Atechnological approach is proposed making it possible to prepare highly porous ceramic based on Al2O3 using a method of filtration combustion (FC). The method is based on an exothermic oxidation reaction (in an FC regime) of aluminum powder PAP-2 particles mixed with spherulites of commercial alumina (type 1 material) or kaolin fibers (type 2 material). Depending on the volume faction of PAP-2 open porosity for sintered type 1 material is from 38 to 50%, and ultimate strength in bending is from 10 to 50 MPa. Good material thermal shock resistance, evaluated from the relative loss of crack resistance after thermal cycling by a regime of heating to 850°C and cooling in an air stream (18°C), comprises 12 – 15% and is unchanged after five successive thermal cycles. Material of type 2 is an ultra-lightweight heat insulator with density of 0.25 – 0.50 g/cm3. The bearing strength is 0.10 – 0.15 MPa, and thermal conductivity in the range 20 to 1000°C is 0.06 – 0.17 W/(m·K).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Porous ceramic materials based on Al2O3 are used extensively in high-temperature engineering, for example as thermally stable structural elements, heat insulation, and gas and liquid media filters [1,2,3,4]. Creation of a pore space in material may be achieved by normal methods, for example use of additions that burn-off, implementation of a foam method, and also due to forming objects from fibers or hollow spherical particles, i.e., micro-balloons [5].
The possibility has been demonstrated in [6] of creating significant porosity in aluminum oxide material as a result of using the effect of zonal compaction during powder workpiece (PW) sintering consisting of particles of a nanosize range. In addition, in order to prepare porous refractory materials and heat insulation new technology has been developed combining cold swelling and self-propagation high-temperature synthesis (SHS), providing so-called “thermochemical synthesis”, and sintering of the phases formed due to the heat of exothermic reactions [7]. A method is also well known of reaction sintering in a filtration combustion (FC) regime in air of aluminum-magnesium alloy for preparing MgO–Al cermet [8], making it possible to prep[are highly porous objects. This technological approach may be considered as a variety of the SHS method in which combustion of a PWis achieved as a result of surrounding its surface with hot (550 – 600°C) air carrier within the volume of a furnace space. In this case FC is supported as a result of a drop in air oxygen partial pressure, contained within a system of communicating PW pores and flowing round a PW. In this case pumping (filtration) of air is realized within the volume of a workpiece. It should be noted that within the material a significant proportion of unoxidized aluminum is retained due to the protective action of cense aluminum oxide films at its surface.
Features of the structure and physicomechanical properties of porous ceramic based on Al2O3 prepared using the FC method were studied within the scope of the present work.
Experimental Procedure and Research
The starting materials used were commercial alumina powder grade G-00 (GOST 30558) whose particles have a spherulitic structure and also kaolin fibers (GOST 23619), and aluminum powder grade PAP-2 (GOST 5494) with a flaky shape of particles of sub-micron thickness. Previously spherulites of commercial alumina were heat treated in air for 1 h in order to complete modification transition γ-Al2O3→α-Al2O3, accompanied by a reduction in volume by about 14%.
In order to prepare specimens aluminum powder in an amount of 37 – 70 vol.% was mixed with spherulites and the mixture obtained was compacted under a pressure of 200 MPa (PW type 1). Also from kaolin fibers an aqueous suspension was prepared within which with continuous mixing aluminum powder was added (10 – 30 vol.%). Then water was extracted from the mixture obtained by vacuum filtration and the workpiece obtained was dried in air (PW type 2). Both PW types were heated in air to 500 – 550°C with which the FC process was initiated. After its completion heating continued to 1500°C with subsequent isothermal exposure for 1 h providing total oxidation of the metal component. In this case two types of sintered materials were prepared containing spherulites (type 1) and kaolin fibers (type 2).
Material of type 1 was tested for thermal shock resistance, and the value of relative loss of notched specimen crack resistance was evaluated after the action of thermal stresses [9], using a device for local thermal shock [10]. In this case thermal stresses at a notch tip were created by thermal cycling by a regime of heating in air at 850°C, and air stream cooling (18°c). The relative loss of crack resistance was calculated by an by a formula \( \left(1-{K}_{1c}^T/{K}_{1c}\right) \) 100%, where K1c and \( {K}_{1c}^T \) are values of critical stress intensity factor for specimens before and after thermal cycling respectively. Ultimate strength in bending was determined in prismatic specimens with a size of 8 × 8 × 50 mm, using a three-point loading scheme with a deformation rate of 1 mm/min. For type 2 material the collapsing stress was determined in compression for cylindrical specimens 20 mm in diameter and 50 mm long with a deformation rate of 0.1 mm/min. In addition, the index of effective thermal conductivity in the range 20 to 1000°C was determined by a hot wire method under conditions of steady state heat flow using a measuring bridge (ISO 8894-1).
