by CYP11A1, the skin, and the subsequent transformations to the corresponding photoproducts after exposure to UVB. (?) – the enzyme transforming 7DHC to 20(OH)7DHC remains to be identified, since none of the products of 7DHC hydroxylation by CYP11A1 has its retention time. Because of the similarity of 20(OH)7DHC and 20-hydroxycholesterol, it is likely to be the same enzyme that transforms cholesterol into 20-hydroxychaolesterol, which is also detectable in the epidermis. (Reprinted from [208] with permission from Elsevier)
Phenotypic Effects of Active Forms of Vitamin D: An Overview
1,25(OH)2D3, in addition to regulating calcium homeostasis, has important pleiotropic activities that include stimulation of differentiation and inhibition of proliferation of different cell types, anti-cancerogenic effects, stimulation of innate immunity, and inhibition of adaptive immunity and inflammation [13, 15, 28, 46,47,48, 55, 65, 73,74,75, 84, 85, 149, 240]. In the skin, vitamin D3 plays a significant role in the formation of the epidermal barrier and adnexal structures, including hair follicles, and has a wide variety of ameliorating effects in skin cancer and proliferative and inflammatory cutaneous diseases [12, 14, 23, 63, 84, 85, 94, 143, 157, 158]. These properties of 1,25(OH)2D3 have been extensively reviewed as listed above and, therefore, will not be detailed.
Similar effects are exerted by CYP11A1-derived hydroxyderivatives of vitamin D3, including mono, dihydroxy, and trihydroxy forms with or without the hydroxyl group at position C1α (reviewed in [197, 205, 207, 208]). Specifically, they exert antiproliferative, pro-differentiation, and anti-inflammatory effects in cultured cells that are comparable or stronger than those of 1,25(OH)2D3 [41, 95, 96, 112, 114,115,116, 119, 123, 189, 190, 194, 195, 207, 225, 248]. In addition, they exhibit antifibrotic activities both in vitro [189, 194, 195] and in vivo [194]. They also display anti-melanoma and antitumor properties that are cell type-dependent [44, 97, 173, 187, 188, 190, 195, 207, 234, 235, 237]. Moreover, similar to 1,25(OH)2D3, they can stimulate different elements of the cutaneous hypothalamus-pituitary-adrenal axis in human keratinocytes including CRH, urocortins, and POMC, together with their corresponding receptors CRHR1, CRHR2, MC1, MC2, MC3, and MC4 [238]. The newly identified hydroxyderivatives of lumisterol also show antiproliferative and pro-differentiation properties in human normal and malignant epidermal keratinocytes [41, 206]. Finally, vitamin D-, lumisterol-, and tachysterol-like compounds with a short or absent side chain also show antiproliferative and antitumor properties [102, 145, 186, 187, 195, 235, 252, 253]. Importantly, 20(OH)D3 and 20,23(OH)2D3 are non-calcemic, while 1,20(OH)2D3 show low-calcemic activity [44, 187, 194, 234].
Receptors for Vitamin D in the Skin
Vitamin D Receptor (VDR)
An Overview
The main phenotypic activities of canonical hydroxyderivatives of vitamin D are mediated through their interaction with the ligand-binding domain of the nuclear receptor, vitamin D receptor (VDR , NR1I1) [22, 28, 39, 46, 81, 130, 131, 142]. This interaction promotes heterodimerization of the VDR with the retinoid X receptor (RXR) and its translocation to the nucleus where it interacts with VDR-responsive elements (VDRE) to regulate the transcription of target genes (transactivation or repression). VDR is expressed in all tissues, including the skin [22, 28, 39, 157], and is reported to regulate approximately 3% of the mammalian genome. The human epidermis is rather unique in this context in that it is both the source of vitamin D3 and a target tissue. The CYP11A1-derived secosteroids with a full-length side chain can bind to the VDR and act via a VDRE-dependent mechanism, with compounds containing a hydroxyl group at C1α exhibiting a higher affinity than those without it [102, 118, 119, 188, 207]. Most importantly, the crystal structures of 20(OH)D3, 1,20(OH)2D3, and 1,25(OH)2D3 bound to the genomic LBD of the VDR were obtained [118, 119] which illustrated similarities and differences between these compounds in their interaction with the VDR receptor (Fig. 13.5), as reported in [119].
Crystal structures of 20(OH)D3, 1,20(OH)2D3, and 1,25(OH)2D3 in complexes with the VDR ligand-binding domain
The crystal structures of 20S(OH)D3, in complex with the Danio Rerio VDR (zVDR) LBD, were determined and compared to those of 1,20(OH)2D3 and 1,25(OH)2D3 VDR complexes as described previously [119]. The complexes with 20(OH)D3 (PDB ID 5OW9), 1,20(OH)2D3 (PDB ID 5MX7), and 1,25(OH)2D3 (PBD ID 2HC4) are shown in cyan, yellow, and salmon, respectively. Hydrogen bonds between the ligands and LBD are represented by purple dashed lines. Details of the interactions mediated by the side chains of 20(OH)D3 are in the second image from the left. Hydrophobic interactions are indicated by gray dashed lines, and hydrogen bonds are depicted as pink dashed lines. Only residues within 4 Å of the ligand are shown by stick representation. The residue numbers correspond to human VDR. The detailed description and analysis are in [119]. (The work is reprinted from [119] under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) with small modifications)
VDR transcriptional activity is dependent on the availability of VDR agonists and antagonists and their effect on receptor conformation (allostery [160], the recruitment of different cofactors [18, 22], and chromatin accessibility [39, 136, 142]). Moreover, VDR activity can be influenced by single-nucleotide polymorphisms (SNPs) [254]. This plays, for example, a role in the etiology of nonmelanoma skin cancers (NMSC) and melanoma [38, 54, 104, 111, 117]. Interestingly, CYP11A1-derived D3 hydroxyderivatives without a hydroxyl group at C1α display a subset of the activities possessed by 1,25(OH)2D3 (see above) and lack calcemic activity, acting as biased agonists on the VDR [197, 207].
In addition to genomic (G), VDRE-mediated regulation of gene expression, the VDR can also induce rapid responses via a non-genomic, membrane-associated mechanism that involves an alternative ligand-binding site (A-pocket) [81, 130, 131]. The list of ligands interacting with the A-pocket of VDR includes 25(OH)D3, 1,25(OH)2D3, 1,25(OH)2L3 [57, 130], and some CYP11A1-derived hydroxylumisterol derivatives [206], but not CYP11A1-derived vitamin D3 hydroxyderivatives [207]. An additional cell membrane-linked mechanism of action includes the interaction between VDR and caveolin-associated signal transducers [249].
Finally, different alternatively spliced forms of VDR have been described [5, 64, 68, 212]. It has been suggested that they can have different transcriptional activity and promote VDR-ligand-independent functions [5]. Most recently, alternatively spliced forms have been detected in human melanoma cells [236]. Using the same methodology and the same primers with sequencing of the resulting cDNA fragments [49, 236], we identified VDR isoforms a, b, c, 1a, 1d, and 1f, similar to those described previously [49, 236], in normal adult and neonatal human epidermal keratinocytes and the skin fragments from white and black subjects. The immediate challenges in this area are to determine whether alternatively spliced VDR isoforms exhibit distinct functions in skin cells and regulate the expression of different genes and whether the alternative splicing is regulated by endogenous or environmental factors, as has been shown for other receptors such as CRH-R1 [146, 147, 196, 250]. In addition, it further needs to be determined whether these isoforms display different affinities for different vitamin D3 hydroxyderivatives and exhibit differences in their interaction with RXR, cofactors, and DNA or to understand mechanisms by which they regulate VDR-ligand-independent functions.
VDR in the Skin
VDR is expressed in all skin cell types [17]. However, its level of expression can change depending on the specific pathology, as documented in VDR knockout mice. For example, VDR-/- mice show significant defects in cutaneous structures, alopecia [46, 143], and have significantly increased propensity to develop epidermal skin cancer [21, 23, 216]. The later indicates that VDR functions as a tumor suppressor [19, 20].
With respect to melanomagenesis, significant changes in the level of VDR expression were observed during progression of melanocytic tumors, with reduced nuclear and cytoplasmic VDR levels correlating with tumor progression and Clark levels, with highest VDR levels in normal skin and common melanocytic nevi, and with lowest VDR levels in advanced and metastatic melanomas [33, 35]. Low or lack of VDR expression also positively correlated with poor prognostic markers of melanoma and poorer outcome of the diseases as measured by shortening of the survival and disease-free times [33, 35]. The combined analysis of CYP27B1 and VDR showed an even stronger correlation with disease progression, with the lowest levels of expression in highly advanced melanomas and metastases [34]. Interestingly, an inverse correlation between VDR and nuclear expression of HIF-1α was found with the highest HIF-1α expression observed in pT3-pT4 VDR-negative melanomas [37]. Also, nuclear VDR expression was significantly lower than in normal uveal cells including melanocytes [125]. Finally, VDR single-nucleotide gene polymorphisms are associated with a higher probability of developing melanoma and a poorer disease outcome (reviewed in [205]).
NMSC studies performed in animal models have convincingly demonstrated a role for VDR in photoprotection and prevention or attenuation of skin cancer development [12, 21,22,23, 40, 57, 92, 94, 233]. The latter involves inhibition of the hedgehog and Wnt signaling pathways and induction of keratinocyte differentiation [3, 79, 120, 216, 230]. Inhibition of the hedgehog pathway has also been implicated in the attenuation of other tumors, including rhabdomyosarcoma [231] and renal carcinomas [60]. The inhibition of hedgehog signaling by vitamin D compounds might be mediated by VDR-dependent and VDR-independent mechanisms [214].
Although VDR polymorphisms have been linked to various malignancies, including cutaneous melanomas [205, 208], studies on the relationship between VDR polymorphisms and the risk of developing NMSC (ApaI, BsmI, and TaqI) [54, 80, 111] were not fully conclusive with some, but limited, evidence indicating a relationship between VDR SNPs and NMSCs. In a German population, a correlation between the combined ApaI/TaqI/BglI AaTtBb genotypes of VDR with BCC risk was observed (aaTTBB VDR genotype was found only in controls). The aaTTbb VDR genotype was much more frequent in BCCs and SCCs that in the control population. Also, a higher frequency of the BB VDR genotype on sun-exposed versus nonexposed areas both in BCCs and SCCs was identified. In addition, Apa1 and Taq1 genotypes were associated with BCCs, but not with SCC photocarcinogenesis [104]. In a Polish population, the TT genotype of FokI VDR polymorphism was correlated with greater than tenfold higher risk of BCC development [111]. Burns et al. found that the BsmI b or TaqI t genotypes of VDR were more frequent in NMSC patients, suggesting that individuals with these genotypes are more likely to develop skin cancer [38]. A very recent nested case control study and meta-analysis showed that patients with rs2228570, rs927650, and rs1544410 recessive genotypes were characterized by a lower risk of SCC development, while rs7975232 and rs739837 recessive genotypes were related to decreased BCC risk [107]. Another study identified two new SNPs in VDR binding sites (rs16917546 and rs79824801) associated with BCC risk. This study also confirmed the association of the rs3769823 SNP in the VDR binding site with increased BCC risk [117], while a study performed on a population in the mid-south of the USA (96 cases vs. 100 controls) showed that subjects with BsmI SNP had two times higher probability of developing NMSC in comparison to controls [38]. Thus, VDR polymorphisms should be considered as factors related to NMCS risk; however, additional studies are needed with larger population cohorts.
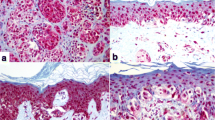
The vitamin D system has been analyzed in cell cultures and clinical samples of NMSCs. Reichrath’s group found a significant increase in nuclear VDR expression (as detected with immunohistochemistry) in SCC samples compared to normal skin, however no correlation with histological type, grading or markers for proliferation, differentiation, or apoptosis, and increased expression of VDR, CYP27A1, CYP27B1 and CYP24A1 in SCC was observed [155]. Reichrath et al. [154] and Mitschelle et al. [129] also analyzed the expression of VDR in BCCs and found a pattern similar to SCC, with significantly elevated nuclear expression of VDR in BCCs in comparison to normal skin, adjacent epidermis, and unaffected epidermis. VDR expression was moderate or strong, and the strongest VDR expression was found in peripheral palisade cells. VDR expression was not correlated with a particular histological type of BCC. Similar to SCCs, the expression of VDR, CYP27B1, and CYP24A1, but not of CYP27A1, was increased in comparison to normal skin [129]. We also detected the VDR in human biopsies of BCC and SCC (Fig. 13.6). These studies show that both receptors for active forms of vitamin D and enzymes activating or inactivating vitamin D are expressed in NMSC, providing a rationale for targeting vitamin D signaling in the therapy of NMSC.
Immunohistochemical detection of RORα (upper), RORγ (middle), and VDR (lower) in normal skin (left panel), BCC (middle), and SCC (right). Scale bar: 50 μm. Archival formalin-fixed paraffin-embedded sections, after heat-induced antigen retrieval in Tris-based antigen unmasking solution (Vector Laboratories, Inc., Burlingame, CA) and endogenous peroxidase blocking, were incubated over night at 4 °C with primary antibodies (rabbit anti-RORα (provided by Dr. Anton M. Jetten), 1:400; rabbit anti-RORγ (provided by Dr. Anton M. Jetten), 1:50; rat anti-VDR (Abcam, MA1-710; Thermo Fisher Scientific, Waltham, MA)). Next, sections were incubated with secondary antibodies conjugated with HRP (anti-rabbit ImmPRESS antibody (ready to use, Vector Laboratories, Inc., Burlingame, CA) for RORα and RORγ; anti-rat antibody (1:200, Abcam, Cambridge, UK) for VDR), followed by peroxidase substrate ImmPACT NovaRED (Vector Laboratories Inc., Burlingame, CA, USA) application and mounting with permanent mounting media and glass coverslip (Thermo Fisher Scientific, Waltham, MA)
Other Receptors for Vitamin D: An Overview
Other receptor candidates for 1,25(OH)2D3 include the 1,25D3-membrane-associated, rapid-response steroid-binding protein (1,25D3-MARRS), which is also known as ERp57/GRp58 and also serves as a protein disulfide isomerase A3 (PDIA3) that acts as a chaperone protein [101, 137] and has additional unexpected functions [138, 219]. According to some reports, it functions as a membrane-bound receptor for active forms of D3 and is involved in the regulation of some of its phenotypic functions [101, 137]. Other studies have shown interactions between plasma membrane 1,25D3-MARRS, VDR, and calveolin-1 via a non-genomic signal transduction pathway initiated by 1,25(OH)2D3 [43, 169]. Our molecular modeling predicts that the CYP11A1-derived secosteroids are unlikely to interact with 1,25D3-MARRS [207].
Retinoic acid-related orphan receptors (ROR) α and γ, members of the nuclear receptor superfamily, provide an alternative mechanism by which vitamin D3 and its derivatives can regulate biological functions and gene expression and affect pathology [99, 199, 207]. CYP11A1-derived hydroxyderivatives of D3 can act as inverse agonists on RORα and RORγ. Similarly, hydroxyderivatives of lumisterol can function as RORα and RORγ inverse agonists [206]. Molecular modeling where these vitamin D3 metabolites exhibit high docking scores predicts that they interact strongly with the ligand-binding pocket of RORα/RORγ [208]. These receptors are expressed in normal and pathological skin [36, 199], including BCC and SCC (Fig. 13.6). Their expression inversely correlates with human melanoma progression, and higher expression in the nucleus correlates with significantly longer overall and disease-free survival times [36]. Interestingly, RORα and RORγ expression positively correlates with HIF-1 expression in cutaneous melanomas [37]. In uveal melanoma, expression of RORs was lower than in normal uveal cells [125]. This suggests that RORs may play an important role in melanomagenesis, melanoma progression, and host responses against the tumor [205, 208]. RORγ is essential for the generation of T-helper 17 (Th17) cells and production of the pro-inflammatory cytokine interleukin 17 (IL-17) which plays a critical role in various autoimmune diseases, including psoriasis, and also has antitumor as well as pro-tumor effects in melanoma [42, 99, 211]. Thus, these hydroxyderivatives could potentially inhibit inflammation and tumor progression in the skin through an RORγ-mediated mechanism.
Most surprising was a recent discovery showing that hydroxyderivatives of vitamin D3, including 20(OH)3, 20,23(OH)2D3, 17,20,23(OH)3D3, and classical 1,25(OH)2D3, can act on the aryl hydrocarbon receptor (AhR) in a manner dependent on the positions of hydroxyl groups on the structure [209]. This discovery is consistent with the promiscuous nature of AhR and its activity [134]. It opens up an exciting opportunity to study the regulation of the skin phenotype by different vitamin D3 hydroxyderivatives acting via AhR signaling, taking into consideration its complex role in skin physiology and pathology [27, 67, 93, 133] (Fig. 13.7).
Thus, different forms of vitamin D3, in addition to acting via the genomic canonical pathway of VDR, can potentially act via noncanonical pathways, including those involving the nuclear receptors, RORs and AhR. While the classical 1,25(OH)2D3 can exert non-genomic activities through action via the non-genomic binding site of VDR or via 1,25D3-MARRS, similar functions for CYP11A1-derived secosteroids are less likely [207] and remain to be established experimentally. The receptors for pregnacalciferol derivatives [195] remain to be identified.
In summary, vitamin D hydroxyderivatives exhibit different affinities for multiple receptor targets and through their modulation of these distinct receptor signaling pathways regulate different physiological functions and influence pathologies in different ways.
Nonmelanoma Skin Cancers
Human Skin Cancer: An Overview
NMSCs, encompassing SCC and BCC, are the most common malignancies in humans. The cost of their treatment is an enormous economic burden to the healthcare system of the USA and to healthcare systems worldwide [113]. The role that UV radiation plays in the pathogenesis was first proposed in the late nineteenth century by Unna, who made the important observation that sailors, who had chronic exposure to sunlight, had a disproportionate increase in the incidence of skin cancer [25]. In fact, over 80% of NMSCs occur in sun-exposed skin sites, i.e., head and neck and back of the hands [11, 51, 100, 162, 200]. Studies in experimental animal models have demonstrated that wavelengths within the UVB range are primarily responsible for these malignancies [25, 66]. Immunocompromised patients, including solid organ transplant recipients who require drugs that suppress immunological function in order to prevent rejection of their transplanted organ, are at greatly increased risk of developing nonmelanoma skin cancers, particularly cutaneous squamous cell carcinomas [6]. Tumors in this population behave more aggressively and are more likely to metastasize [6]. Military personnel also have an increased risk of NMSCs [7]. They are exposed to high doses of UVR during deployment to locations with high solar radiation including the desert and high-altitude environments. This often happens in situations in which adequate attention to photoprotective measures is unavoidable. It should be noted that there was an unusually high incidence of NMSCs in World War II veterans who served in the Pacific and elsewhere in the tropics. Currently, the incidence of skin cancer in the military is greater than in the general population.