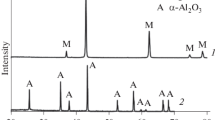
The material phase composition was studied using x-ray phase analysis (XPA) in a DRON-3 unit (Cu Kα radiation) by a standard procedure, and the structure was studied in a Hitachi-F405A electron microscope from a specimen fracture surface obtained after mechanical tests. The density ρ of sintered specimens was calculated as the ratio of their weight to volume. Overall porosity was calculated from a relationship (1 – ρ/γ) · 100%, where γ = 3.99 g/cm3. Open porosity was determined by hydrostatic weighing.
Discussion of Results
Porous ceramic materials were prepared by the FC method. It duration for specimens of material type 1 was 7 – 10 min, and for materials of type 2 it was 3 – 5 min. in the first case maximum brightness temperature was 1200°C, and in the second it was 1500°C.
In the initial stage of the FC process there is synthesis of Al2O3-phase as a result of exothermic reaction in the system melt (a) – gas (O2) as a result of oxygen diffusion through aluminum oxide surface films of flaky particles towards aluminum melt. The strength of these films is sufficient in order to maintain overheated aluminum melt at the FC temperature. Completion of specimen FC is apparently connected with formation of a dense network of nanosize aluminum oxide crystals at the surface of aluminum flaky particles, which it passivates, making them inactive and impermeable for the gaseous oxidizing agent, i.e., air oxygen. In this case aluminum flakes appear within dense aluminum oxide capsules.
As is seen from XPA results (Table 1), it was only possible to provide total aluminum oxidation within the composition of type 1 material after additional heat treatment in air (for completion of FC) at 1500°C for 1 h. Under these conditions the diffusion activity of atomic oxygen becomes adequate for penetration through aluminum oxide capsules of flaky particles and total metal oxidation. Lamellar aluminum oxide particles (α-Al2O3) are shown in Fig. 1 within the structure of the material obtained, which inherits the shape of the original flaky particles of PAP-2 powder. The material with significant porosity (Πopen = 38 – 50%) has quite good strength (σben = 10 – 50 MPa), which increases with an increase in volume fraction (V, %) of aluminum powder in the original mixture with spherulites (Table 2). This feature is explained by the reinforcing action of lamellar aluminum oxide particles forming a continuous framework, receiving the applied load. In this case a significant loss of crack resistance is observed after thermal shock due to rapid elimination of a temperature gradient within structural elements having micron cross section. It should be noted that the index for loss of crack resistance for material type 1 does not change after five successive thermal cycles, whereas for dense aluminum oxide material (No. 4, see Table 2) a marked reduction was observed in crack resistance after repeated thermal cycling as a result of the action of thermal stresses leading to formation of macrocracks within the structure.
Material of type 2 with density of 0.25 – 0.50 g/cm3 is classified as ultra-light weight heat insulation (Table 3). Stresses corresponding to the initial instant of collapse increase with an increase in volume faction of aluminum powder in a mixture with kaolin fibers. In this case an effect is also observed of structural reinforcement by lamellar aluminum oxide particles (similar to type 1 material). Within the material structure bonding of kaolin fibers is achieved by covering them with aluminum oxide lamellar and droplet shaped particles as a result of sintering over contact areas (Fig. 2). It should be noted that formation of particles 3 (see Fig. 2) occurs in the FC stage: agglomerates of nanoparticles of dusty fraction, contained in powder type PAP-2, during heating above the aluminum melting temperature form “sessile” droplets with a typical shape due to limited wetting of the kaolin fiber surface. This mechanism of bonding kaolin fibers within the volume of a specimen is confirmed with consideration of their fracture surface after mechanical tests (Fig. 3).
The ultra-lightweight heat insulation developed is demonstrated by the relatively low thermal conductivity, i.e., 0.06 – 0.17 W/(M·K) in the range from 20 to 1000°C, which may be explained by the screening action of lamellar Al2O3 particles arranged normal with respect to the heat flow. The dependence of thermal conductivity λ for type 2 material (V = 20 vol.%) on temperature T is given below.