Although there has been an intensive effort by healthcare institutions around the world to take preventative measures against excessive sun exposure, the incidence of these malignancies continues to rise [161]. Therefore, there is an urgent need to establish proper measures to stimulate photoprotective or reparative mechanisms in the skin of civilian and military personnel against UVR-induced damage. These measures need to be taken at as early an age as possible for young and older individuals alike, since skin cancers often develop after a long latency period.
Therapy of NMSC
The mortality for most NMSCs is low. However, they, and the treatment required for their removal, can be disfiguring with significant morbidity. Given the frequency with which they occur, the management of NMSC is a tremendous economic burden [113]. In the USA alone, the estimated cost for the treatment of actinically damaged skin is $1.68 billion [113].
Guidelines and appropriate use criteria for the management of both basal cell carcinomas and squamous cell carcinomas have been created by the American Academy of Dermatology and the National Comprehensive Cancer Network [4, 9, 10, 103, 244]. In most instances, the treatment of nonmelanoma skin cancers is surgical. This includes electrodessication and curettage, excision with appropriate tumor-free margins, and Mohs micrographic surgery. Electrodessication and curettage is used primarily for lower risk skin cancers, chiefly on the trunk and extremities. The procedure involves scraping away malignant tissue with a curette followed by electrodessication of the treatment area; the procedure is repeated up to three times. The cure rate has been reported to be up to 95% for low-risk lesions but is considerably lower for higher-risk tumors [9, 45, 108]. Standard excision followed by histological evaluation of margins is another option. Recurrence or metastasis rates of less than 6% can be achieved for primary tumors; cure rates for recurrent lesions, however, are substantially lower [163]. Subclinical involvement for cutaneous squamous cell carcinomas is present in up to 15% of primary tumors and up to 50% of recurrent squamous cell carcinomas [8, 110]. For this reason, Mohs micrographic surgery is the treatment of choice for most high-risk nonmelanoma skin cancers. Mohs micrographic surgery is an outpatient surgical procedure in which the tumor is debulked. Then a thin layer of underlying tissue is removed and examined histologically by frozen section to determine if it is free of tumor. If not, then further surgical layers are removed until there is no microscopic evidence of tumor. Surgery is performed all in one session with the patient remaining in the clinic while tissue sections are evaluated. Mohs micrographic surgery minimizes the amount of normal tissue that must be taken and provides microscopic verification that the tumor has been completely removed. Retrospective studies have found a 5-year cure rate of 97% for primary tumors and 90% for recurrences [229]. This is compared with 92% for primary tumors and 77% for recurrences with other procedures.
Radiotherapy, especially for low-risk tumors, is employed in some situations based on patient preference or other factors [9, 10]. It is contraindicated in patients with certain genodermatoses such as basal cell nevus syndrome and in individuals less than 60 years of age because of the potential for long-term consequences. Five-year cure rates of 93% for primary tumors and 90% for recurrent tumors have been accomplished with radiotherapy [9].
Topical imiquimod has received regulatory approval for treatment of superficial BCCs and premalignant actinic keratoses [10, 70, 139, 150, 166, 171]. It has also been used off-label for nodular BCCs [77, 239]. Imiquimod stimulates innate and acquired immunity by binding to the TLR7 and, as a consequence, stimulates dendritic cells and augments production of interferon-gamma, TNF-alpha, and other pro-inflammatory cytokines [77]. Recent studies have shown that it also has actions independent of TLR7 stimulation [232]. The end result is an antitumor immune response capable of eradicating BCCs. Treatment requires daily application of imiquimod for 6 (superficial BCC) to 12 (nodular BCC) weeks. Five-year response rates with imiquimod are significantly less than with surgical excision [239].
While metastasis of BCC is very rare, it can occur. Furthermore, neglected BCCs can enlarge to the point at which sufficient destruction of cutaneous and even non-cutaneous tissue occurs, making it impossible to remove the lesions surgically. The sonic hedgehog pathway plays an essential role in BCC pathogenesis. Two oral sonic hedgehog inhibitors, vismodegib and sonidegib, are commercially available, and both cause BCC regression [61, 122, 128, 168]. They are employed for the treatment of locally advanced and metastatic BCC. These agents have many adverse effects including hair loss, muscle spasms, weight loss, and dysgeusia, which reduce patient compliance. Moreover, BCCs can develop resistance to these medications, and discontinuation often results in BCC regrowth. Thus, these medications are not used for routine BCCs.
Other treatment options for nonmelanoma skin cancers include cryotherapy, PDT, 5-FU, and intralesional methotrexate but are only utilized in special circumstances [9, 10, 103, 244].
Vitamin D in Chemoprevention of NMSC
Photoprotective Activity of Active Forms of Vitamin D3
A significant number of studies have shown protective effect of different vitamin D analogs against UVR in human skin cells and hairless mice [53, 56, 58, 59, 72, 76, 109, 127, 220, 243]. Specifically, 1,25(OH)2D3 and 1,25(OH)2L3 reduced UV-induced DNA damage including formation of CPD and reduced production of pro-inflammatory cytokines in human keratinocytes in culture and in mouse and human skin [127]. The photoprotective effects of these compounds were also connected with increased expression of P53 in the nucleus and a decrease in the number of apoptotic sunburn cells and attenuation of UVB-induced immunosuppression [57]. The authors suggested non-genomic actions of 1,25(OH)2D3 and 1,25(OH)2L3 [57]. Similarly, topical application of CYP11A1-derived 20(OH)D at 23 or 46pmol/cm2 protected mouse skin against UVB-induced DNA damage at comparable level to that of 1,25(OH)2D3 [221]. It also reduced the sunburn edema and protected against UVR-induced immunosuppression in a similar manner to 1,25(OH)2D3. Thus, these in vivo photoprotective effects were independent of C1α-hydroxylation [221]. The same group demonstrated that in addition to 1,25(OH)2D3, low-calcemic analogs of D3 reduced UV-induced CPDs in both skin fibroblasts and keratinocytes and their cell death after UV exposure [58]. They were equally effective as 125(OH)2D3 in increasing levels of p53 in cultured human keratinocytes. In a hairless mouse line, these compounds reduced UV immunosuppression. However, the low-calcemic analog was not as effective as 1,25(OH)2D3 in reducing tumorigenesis [58]. Most recently, an interesting mechanism of action for 1,25(OH)2D3 in UVB-irradiated keratinocytes was demonstrated. Specifically, it enhanced glycolysis along with energy-conserving processes such as autophagy and mitophagy, resulting in increased repair of CPDs and decreased oxidative DNA damage [165]. Finally, high doses of vitamin D3 given orally shortly after exposure to UVB could reverse the induced skin damage with attenuation of the inflammation and induction of barrier repair mechanisms [167].
Our studies on photoprotective functions of 20(OH)D3 and 20,23(OH)2D3 in cultured human epidermal keratinocytes, melanocytes, and HaCaT keratinocytes have shown that they can attenuate ROS, H2O2, and NO production induced by UVB to a similar level to that for 1,25(OH)2D3, with 25(OH)D3 and 20(OH)7DHC having lower efficiency [201]. The photoprotection was accompanied by increased expression of genes involved in defense against oxidative stress. Furthermore, these compounds reduced the UVB-induced CPDs and DNA fragmentation in the comet assay and enhanced expression of p53 phosphorylated at Ser-15, but not at Ser-46 [201]. The most recent tests on an extended list of CYP11A1-derived vitamin D3 and lumisterol hydroxymetabolites (1,25(OH)2D3, 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3, 1,20,23(OH)3D3, 20(OH)L3, 22(OH)L3, 20,22(OH)2L3, and 24(OH)L3), and lumisterol itself, have shown that they can protect human epidermal keratinocytes against UVB [41]. Treatment of cells with the D3 or lumisterol derivatives showed a dose-dependent reduction in UVB-induced oxidant formation, protection against DNA damage, and/or induction of DNA repair by enhancing the repair of 6-4PP and attenuating CPD levels and the tail moment of comets. They also stimulated the expression of antioxidant response genes downstream of Nrf-2 (GR, HO-1, CAT, SOD1, and SOD2) and expression at the protein level of HO-1, CAT, and MnSOD [41]. With respect to their mechanism of action, these compounds increased the phosphorylation of p53 at Ser-15 with stimulation of p53 and Nrf2 translocation into the nucleus. We have also shown that not only pre-treatment but also posttreatment of keratinocytes with D3 and lumisterol derivatives can reverse UVB-induced keratinocyte damage [41] which is similar to other natural products [98, 175]. Thus, CYP11A1-derived D3 or lumisterol derivatives, and to some degree lumisterol itself, act as photoprotectors with their mechanism of action involving stimulation of the Nrf2-dependent and p53 responses, as well as stimulation of the DNA repair system.
Chemoprevention Against UVR and Chemically Induced NMSC in Animal Models
As discussed in subheading “Vitamin D Receptor (VDR)”, the chemopreventive and potentially therapeutic roles of D3 hydroxyderivatives in NMSC are indicated by experiments with VDR-/- and RXR-/- (partner for VDR) mice on cutaneous carcinogenesis [12, 21,22,23, 40, 57, 92, 94, 233]. For example, Dixon et al. [57] have shown that 1,25(OH)2D3 and 1,25(OH)2L3 inhibited UVB-induced development of papillomas and squamous cell carcinomas in immunocompetent mice (Skh:hr1). They suggested a non-genomic mechanism of action, at least in part [57]. Studies on low-calcemic analog, 1α-hydroxymethyl-16-ene-24,24-difluoro-25-hydroxy-26,27-bis-homovitamin D3, have shown that while it protected against UVB-induced damage, it was not as effective as 1,25(OH)2D3 in reducing tumor formation and progression [58].
Others using 1,25(OH)2D3 have shown that it inhibits proliferation and growth of BCC of Ptch mutant mice in vivo and of established murine BCC lines in vitro [230]. Two mechanisms of action have been shown, e.g., the activation of the VDR and induction of keratinocyte differentiation and inhibition of Hh signaling at the level of Smo in a VDR-independent manner [230]. The 1,25(OH)2D3 effects on BCC growth were stronger than those of the cyclopamine (Hh inhibitor), indicating that its dual action makes 1,25(OH)2D3 an excellent therapeutic for BCC and other tumors in which Hh signaling is disrupted [230]. Of great interest was the study showing that unmodified D3 inhibited Hh signaling and growth of murine BCCs both in vitro and in vivo [214]. D3 blocked both proliferation and Hh signaling to similar degree as cyclopamine. 7DHC, 25(OH)D3, and 1,25(OH)2D3 were less effective in these actions. The D3 effect appeared to be independent of the VDR [214]. An important study led by Epstein on UVB-induced BCC carcinogenesis in Ptch1(+/−) mice showed that inhibition of UVB-induced production of D3 in the skin accelerated BCC carcinogenesis [124]. Furthermore, topical application of the D3 prohormone inhibited UVB-induced BCC tumorigenesis, while orally delivered D3 had no protective effect [124]. The authors concluded that UVB-induced production of D3 in keratinocytes significantly restrains murine BCC tumorigenesis and that UVB has anti-BCC carcinogenic effects through induction of D3 formation [124].
Studies on the chemically induced development and progression of SCC in mice showed that calcipotriol (analog of 1,25(OH)2D3) inhibited the cancerogenesis and growth of tumors [50]. The mechanism of anti-cancerogenic action included induction of thymic stromal lymphopoietin [50].
Vitamin D in Chemoprevention or Adjuvant Therapy in NMSC in Humans
Currently, a few clinical trials have investigated the effects of vitamin D on NMSCs. The synergistic effects of calcipotriol and 5-FU treatment in optimally activating a CD4+ T cell-mediated immunity against actinic keratoses in randomized, double-blind clinical trial involving 131 participants were reported [50]. Another human trial has shown that calcipotriol combined with methyl aminolaevulinate photodynamic therapy (MAL-PDT) was more efficacious than MAL-PDT alone and well tolerated [222]. The already completed Dutch phase II clinical trial (ClinicalTrials.gov Identifier: NCT01358045, start date November 2011, completed date May 2013) ([31], https://clinicaltrials.gov/ct2/show/NCT01358045?term=vitamin+d&cond=BCC&rank=3) was a randomized trial on the treatment of primary, histologically confirmed BCC (nodular of superficial subtype) with topical application of vitamin D3, diclofenac, or a combination of both twice daily under occlusion on BCC lesion. After 8 weeks, tumors were excised, and proliferation (Ki-67) and antiapoptotic (Bcl-2) markers were examined, and no effect of calcitriol alone was found. Combination therapy resulted in decreasing Ki-67 level in superficial BCC subtype, while diclofenac application was related to a significantly reduced expression of both Ki-67 and B-cl2 in superficial BCC. Another two clinical trials are related to BCC in basal cell nevus syndrome (BCNS) treatment with photodynamic therapy (PDT) and vitamin D as neoadjuvant. The first one is a clinical, double-blinded, randomized trial (ClinicalTrials.gov Identifier: NCT03467789, start date October 2018) (https://clinicaltrials.gov/ct2/show/NCT03467789?term=vitamin+d&cond=BCC&rank=2) on the vitamin D effect (10,000 IU/day) prior to the first or second PDT visit (treatment for 14 days when patients are deficient for 25-hydroxy-D3 serum levels or 5 days when 25-hydroxy-D3 levels are normal, and to maintain vitamin D3 level patients are supplemented with 2000 IU/day or 1000 IU/day for adults and children, respectively). The tumor clearance measured as change in lesion diameter per month is the primary outcome of this trial. The second one is randomized Phase 1 clinical trial (ClinicalTrials.gov Identifier: NCT03483441, start date March 2018, (https://clinicaltrials.gov/ct2/show/study/NCT03483441?term=vitamin+d&cond=BCC&rank=1), with a similar study design. Patients will take 10,000 units of cholecalciferol for several days prior to PDT, and differences in tumor BCC tumor diameter between treatments will be measured. The recruitment to these clinical trials has been opened; however, no results are available yet. There is also a completed early Phase 1, double-blinded clinical trial on actinic keratosis, a precursor of SCC, treated with calcipotriol plus 5-fluorouracil (5-FU) in patients with multiple actinic keratoses (ClinicalTrials.gov Identifier: NCT02019355, start date October 2013, completed date March 2015, ([50], https://clinicaltrials.gov/ct2/show/NCT02019355)). A significantly reduced number of actinic keratosis was found in patients treated for 4 days with calcipotriol plus 5-FU when compared to only 5-FU treated patients. Currently, there is no open clinical trial on SCC treatment with vitamin D. Thus, vitamin D could enhance NMSC treatment; however, additional clinical trials are needed to fully justify its use and to select the most optimal vitamin D derivative for treatment of keratinocyte-derived cancers.
Perspective and Conclusions
The pleiotropic activities of D3 that are in addition to the regulation of body calcium homeostasis and include radioprotective and anticarcinogenic activities are consistent with the actions of multiple vitamin D derivatives produced in the human body and multiple target receptors in addition to the VDR. In vivo and in vitro studies reviewed above clearly document an important if not crucial role for different vitamin D compounds and the VDR, not only in photoprotection but also in the prevention or attenuation of NMSCs. With respect to cutaneous carcinogenesis, a key question is which chemical configurations of vitamin D compounds are the most efficacious with relatively minimal site effects and what is their mechanism of action, e.g., genomic or no-genomic. For genomic activities, new receptor candidates in addition to the VDR are emerging such as RORα and RORγ and AhR, which may be targeted in addition to the targeting of the Hh signaling pathway. Finally, different routes of delivery with preferred topical application have to be considered that require proper formulation.
Due to toxic (calcemic) effects, the therapeutic use of 1,25(OH)2D3 at pharmacological doses or chronic oral use of D3 has its limitations. The discovery of an alternative pathway of D3 activation initiated by CYP11A1, producing at least 15 metabolites (OH)nD3) with a full-length side chain and potentially several others with a short or absent side chain, opens new possibilities for treatment, since they have antiproliferative, pro-differentiation, anti-inflammatory photoprotective effects on normal and malignant epidermal cells. Many of them are non-calcemic and non-toxic at suprapharmacological doses. Furthermore, with the contribution of UVB acting on Δ7-steroids or sterols produced in the skin, the corresponding lumisterol and tachysterol compounds can be produced with photoprotective properties. Thus, novel secosteroids, lumisterol, and/or tachysterol compounds are excellent candidates to serve as radioprotectors and chemopreventive agents for skin cancers. They potentially can induce the repair of damaged DNA and/or attenuate or reverse UVR-induced skin aging [26].
In summary, recent advances in vitamin D, lumisterol and 7DHC biochemistry, skin biology, and pharmacology are opening up new exciting opportunities in skin healthcare and treatment of different cutaneous pathologies.
References
Addison CC, Gamlen GA, Thompson R. The ultra-violet absorption spectra of sodium hyponitrite and sodium α-oxyhyponitrite: the analysis of mixtures with sodium nitrite and nitrate. J Chem Soc. 1952:338–45.
Ahmad N, Mukhtar H. Cytochrome p450: a target for drug development for skin diseases. J Invest Dermatol. 2004;123:417–25.
Albert B, Hahn H. Interaction of hedgehog and vitamin D signaling pathways in basal cell carcinomas. Adv Exp Med Biol. 2014;810:329–41.
American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, American Society For Mohs Surgery, Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Begolka WS, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, Mcburney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582–603.
Annalora AJ, Jozic M, Marcus CB, Iversen PL. Alternative splicing of the vitamin D receptor modulates target gene expression and promotes ligand-independent functions. Toxicol Appl Pharmacol. 2019;364:55–67.
Athar M, Walsh SB, Kopelovich L, Elmets CA. Pathogenesis of nonmelanoma skin cancers in organ transplant recipients. Arch Biochem Biophys. 2011;508:159–63.
Bain J. At risk in the military? [Online]. 2018. Available: https://blog.skincancer.org/2018/07/11/skin-cancer-risk-military/. Accessed 21 July 2019.