T, °C......... | 20 | 200 | 400 | 600 | 800 | 1000 |
|---|---|---|---|---|---|---|
λ, W/(m·K) .... | 0.06 | 0.08 | 0.09 | 0.10 | 0.12 | 0.17 |
Conclusion
In order to prepare porous ceramic materials based on Al2O3 an FC method is used for a PW in air in air consisting of a mixture of aluminum powder PAP-2 with spherulites of commercial alumina (material type 1) and with kaolin fibers (material type 2). After the end of FC specimens are additionally treated in air at 1500°C for 1 in order to complete aluminum oxidation. In this case from aluminum flaky particles lamellar aluminum oxide (α-Al2O3) particles are synthesized, inheriting the shape of the original aluminum flakes.
It has been established that for type 1 material with variation of the volume fraction of PAP-2 powder from 30 to 70% in a mixture with spherulites the open porosity is reduced from 50 to 38% and ultimate strength in bending increases from 10 – 20 to 30 – 50 MPa. This is connected with the reinforcing action of lamellar aluminum oxide particles that form a continuous framework withstanding an applied load. For type 1 material an insignificant loss of crack resistance (12 – 155) is recorded after thermal cycling by regime of heating at 850°C – air stream cooling at 18°C (this index did not change after five subsequent thermal cycles). This fact is possible due to rapid avoidance of a temperature gradient in structural elements having a micron cross section.
Type 2 material, with density of 0.25 – 0.50 g/cm3, is classified as ultra-lightweight heat insulation. Its density increases with an increase in volume fraction of aluminum powder (10 – 30%) in a mixture with kaolin fibers, and the collapsing strength with action of a compressive load is 0.10 – 0.15 MPa. The low thermal conductivity of the heat insulation obtained, i.e., 0.06 – 0.17 W/(m·K) in the range to 1000°C is explained by the screening action of lamellar Al2O3 particles arranged normal with respect to heat flow.
References
R. V. Zubashchenko, “Heat-resistant high-temperature heat insulation object based on aluminum silicate fiber,” Novye Ogneupory, No. 12, 3 – 5 (2016).
R. V. Zubashchenko, “Lining of a small tunnel kiln with high-alumina objects based on aluminosilicate fiber,” Novye Ogneupory, No. 2, 3 – 5 (2017).
A. Mocciaro, M. B. Lombardi, and A. N. Scian, “Ceramic material porous structure prepared using pore-forming additives,” Refract. Indust. Ceram., 58(1), 65 – 68 (2017).
A. V. Belyakov, Zaw Ye Maw Oo, N. A. Popova, et al., “Strengthening binders for porous permeable ceramic with electromelted corundum filler,” Refract. Indust. Ceram., 58(1), 89 – 93 (2017).
I. Ya. Guzman (editor) Ceramic Chemical technology: Higher School Textbook [in Russian], RIF Stroimatewrialy, Moscow (2003).
S. M. Barinov, V. A. Demin, A. V. Ivanov, D. A. Ivanov, A. Yu. Omarov, A. D. Shlyapin, and S. D. Shlyapin, RF Patent. 2522487, MPK C 04 B 35/111, 35/626. Method for preparing structural aluminum oxide ceramic, No. 2012146829/03, Claim 11.02.12, Publ. 07.20.14, Bull. No. 20.
V. S. Vladimirov, A. P. Galagan, M. A. Ilyukhin, et al., “New refractories and heat insulation materials and the manufacturing technology,” Novye Ogneupory, No. 1 (April), 81 – 88 (2002).
A. A. Vasin, V. P. Tarasovskii, A. Yu. Omarov, and V. V. Rybalchenko, “Study of cermet synthesis from powders prepared by chemical dispersion of Al–Mg (20 wt.%)-alloy,” Refract. Indust. Ceram., 56(3), 310 – 314 (2015).
D. A. Ivanov, A. I. Sitnikov, and G. E. Val’yano, “Evaluation of the thermal resistance of structural ceramics in testing notched prismatic samples,” Glass and Ceramics, 58(5/6), 169 – 173 (2001).
D. A. Ivanov and A. I. Sitnikov, “Features of ceramic material breakage during thermal loading by local thermal shock method,” Ogneupory Tekhn. Keram., No. 12, 30 – 35 (2004).
Research was carried out within the scope of the main part of a state assignment to higher education establishments No. 11.7568.2017/B4 using equipment of the collective use resource center “Aerospace materials and technology” MAI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 9, pp. 40 – 43, September, 2017.
Rights and permissions
About this article
Cite this article
Ivanov, D.A., Shlyapin, S.D., Val’yano, G.E. et al. Structure and Physicomechanical Properties of Porous Ceramic Based on Al2O3 Prepared Using a Filtration Combustion Method. Refract Ind Ceram 58, 538–541 (2018). https://doi.org/10.1007/s11148-018-0140-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-018-0140-5