Batra RS, Kelley LC. Predictors of extensive subclinical spread in nonmelanoma skin cancer treated with Mohs micrographic surgery. Arch Dermatol. 2002;138:1043–51.
Bichakjian CK. NCCN guidelines on squamous cell skin cancer [Online]. 2019. Available: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf. Accessed 21 July 2019.
Bichakjian CK, Olencki T, Aasi SZ, Alam M, Andersen JS, Berg D, Bowen GM, Cheney RT, Daniels GA, Glass LF, Grekin RC, Grossman K, Higgins SA, Ho AL, Lewis KD, Lydiatt DD, Nehal KS, Nghiem P, Olsen EA, Schmults CD, Sekulic A, Shaha AR, Thorstad WL, Tuli M, Urist MM, Wang TS, Wong SL, Zic JA, Hoffmann KG, Engh A. Basal cell skin cancer, Version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2016;14:574–97.
Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–75.
Bikle DD. Vitamin D receptor, UVR, and skin cancer: a potential protective mechanism. J Invest Dermatol. 2008;128:2357–61.
Bikle DD. Vitamin D and the skin. J Bone Miner Metab. 2010a;28:117–30.
Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010b;21:375–84.
Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011a;347:80–9.
Bikle DD. Vitamin D: an ancient hormone. Exp Dermatol. 2011b;20:7–13.
Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19.
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014a;21:319–29.
Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Adv Exp Med Biol. 2014b;810:282–302.
Bikle DD. Vitamin D receptor, a tumor suppressor in skin. Can J Physiol Pharmacol. 2015;93:349–54.
Bikle DD, Elalieh H, Welsh J, Oh D, Cleaver J, Teichert A. Protective role of vitamin D signaling in skin cancer formation. J Steroid Biochem Mol Biol. 2012;136:271–9.
Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2015;148:47–51.
Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Disruption of vitamin D and calcium signaling in keratinocytes predisposes to skin cancer. Front Physiol. 2016;7:296.
Bjorn LO. Photobiology: the science of life and light. New York: Springer; 2008.
Blum HF. Carcinogenesis by ultraviolet light. Princeton: Princeton University Press; 1959.
Bocheva G, Slominski RM, Slominski AT. Neuroendocrine aspects of skin aging. Int J Mol Sci. 2019;20
Bock KW. From TCDD-mediated toxicity to searches of physiologic AHR functions. Biochem Pharmacol. 2018;155:419–24.
Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2018;40:1109–51.
Brash DE. UV signature mutations. Photochem Photobiol. 2015;91:15–26.
Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br J Dermatol. 2005;153:733–9.
Brinkhuizen T, Frencken KJ, Nelemans PJ, Hoff ML, Kelleners-Smeets NW, Zur Hausen A, Van Der Horst MP, Rennspiess D, Winnepenninckx VJ, Van Steensel MA, Mosterd K. The effect of topical diclofenac 3% and calcitriol 3 mug/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): a phase II, randomized controlled trial. J Am Acad Dermatol. 2016;75:126–34.
Brozyna A, Zbytek B, Granese J, Carlson AJ, Ross J, Slominski A. Mechanism of UV-related carcinogenesis and its contribution to nevi/melanoma. Expert Rev Dermatol. 2007;2:451–69.
Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol. 2011;42:618–31.
Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of the vitamin D-activating enzyme 1alpha-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2013;44:374–87.
Brozyna AA, Jozwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: new data and analyses. Anticancer Res. 2014;34:2735–43.
Brozyna AA, Jozwicki W, Skobowiat C, Jetten A, Slominski AT. RORalpha and RORgamma expression inversely correlates with human melanoma progression. Oncotarget. 2016;7:63261–82.
Brożyna AA, Jóźwicki W, Jetten AM, Slominski AT. On the relationship between VDR, RORα and RORγ receptors expression and HIF1-α levels in human melanomas. Exp Dermatol. 2019; 28:1036-1043.
Burns EM, Guroji P, Ahmad I, Nasr HM, Wang Y, Tamimi IA, Stiefel E, Abdelgawwad MS, Shaheen A, Muzaffar AF, Bush LM, Hurst CB, Griffin RL, Elmets CA, Yusuf N. Association of vitamin D receptor polymorphisms with the risk of nonmelanoma skin cancer in adults. JAMA Dermatol. 2017;153(10):983–9.
Carlberg C. Vitamin D genomics: from in vitro to in vivo. Front Endocrinol (Lausanne). 2018;9:250.
Chagani S, Kyryachenko S, Yamamoto Y, Kato S, Ganguli-Indra G, Indra AK. In vivo role of vitamin D receptor signaling in UVB-induced DNA damage and melanocyte homeostasis. J Invest Dermatol. 2016;136:2108–11.
Chaiprasongsuk A, Janjetovic Z, Kim TK, Jarrett SG, D’orazio JA, Holick MF, Tang EKY, Tuckey RC, Panich U, Li W, Slominski AT. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019;24:101206.
Chen C, Gao FH. Th17 cells paradoxical roles in melanoma and potential application in immunotherapy. Front Immunol. 2019;10:187.
Chen J, Doroudi M, Cheung J, Grozier AL, Schwartz Z, Boyan BD. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1alpha,25(OH)(2)D(3). Cell Signal. 2013;25:2362–73.
Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014;34:2153–63.
Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188–96.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408.
Christakos S, Li S, De La Cruz J, Bikle DD. New developments in our understanding of vitamin metabolism, action and treatment. Metabolism. 2019;98:112–20.
Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151.
Crofts LA, Hancock MS, Morrison NA, Eisman JA. Isoforms of the human Vitamin D receptor. United States patent application 11/156,272. 2006.
Cunningham TJ, Tabacchi M, Eliane JP, Tuchayi SM, Manivasagam S, Mirzaalian H, Turkoz A, Kopan R, Schaffer A, Saavedra AP, Wallendorf M, Cornelius LA, Demehri S. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106–16.
D’orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–48.
De Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:316–20.
De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141–8.
Denzer N, Vogt T, Reichrath J. Vitamin D receptor (VDR) polymorphisms and skin cancer: a systematic review. Dermatoendocrinol. 2011;3:205–10.
Dimitrov V, Bouttier M, Boukhaled G, Salehi-Tabar R, Avramescu RG, Memari B, Hasaj B, Lukacs GL, Krawczyk CM, White JH. Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J Biol Chem. 2017;292:20657–68.
Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, Mason RS. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007;103:451–6.
Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, Halliday GM, Mason RS. 1alpha,25(OH)(2)-vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila). 2011;4:1485–94.
Dixon KM, Sequeira VB, Deo SS, Mohan R, Posner GH, Mason RS. Differential photoprotective effects of 1,25-dihydroxyvitamin D3 and a low calcaemic deltanoid. Photochem Photobiol Sci. 2012;11:1825–30.
Dixon KM, Tongkao-On W, Sequeira VB, Carter SE, Song EJ, Rybchyn MS, Gordon-Thomson C, Mason RS. Vitamin D and death by sunshine. Int J Mol Sci. 2013;14:1964–77.
Dormoy V, Beraud C, Lindner V, Coquard C, Barthelmebs M, Brasse D, Jacqmin D, Lang H, Massfelder T. Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma. Carcinogenesis. 2012;33:2084–93.
Dummer R, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, Herd RM, Kaatz M, Loquai C, Stratigos AJ, Schulze HJ, Plummer R, Gogov S, Pallaud C, Yi T, Mone M, Chang AL, Cornelis F, Kudchadkar R, Trefzer U, Lear JT, Sellami D, Migden MR. The 12-month analysis from basal cell carcinoma outcomes with LDE225 treatment (BOLT): a phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J Am Acad Dermatol. 2016;75:113–125 e5.
Dunaway S, Odin R, Zhou L, Ji L, Zhang Y, Kadekaro AL. Natural antioxidants: multiple mechanisms to protect skin from solar radiation. Front Pharmacol. 2018;9:392.
Elias PM. Structure and function of the stratum corneum extracellular matrix. J Invest Dermatol. 2012;132:2131–3.
Esteban LM, Fong C, Amr D, Cock TA, Allison SJ, Flanagan JL, Liddle C, Eisman JA, Gardiner EM. Promoter-, cell-, and ligand-specific transactivation responses of the VDRB1 isoform. Biochem Biophys Res Commun. 2005;334:9–15.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57.
Freeman RG. Action spectrum for ultraviolet carcinogenesis. Natl Cancer Inst Monogr. 1978;50:27–9.
Furue M, Takahara M, Nakahara T, Uchi H. Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res. 2014;306:769–79.
Gardiner EM, Esteban LM, Fong C, Allison SJ, Flanagan JL, Kouzmenko AP, Eisman JA. Vitamin D receptor B1 and exon 1d: functional and evolutionary analysis. J Steroid Biochem Mol Biol. 2004;89–90:233–8.
Gasparro FP. Sunscreens, skin photobiology, and skin cancer: the need for UVA protection and evaluation of efficacy. Environ Health Perspect. 2000;108(Suppl 1):71–8.
Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol. 2004;50:722–33.
Gilchrest BA. Photodamage. Cambridge, MA: Blackwell Sci, Inc; 1995.
Gordon-Thomson C, Tongkao-On W, Song EJ, Carter SE, Dixon KM, Mason RS. Protection from ultraviolet damage and photocarcinogenesis by vitamin D compounds. Adv Exp Med Biol. 2014;810:303–28.
Grant WB. Roles of solar UVB and vitamin D in reducing cancer risk and increasing survival. Anticancer Res. 2016;36:1357–70.
Grant WB, Garland CF. Vitamin D has a greater impact on cancer mortality rates than on cancer incidence rates. BMJ. 2014;348:g2862.
Grant WB, Bhattoa HP, Boucher BJ. Seasonal variations of U.S. mortality rates: roles of solar ultraviolet-B doses, vitamin D, gene expression, and infections. J Steroid Biochem Mol Biol. 2017;173:5–12.
Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–15.
Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415.
Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci U S A. 2003;100:14754–9.
Hadden MK. Hedgehog and vitamin D signaling pathways in development and disease. Vitam Horm. 2016;100:231–53.
Han J, Colditz GA, Hunter DJ. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. 2007;28:390–7.
Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59.
Herlyn M, Berking C, Li G, Satyamoorthy K. Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. 2000;10:303–12.
Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–88.
Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Holick MF, Maclaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–3.
Holick MF, Tian XQ, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc Natl Acad Sci U S A. 1995;92:3124–6.
https://clinicaltrials.gov/ct2/show/nct01358045?term=vitamin+d&cond=bcc&rank=3.
https://clinicaltrials.gov/ct2/show/nct03467789?term=vitamin+d&cond=bcc&rank=2.
https://clinicaltrials.gov/ct2/show/study/nct03483441?term=vitamin+d&cond=bcc&rank=1.
Hu L, Bikle DD, Oda Y. Reciprocal role of vitamin D receptor on beta-catenin regulated keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol. 2013;144 Pt A:237–41.
Ikuta T, Namiki T, Fujii-Kuriyama Y, Kawajiri K. AhR protein trafficking and function in the skin. Biochem Pharmacol. 2009;77:588–96.
Indra AK, Castaneda E, Antal MC, Jiang M, Messaddeq N, Meng X, Loehr CV, Gariglio P, Kato S, Wahli W, Desvergne B, Metzger D, Chambon P. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol. 2007;127:1250–60.
Janjetovic Z, Zmijewski MA, Tuckey RC, Deleon DA, Nguyen MN, Pfeffer LM, Slominski AT. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988.
Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr, Slominski AT. 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48.
Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–84.
Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: involvement of NRF2-mediated pathways. Sci Rep. 2017;7:1274.
Jetten AM, Takeda Y, Slominski A, Kang HS. Retinoic acid-related Orphan Receptor gamma (RORgamma): connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr Opin Toxicol. 2018;8:66–80.
Kauvar ANB, Cronin TJ, Roenigk R, Hruza G, Bennett R. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550–71.
Khanal RC, Nemere I. The ERp57/GRp58/1,25D3-MARRS receptor: multiple functional roles in diverse cell systems. Curr Med Chem. 2007;14:1087–93.
Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361:143–52.
Kim JYS, Kozlow JH, Mittal B, Moyer J, Olenecki T, Rodgers P. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560–78.
Kostner K, Denzer N, Koreng M, Reichrath S, Graber S, Klein R, Tilgen W, Vogt T, Reichrath J. Association of genetic variants of the vitamin D receptor (VDR) with cutaneous squamous cell carcinomas (SCC) and basal cell carcinomas (BCC): a pilot study in a German population. Anticancer Res. 2012;32:327–33.
Krengel S, Stark I, Geuchen C, Knoppe B, Scheel G, Schlenke P, Gebert A, Wunsch L, Brinckmann J, Tronnier M. Selective down-regulation of the alpha6-integrin subunit in melanocytes by UVB light. Exp Dermatol. 2005;14:411–9.
Kripke ML. Ultraviolet radiation and immunology: something new under the sun–presidential address. Cancer Res. 1994;54:6102–5.
La VONS, Law MH, Montgomery GW, Green AC, Jc VDP. Vitamin D pathway gene polymorphisms and keratinocyte cancers: a nested case-control study and meta-analysis. Anticancer Res. 2016;36:2145–52.
Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153.
Lee J, Youn JI. The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci. 1998;18:11–8.
Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol. 2005;53:253–60.
Lesiak A, Norval M, Wodz-Naskiewicz k, Pawliczak r, Rogowski-Tylman m, Sysa-Jedrzejowska A, Sobjanek M, Wlodarkiewicz A, Narbutt J. An enhanced risk of basal cell carcinoma is associated with particular polymorphisms in the VDR and MTHFR genes. Exp Dermatol. 2011;20:800–4.
Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, Zjawiony J, Tuckey RC, Miller D, Slominski A. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–35.
Lim HW, Collins SAB, Resneck JS Jr, Bolognia JL, Hodge JA, Rohrer TA, Van Beek MJ, Margolis DJ, Sober AJ, Weinstock MA, Nerenz DR, Smith Begolka W, Moyano JV. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958–972.e2.
Lin Z, Marepally SR, Ma D, Myers LK, Postlethwaite AE, Tuckey RC, Cheng CY, Kim TK, Yue J, Slominski AT, Miller DD, Li W. Chemical synthesis and biological activities of 20S,24S/R-dihydroxyvitamin D3 epimers and their 1alpha-hydroxyl derivatives. J Med Chem. 2015;58:7881–7.
Lin Z, Marepally SR, Kim TK, Janjetovic Z, Oak AS, Postlethwaite AE, Myers LK, Tuckey RC, Slominski AT, Miller DD, Li W. Design, synthesis and biological activities of novel gemini 20S-hydroxyvitamin D3 analogs. Anticancer Res. 2016a;36:877–86.
Lin Z, Marepally SR, Ma D, Kim TK, Oak AS, Myers LK, Tuckey RC, Slominski AT, Miller DD, Li W. Synthesis and biological evaluation of vitamin D3 metabolite 20S,23S-dihydroxyvitamin D3 and its 23R epimer. J Med Chem. 2016b;59:5102–8.
Lin Y, Chahal HS, Wu W, Cho HG, Ransohoff KJ, Dai H, Tang JY, Sarin KY, Han J. Association between genetic variation within vitamin D receptor-DNA binding sites and risk of basal cell carcinoma. Int J Cancer. 2017a;140:2085–91.
Lin Z, Chen H, Belorusova AY, Bollinger JC, Tang EKY, Janjetovic Z, Kim TK, Wu Z, Miller DD, Slominski AT, Postlethwaite AE, Tuckey RC, Rochel N, Li W. 1alpha,20S-dihydroxyvitamin D3 interacts with vitamin D receptor: crystal structure and route of chemical synthesis. Sci Rep. 2017b;7:10193.
Lin Z, Marepally SR, Goh ESY, Cheng CYS, Janjetovic Z, Kim TK, Miller DD, Postlethwaite AE, Slominski AT, Tuckey RC, Peluso-Iltis C, Rochel N, Li W. Investigation of 20S-hydroxyvitamin D3 analogs and their 1alpha-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities. Sci Rep. 2018;8:1478.
Lisse TS, Saini V, Zhao H, Luderer HF, Gori F, Demay MB. The vitamin D receptor is required for activation of cWnt and hedgehog signaling in keratinocytes. Mol Endocrinol. 2014;28:1698–706.
Lo HL, Nakajima S, Ma L, Walter B, Yasui A, Ethell DW, Owen LB. Differential biologic effects of CPD and 6-4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer. 2005;5:135.
Lorusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, Graham RA, Zerivitz KL, Low JA, Von Hoff DD. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–11.
Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, Tuckey RC, Slominski AT, Li W, Miller DD. Design, synthesis, and biological action of 20R-hydroxyvitamin D3. J Med Chem. 2012;55:3573–7.
Makarova A, Wang G, Dolorito JA, Kc S, Libove E, Epstein EH Jr. Vitamin D3 produced by skin exposure to UVR inhibits murine basal cell carcinoma carcinogenesis. J Invest Dermatol. 2017;137:2613–9.
Markiewicz A, Brozyna AA, Podgorska E, Elas M, Urbanska K, Jetten AM, Slominski AT, Jozwicki W, Orlowska-Heitzman J, Dyduch G, Romanowska-Dixon B. Vitamin D receptors (VDR), hydroxylases CYP27B1 and CYP24A1 and retinoid-related orphan receptors (ROR) level in human uveal tract and ocular melanoma with different melanization levels. Sci Rep. 2019;9:9142.
Marr DG, Poser I, Shellman YG, Bosserhoff AK, Norris DA. Ultraviolet radiation induces release of MIA: a new mechanism for UVR-induced progression of melanoma. Int J Oncol. 2004;25:105–11.
Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, Mizwicki MT, Norman AW, Feldman D, Halliday GM, Reeve VE. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010;121:164–8.
Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, Herd RM, Kudchadkar R, Trefzer U, Gogov S, Pallaud C, Yi T, Mone M, Kaatz M, Loquai C, Stratigos AJ, Schulze HJ, Plummer R, Chang AL, Cornelis F, Lear JT, Sellami D, Dummer R. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716–28.
Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gartner BC, Kamradt J, Meese E, Tilgen W, Reichrath J. Analysis of the vitamin D system in basal cell carcinomas (BCCs). Lab Invest. 2004;84:693–702.
Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal. 2009;2:re4.
Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, Moras D, Norman AW. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101:12876–81.
Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–70.
Muenyi CS, Carrion SL, Jones LA, Kennedy LH, Slominski AT, Sutter CH, Sutter TR. Effects of in utero exposure of C57BL/6J mice to 2,3,7,8-tetrachlorodibenzo-p-dioxin on epidermal permeability barrier development and function. Environ Health Perspect. 2014;122:1052–8.
Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45.
Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–86.
Neme A, Seuter S, Malinen M, Nurmi T, Tuomainen TP, Virtanen JK, Carlberg C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J Steroid Biochem Mol Biol. 2018;S0960-0760(18):30624–1.
Nemere I, Garbi N, Hammerling G, Hintze KJ. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids. 2012;77:897–902.
Nemere I, Garbi N, Winger Q. The 1,25D3-MARRS receptor/PDIA3/ERp57 and lifespan. J Cell Biochem. 2015;116:380–5.
Neugebauer R, Su KA, Zhu Z, Sokil M, Chren MM, Friedman GD, Asgari MM. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998–1005.
Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin d2 to 17,20,24-trihydroxyvitamin d2 by cytochrome p450scc (CYP11A1). Drug Metab Dispos. 2009;37:761–7.
Nilsen LT, Hannevik M, Veierod MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730–40.
Nurminen V, Neme A, Seuter S, Carlberg C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim Biophys Acta Gene Regul Mech. 2018;1861:697–705.
Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2016;164:379–85.
Pastila R, Leszczynski D. Ultraviolet A exposure alters adhesive properties of mouse melanoma cells. Photodermatol Photoimmunol Photomed. 2005;21:234–41.
Piotrowska A, Wierzbicka J, Slebioda T, Wozniak M, Tuckey RC, Slominski AT, Zmijewski MA. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids. 2016;110:49–61.
Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–6.
Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–30.
Plonka PM, Passeron T, Brenner M, Tobin DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D, Peters E, Nordlund JJ, Abdel-Malek Z, Takeda K, Paus R, Ortonne JP, Hearing VJ, Schallreuter KU. What are melanocytes really doing all day long…? Exp Dermatol. 2009;18:799–819.
Plum LA, Deluca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–55.
Quirk C, Gebauer K, De’ambrosis B, Slade HB, Meng TC. Sustained clearance of superficial basal cell carcinomas treated with imiquimod cream 5%: results of a prospective 5-year study. Cutis. 2010;85:318–24.
Rass K. UV damage and DNA repair in basal cell and squamous cell carcinomas. In: Reichrath J, editor. Molecular mechanisms of basal cell and squamous cell carcinomas. Boston: Springer; 2018.
Reichrath J, Rass K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: an update. Adv Exp Med Biol. 2014;810:208–33.
Reichrath J, Reichrath S. Sunlight, vitamin D and malignant melanoma: an update. Adv Exp Med Biol. 2014;810:390–405.
Reichrath J, Kamradt J, Zhu XH, Kong XF, Tilgen W, Holick MF. Analysis of 1,25-dihydroxyvitamin D(3) receptors (VDR) in basal cell carcinomas. Am J Pathol. 1999;155:583–9.
Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gartner BC, Tilgen W, Holick MF. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol. 2004;31:224–31.
Reichrath J, Reichrath S, Heyne K, Vogt T, Roemer K. Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Front Physiol. 2014;5:166.
Reichrath J, Zouboulis CC, Vogt T, Holick MF. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: an historical view and outlook. Rev Endocr Metab Disord. 2016;17:405–17.
Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96–102.
Reichrath J, Lindqvist PG, Fr DEG, Pilz S, Kimball SM, Grant WB, Holick MF. A critical appraisal of the recent reports on sunbeds from the European Commission’s Scientific Committee on health, environmental and emerging risks and from the World Health Organization. Anticancer Res. 2018;38:1111–20.
Rochel N, Molnar F. Structural aspects of Vitamin D endocrinology. Mol Cell Endocrinol. 2017;453:22–35.
Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (Keratinocyte Carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081–6.
Rosenthal A, Stoddard M, Chipps L, Herrmann J. Skin cancer prevention: a review of current topical options complementary to sunscreens. J Eur Acad Dermatol Venereol. 2019;33(7):1261–7.
Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976–90.
Runger TM. Mechanisms of melanoma promotion by ultraviolet radiation. J Invest Dermatol. 2016;136:1751–2.
Rybchyn MS, De Silva WGM, Sequeira VB, Mccarthy BY, Dilley AV, Dixon KM, Halliday GM, Mason RS. Enhanced repair of UV-induced DNA damage by 1,25-dihydroxyvitamin D3 in skin is linked to pathways that control cellular energy. J Invest Dermatol. 2018;138:1146–56.
Schulze HJ, Cribier B, Requena L, Reifenberger J, Ferrandiz C, Garcia Diez A, Tebbs V, Mcrae S. Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in Europe. Br J Dermatol. 2005;152:939–47.
Scott JF, Das LM, Ahsanuddin S, Qiu Y, Binko AM, Traylor ZP, Debanne SM, Cooper KD, Boxer R, Lu KQ. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078–86.
Sekulic A, Migden MR, Lewis K, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang AL, Dirix L, Hou J, Yue H, Hauschild A, Investigators EB. Pivotal Erivance basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72:1021–6 e8.
Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, Norman AW, Reeve VE, Halliday GM, Feldman D, Mason RS. The role of the vitamin D receptor and ERp57 in photoprotection by 1alpha,25-dihydroxyvitamin D3. Mol Endocrinol. 2012;26:574–82.
Sexton DJ, Muruganandam A, Mckenney DJ, Mutus B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: potential photochemotherapeutic applications. Photochem Photobiol. 1994;59:463–7.
Sharma M, Sharma G, Singh B, Katare OP. Actinic keratosis and imiquimod: a review of novel carriers and patents. Expert Opin Drug Deliv. 2019;16:101–12.
Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J Invest Dermatol. 2015;135:1638–48.
Skobowiat C, Oak AS, Kim TK, Yang CH, Pfeffer LM, Tuckey RC, Slominski AT. Noncalcemic 20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and in vivo models. Oncotarget. 2017a;8:9823–34.
Skobowiat C, Postlethwaite AE, Slominski AT. Skin exposure to ultraviolet B rapidly activates systemic neuroendocrine and immunosuppressive responses. Photochem Photobiol. 2017b; 93:1008–1015.
Skobowiat C, Brozyna AA, Janjetovic Z, Jeayeng S, Oak ASW, Kim TK, Panich U, Reiter RJ, Slominski AT. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J Pineal Res. 2018;65:e12501.
Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18:760–3.
Slominski AT, Carlson JA. Melanoma resistance: a bright future for academicians and a challenge for patient advocates. Mayo Clin Proc. 2014;89:429–33.
Slominski A, Pawelek J. Animals under the sun: effects of ultraviolet radiation on mammalian skin. Clin Dermatol. 1998;16:503–15.
Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87.
Slominski A, Wortsman J, Nickoloff B, Mcclatchey K, Mihm MC, Ross JS. Molecular pathology of malignant melanoma. Am J Clin Pathol. 1998;110:788–94.
Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020.
Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004a;84:1155–228.
Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RA. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004b;271:4178–88.
Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–90.
Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–901.
Slominski AT, Zmijewski MA, Semak I, Sweatman T, Janjetovic Z, Li W, Zjawiony JK, Tuckey RC. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4:e4309.
Slominski AT, Janjetovic z, Fuller b e, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, Chen TC, Lozanski G, Holick MF. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907.
Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, Li W, Chen J, Nguyen MN, Tang EK, Miller D, Chen TC, Holick M. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011a;300:C526–41.
Slominski AT, Li W, Bhattacharya SK, Smith RA, Johnson PL, Chen J, Nelson KE, Tuckey RC, Miller D, Jiao Y, Gu W, Postlethwaite AE. Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J Invest Dermatol. 2011b;131:1167–9.
Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012a;32:3733–42.
Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, Sweatman T, Janjetovic Z, Tuckey RC. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012b;44:2003–18.
Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, Yates CR, Postlethwaite A, Li W, Tuckey RC. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012c;26:3901–15.
Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012d;212:v, vii, 1–115.
Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, Li W, Jiao Y, Gu W, Brown M, Postlethwaite AE. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013a;98:E298–303.
Slominski A, Kim TK, Zmijewski MA, Janjetovic Z, Li W, Chen J, Kusniatsova EI, Semak I, Postlethwaite A, Miller DD, Zjawiony JK, Tuckey RC. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013b;5:7–19.
Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013c;34:827–84.
Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014a;144 Pt A:28–39.
Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014b;383:181–92.
Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014c;28:2775–89.
Slominski AT, Zmijewski MA, Semak I, Zbytek B, Pisarchik A, Li W, Zjawiony J, Tuckey RC. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med Chem. 2014d;14:77–96.
Slominski AT, Janjetovic Z, KIM TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015a;148:52–63.
Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015b;5:14875.
Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015c;151:25–37.
Slominski AT, Kim TK, Li W, Tuckey RC. Classical and non-classical metabolic transformation of vitamin D in dermal fibroblasts. Exp Dermatol. 2016;25:231–2.
Slominski AT, Brozyna AA, Zmijewski MA, Jozwicki W, Jetten AM, Mason RS, Tuckey RC, Elmets CA. Vitamin D signaling and melanoma: role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017a;97:706–24.
Slominski AT, Kim TK, Hobrath JV, Janjetovic Z, Oak ASW, Postlethwaite A, Lin Z, Li W, Takeda Y, Jetten AM, Tuckey RC. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci Rep. 2017b;7:11434.
Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, Li W, Tuckey RC, Jetten AM. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma. J Steroid Biochem Mol Biol. 2017c;173:42–56.
Slominski AT, Brozyna AA, Skobowiat C, Zmijewski MA, Kim TK, Janjetovic Z, Oak AS, Jozwicki W, Jetten AM, Mason RS, Elmets C, Li W, Hoffman RM, Tuckey RC. On the role of classical and novel forms of vitamin D in melanoma progression and management. J Steroid Biochem Mol Biol. 2018a;177:159–70.
Slominski AT, Kim TK, Janjetovic Z, Brozyna AA, Zmijewski MA, Xu H, Sutter TR, Tuckey RC, Jetten AM, Crossman DK. Differential and overlapping effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on gene expression in human epidermal keratinocytes: identification of AhR as an alternative receptor for 20,23(OH)(2)D3. Int J Mol Sci. 2018b;19:3072.
Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018c;159:1992–2007.
Smith SH, Peredo CE, Takeda Y, Bui T, Neil J, Rickard D, Millerman E, Therrien JP, Nicodeme E, Brusq JM, Birault V, Viviani F, Hofland H, Jetten AM, Cote-Sierra J. Development of a topical treatment for psoriasis targeting RORgamma: from bench to skin. PLoS One. 2016;11:e0147979.
Sunn KL, Cock TA, Crofts LA, Eisman JA, Gardiner EM. Novel N-terminal variant of human VDR. Mol Endocrinol. 2001;15:1599–609.
Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, Tuckey RC. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Dispos. 2010;38:1553–9.
Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, So PL, Hebert J, Bikle D, Epstein EH Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila). 2011;4:744–51.
Tang EK, Chen J, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab Dispos. 2013;41:1112–24.
Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131:2289–97.
Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol. 2012a;129:163–71.
Tieu EW, Tang EK, Chen J, Li W, Nguyen MN, Janjetovic Z, Slominski A, Tuckey RC. Rat CYP24A1 acts on 20-hydroxyvitamin D(3) producing hydroxylated products with increased biological activity. Biochem Pharmacol. 2012b;84:1696–704.
Tohda C, Urano T, Umezaki M, Nemere I, Kuboyama T. Diosgenin is an exogenous activator of 1,25D3-MARRS/Pdia3/ERp57 and improves Alzheimer's disease pathologies in 5XFAD mice. Sci Rep. 2012;2:535.
Tongkao-On W, Gordon-Thomson C, Dixon KM, Song EJ, Luu T, Carter SE, Sequeira VB, Reeve VE, Mason RS. Novel vitamin D compounds and skin cancer prevention. Dermatoendocrinol. 2013;5:20–33.
Tongkao-On W, Carter S, Reeve VE, Dixon KM, Gordon-Thomson C, Halliday GM, Tuckey RC, Mason RS. CYP11A1 in skin: an alternative route to photoprotection by vitamin D compounds. J Steroid Biochem Mol Biol. 2015;148:72–8.
Torezan L, Grinblat B, Haedersdal M, Valente N, Festa-Neto C, Szeimies RM. A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br J Dermatol. 2018;179:829–35.
Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008a;112:213–9.
Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008b;275:2585–96.
Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T, Baldisseri DM, Slominski A. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39:1577–88.
Tuckey RC, Slominski AT, Cheng CY, Chen J, Kim TK, Xiao M, Li W. Lumisterol is metabolized by CYP11A1: discovery of a new pathway. Int J Biochem Cell Biol. 2014;55:24–34.
Tuckey RC, Li W, Ma D, Cheng CYS, Wang KM, Kim TK, Jeayeng S, Slominski AT. CYP27A1 acts on the pre-vitamin D3 photoproduct, lumisterol, producing biologically active hydroxy-metabolites. J Steroid Biochem Mol Biol. 2018;181:1–10.
Tuckey RC, Cheng CYS, Slominski AT. The serum vitamin D metabolome: what we know and what is still to discover. J Steroid Biochem Mol Biol. 2019;186:4–21.
Turner RJ, Leonard N, Malcolm AJ, Lawrence CM, Dahl MG. A retrospective study of outcome of Mohs’ micrographic surgery for cutaneous squamous cell carcinoma using formalin fixed sections. Br J Dermatol. 2000;142:752–7.
Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Nitzki F, Fritsch A, Prufer N, Rosenberger A, Dullin C, Schraepler A, Reifenberger J, Schweyer S, Pietsch T, Strutz F, Schulz-Schaeffer W, Hahn H. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther. 2011;10:2179–88.
Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Rosenberger A, Dullin C, Schraepler A, Schulz-Schaeffer W, Hahn H. Calcitriol inhibits hedgehog signaling and induces vitamin d receptor signaling and differentiation in the patched mouse model of embryonal rhabdomyosarcoma. Sarcoma. 2012;2012:357040.
Walter A, Schafer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, Schonewolf N, Dummer R, Bloch W, Werner S, Beer HD, Knuth A, Van Den Broek M. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560.
Wang Z, Coleman DJ, Bajaj G, Liang X, Ganguli-Indra G, Indra AK. RXRalpha ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. J Invest Dermatol. 2011;131:177–87.
Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D(3) inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–46.
Wasiewicz T, Szyszka P, Cichorek M, Janjetovic Z, Tuckey RC, Slominski AT, Zmijewski MA. Antitumor effects of vitamin D analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int J Mol Sci. 2015;16:6645–67.
Wasiewicz T, Piotrowska A, Wierzbicka J, Slominski AT, Zmijewski MA. Antiproliferative activity of non-calcemic vitamin D analogs on human melanoma lines in relation to VDR and PDIA3 receptors. Int J Mol Sci. 2018;19:2583.
Wierzbicka JM, Binek A, Ahrends T, Nowacka JD, Szydlowska A, Turczyk L, Wasiewicz T, Wierzbicki PM, Sadej R, Tuckey RC, Slominski AT, Chybicki J, Adrych K, Kmiec Z, Zmijewski MA. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int J Oncol. 2015;47:1084–96.
Wierzbicka JM, Zmijewski MA, Piotrowska A, Nedoszytko B, Lange M, Tuckey RC, Slominski AT. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol Cell Endocrinol. 2016;437:312–22.
Williams HC, Bath-Hextall F, Ozolins M, Armstrong SJ, Colver GB, Perkins W, Miller PSJ. Surgery versus 5% imiquimod for nodular and superficial basal cell carcinoma: 5-year results of the SINS randomized controlled trial. J Investig Dermatol. 2017;137:614–9.
Wobke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244.
Wondrak GT. Let the sun shine in: mechanisms and potential for therapeutics in skin photodamage. Curr Opin Investig Drugs. 2007;8:390–400.
Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. 3-hydroxypyridine chromophores are endogenous sensitizers of photooxidative stress in human skin cells. J Biol Chem. 2004;279:30009–20.
Wong G, Gupta R, Dixon KM, Deo SS, Choong SM, Halliday GM, Bishop JE, Ishizuka S, Norman AW, Posner GH, Mason RS. 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J Steroid Biochem Mol Biol. 2004;89–90:567–70.
Work G, Invited R, Kim JYS, Kozlow JH, Mittal B, Moyer J, Olencki T, Rodgers P. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540–59.
Young AR. Chromophores in human skin. Phys Med Biol. 1997;42:789.
Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76:S100–9.
Yu S, Liu C, Li L, Tian T, Wang M, Hu Y, Yuan C, Zhang L, Ji C, Ma D. Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Lab Invest. 2015;95:157–67.
Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–80.
Zhao G, Simpson RU. Membrane localization, Caveolin-3 association and rapid actions of vitamin D receptor in cardiac myocytes. Steroids. 2010;75:555–9.
Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol. 2009;218:593–602.
Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Synthesis and photo-conversion of androsta- and pregna-5,7-dienes to vitamin D3-like derivatives. Photochem Photobiol Sci. 2008;7:1570–6.
Zmijewski MA, Li W, Zjawiony JK, Sweatman TW, Chen J, Miller DD, Slominski AT. Photo-conversion of two epimers (20R and 20S) of pregna-5,7-diene-3beta, 17alpha, 20-triol and their bioactivity in melanoma cells. Steroids. 2009;74:218–28.
Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3beta,21-dihydroxypregna-5,7-dien-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203.
Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–17.
Acknowledgment
The support of NIH grants 1R01AR073004-01A1 and R01AR071189-01A1 and VA merit grant 1I01BX004293-01A1 to ATS; P01CA210946, R01CA193885, P30 CA013148, and VA grant 101BX003395 to CAE; the National Science Centre of Poland grant 2017/25/B/NZ3/00431 to MAZ; funds for statutory research from Nicolaus Copernicus University to AAB; and the National Health and Medical Research Council of Australia (APP1070688) and Australian Research Council Linkage grant (LP100200680) to RSM are acknowledged. AMJ research was supported by the Intramural Research Program of the NIEHS, NIH (Z01-ES-101585).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Slominski, A.T. et al. (2020). The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers. In: Reichrath, J. (eds) Sunlight, Vitamin D and Skin Cancer. Advances in Experimental Medicine and Biology, vol 1268. Springer, Cham. https://doi.org/10.1007/978-3-030-46227-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-46227-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46226-0
Online ISBN: 978-3-030-46227-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)